Abstract
Background
Long-term oxygen therapy (LTOT) is an established treatment for patients with chronic hypoxemia. Its scientific basis is derived mainly from two trials from the early 1980s that showed a survival advantage for patients with chronic obstructive pulmonary disease (COPD) treated with LTOT. Robust data are not available for other diseases associated with hypoxemia.
Methods
This review is based on pertinent publications retrieved by a selective search in PubMed.
Results
The use of LTOT for 15 to 16 hours per day (or, better, 24 hours per day) is recommended in current guidelines for patients with chronic hypoxemia (PaO2 = 55 mm Hg) because this treatment was found to be associated with a lower mortality rate compared to no LTOT (33% vs. 55%, p <0.05) based on data from the early 1980s. In the short term, oxygen administration to a hypoxemic patient can improve oxygen saturation by nine percentage points and improve physical performance to a clinically relevant extent (6-minute walking test: + 37 m, p <0.001). The available data do not support the use of LTOT for normoxemic patients. LTOT should only be administered for strict indications, in accordance with the guidelines, and only in a form suitable for the individual patient. Skin burns can occur as a side effect of LTOT because of contact explosions with any type of fire.
Conclusion
The acquisition of further robust data would be desirable, particularly with respect to patient-relevant outcome parameters including quality of life, performance status, and mortality. Moreover, the German guidelines on oxygen therapy need to be updated.
In a first pilot study in 1967, Levine and colleagues (1) found that oxygen delivery to patients with chronic obstructive pulmonary disease (COPD) and hypoxemia improved pulmonary hypertension and increased exercise performance (1). As a result, oxygen supplementation developed as a therapeutic measure in chronic hypoxemia. However, there is still insufficient evidence for oxygen therapy, as it is essentially based on two studies from the early 1980s (2, 3).
This review provides an overview of the current evidence, with the aim of examining the practical, day-to-day aspects of long-term oxygen therapy (LTOT).
Methods
This article is based on a selective literature search in PubMed. Publications from January 1980 to April 2018 were searched using the terms “long-term oxygen therapy,” “ambulatory oxygen therapy,” “nocturnal oxygen therapy,” and “supplemental oxygen.” Current German and international guidelines (in German or English) were also included.
Results
Long-Term Oxygen Therapy
For chronic hypoxemic patients with an arterial partial pressure of oxygen (PaO2) =55mmHg, national guidelines for LTOT recommend giving oxygen for at least 15 hours per day (4) or for 16 hours per day (5); this should be extended to 24 hours for increased efficacy (see section “Indication criteria for LTOT”) (4, 5). This includes targeted oxygen replacement during exercise and at night. As studies do not consider these different situations of oxygen deficiency separately, the effects of these three therapeutic approaches cannot be delimited. The goals of LTOT are to improve quality of life and exercise performance, as well as to reduce morbidity and mortality (5).
Effects of LTOT
LTOT for chronic hypoxemia
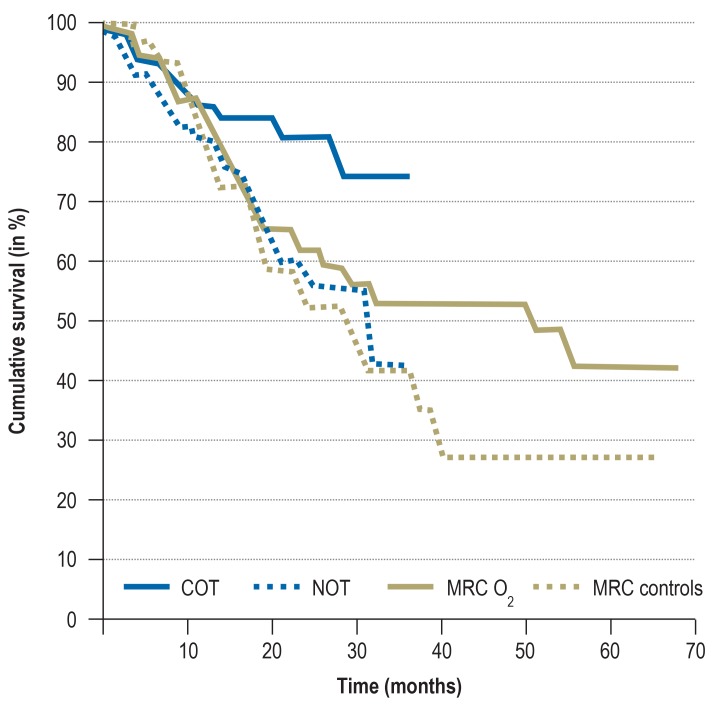
The currently validated evidence for prescribing LTOT is based on two randomized controlled trials published in the early 1980s (2, 3). In the so-called MRC (Medical Research Council) trial, 87 COPD patients (FEV1, 0.6 L) were included who had pronounced chronic hypoxemia (PaO2=51mmHg) and hypercapnia (PaCO2=54mm Hg) in a resting state; they were then randomized to an LTOT group that received oxygen for at least 15 hours per day or to a no-oxygen control group (3). Within the 5-year study period, the probability of survival in the LTOT group was significantly improved (55% versus 33%; p <0.05) (figure 1), with the greatest survival benefit observed for hypercapnia patients (6). The second trial (Nocturnal Oxygen Therapy Trial [NOTT]), which compared continuous (24-hour) oxygen administration with 12-hour nocturnal oxygen supplementation over a period of two years in patients with chronic hypoxemia and COPD, found a survival benefit for 24-hour oxygen administration (87% versus 59%; p<0.05) (2). For COPD patients with lower disease severity (PaO2 56–65mm Hg) (7) or with oxygen saturation levels of 88% to 93% (8), no mortality reduction was detected after six years (mortality for oxygen therapy group, 18%, versus control group, 20%; p=0.53).
Figure 1.
Effects of long-term oxygen therapy on patients with COPD and hypoxemia,
from the Medical Research Council (MRC) trial and the Nocturnal Oxygen Therapy Trial (NOTT).
The figure shows survival data of men <70 years of age.
COT, continuous oxygen therapy (24-h O2 supplementation per day);
NOT, nocturnal oxygen therapy (O2 supplementation only at night);
MRC O2, 15-h O2 supplementation per day), MRC controls, no O2 supplementation
(Figure taken from Stoller et al. 2010 [11]; reprinted with permission from Elsevier)
For patients with isolated nocturnal hypoxemia, a double-blind, randomized trial showed that pulmonary arterial pressure was significantly reduced after three years of nocturnal oxygen delivery (of 3 L/min), but was significantly increased by delivery of compressed room air. No impact on mortality was detectable (9). There is some evidence that oxygen delivery during nocturnal hypoxemia improves sleep duration and sleep quality (as measured by EEG) (10).
LTOT for exercise-induced hypoxemia
Some evidence suggests that isolated, exercise-induced hypoxemia is an independent predictor of increased 5-year mortality risk in patients with COPD (relative risk 2.63 [95% confidence interval (CI): 1.53; 4.51], p<0.001) (11). For the most part, a decrease in oxygen saturation during exercise to below a threshold of 88% to 90%, or a relative decline during exercise of 2% to 5%, is considered clinically relevant (12). A retrospective analysis of 471 patients with COPD and exclusively exercise-induced hypoxemia found no significant differences in mortality, irrespective of whether patients were given continuous LTOT, intermittent oxygen therapy, or no supplemental oxygen (13).
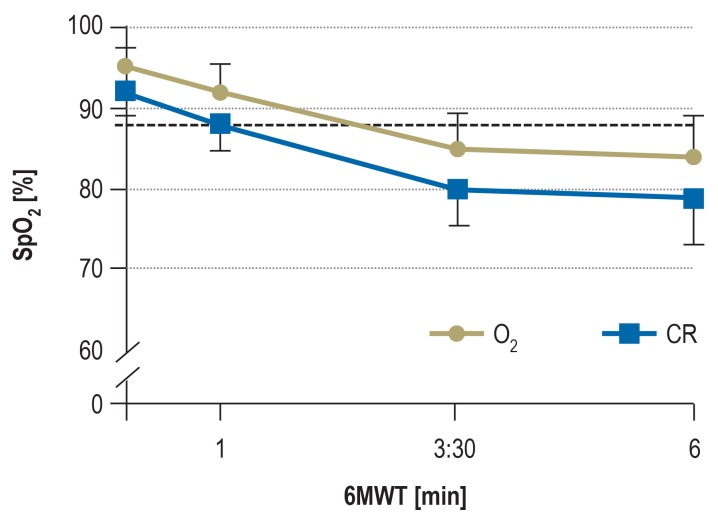
Nevertheless, oxygen administration appears to be useful and beneficial for exercise-induced hypoxemia, for example as part of an exercise training program. Several cross-over trials have shown that oxygen administration to patients with COPD leads to a reduction in both breathing frequency and dynamic hyperinflation, and contributes to a significant increase in short-term exercise tolerance (14, 15). A recent study showed that administering oxygen at a non-individually tailored rate of 2L/min to patients with COPD and exercise-induced hypoxemia led to a relevant proportion (76%) of patients who experienced a drop in oxygen saturation, to either below 88% or by =4% points, during the 6-minute walk test (15) (figure 2).
Figure 2.
About 76% of patients with COPD and exercise-induced hypoxemia who received oxygen supplementation at a set flow rate of 2L/min had a relevant drop in SpO2 during the 6-minute walk test (15).
6MWT, 6-minute walk test; SpO2, arterial oxygen saturation; CR, compressed room air (Reprinted with permission from Elsevier)
LTOT during exercise with normoxemia
The use of LTOT for patients with COPD and normoxemia has only been addressed by a small number of studies. Two double-blind, randomized trials that compared giving normoxemic patients with severe COPD (FEV1, 36% to 44% predicted) either oxygen or compressed room air during exercise arrived at distinct conclusions. Emtner and colleagues (16) showed that, after a 7-week ergometer training program, patients with COPD who were administered oxygen during exercise increased their endurance capacity by 38% as compared to those who received room air. In the double-blind, randomized trial by Spielmanns et al. (17), no difference in either exercise capacity or quality of life could be demonstrated after a 6-month exercise program with administration (at a flow rate of 4 L/min) of either oxygen or compressed air during exercise. In contrast, a small double-blind, cross-over trial found that 29 patients with COPD (FEV1, 46% predicted) who participated in ergometer training for 6 weeks showed an improvement in endurance of 12 watts after supplementation with oxygen (at an unusually high flow rate, of 10 L/min), as compared to only 5 watts without oxygen supplementation (18).
Diagnostic work-up and LTOT prescription
Indication criteria for LTOT
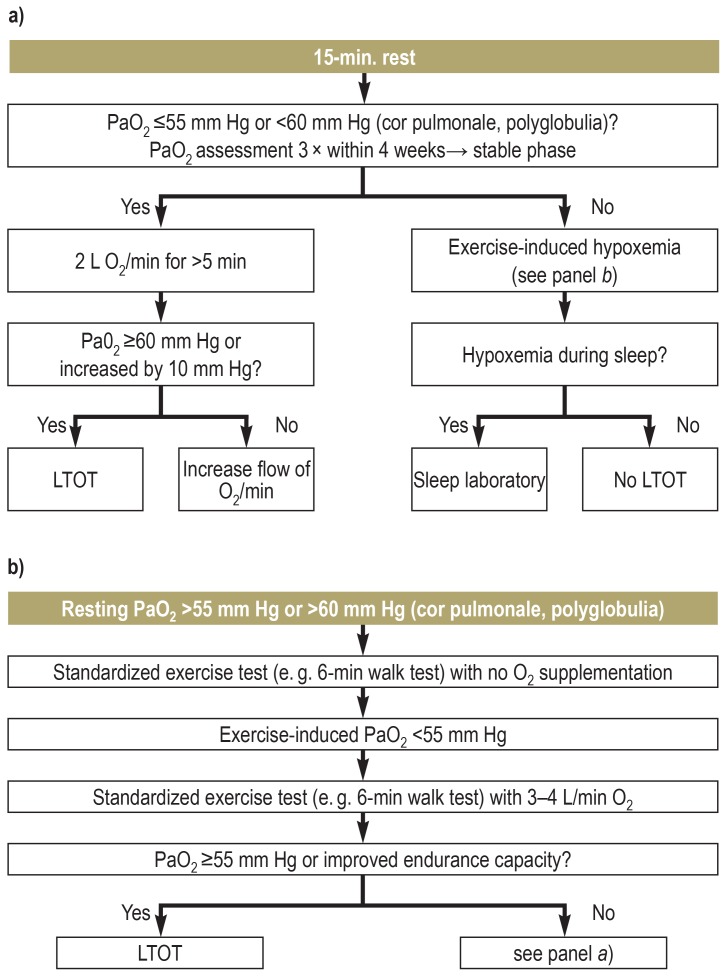
An indication for LTOT exists if, despite adequate therapy of the underlying disease, patients still have chronic hypoxemia at rest, during exercise, or at night. Chronic hypoxemia is present if the arterial partial pressure of oxygen (PaO2) is measured to be =55mm Hg at least three times under resting conditions during a stable disease phase (of around four weeks). If secondary polyglobulia and/or cor pulmonale (with or without right-sided heart failure) are present, an indication for LTOT already exists at =60mmHg (5).
Hypoxemia observed only at night should be further assessed in a medical sleep laboratory (5) (figure 3).
Figure 3.
Long-term oxygen therapy (LTOT)
a) Blood gas analysis at rest and b) blood gas analysis during exercise (19). PaO2, arterial partial pressure of oxygen. (Reprinted with permission from Elsevier)
The available guidelines for LTOT, which are mainly based on the NOTT and MRC trials in patients with COPD, transfer the recommendations for LTOT (shown in Figure 3) also to patients with other types of hypoxemia. Contraindications to LTOT have not yet been defined. The presence of asymptomatic hypercapnia prior to LTOT, or its development during LTOT, is not a strict contraindication (5). However, a critical clinical evaluation may be advisable, to determine whether an indication for non-invasive ventilation therapy (NIV) is present (19). For instance, morning headaches can be symptoms of increased hypercapnia.
A review published in 2017 that compared the British and German guidelines on LTOT found similarities in the duration of use, although it also identified differences in the indications (20) (table).
Table. Overview of differences between the BTS (British Thoracic Society) and the DGP (German Respiratory Society) guidelines for LTOT (taken from Magnet et al. [20]).
|
British guideline of the British Thoracic Society (BTS) |
German guideline of the German Respiratory Society (DGP) |
||
| 1. | Blood gas analysis technique |
Arterial blood gas analysis (ABG) | Capillary blood gas analysis (CBG) |
| 2. | Criteria for initiation of LTOT in stable patients |
Two ABG analyses at least 3 weeks apart | Three CBG analyses within a 4-week period |
| 3. | Initiation of LTOT in patients with COPD after exacerbation | Discharge with oxygen supplementation only if patients cannot manage without oxygen, are breathless, and with SpO2 ≤92% at rest (room air); follow-up assessment of LTOT after 8 weeks | Direct, with later follow-up to re-evaluate indication |
| 4. | Ambulatory oxygen therapy (AOT) for patients without classic indication criteria | Only during rehabilitation | Indicated by resting PaO2 ≤60 mm Hg and by drop in PaO2 during 6MWT or if AOT improves exercise capacity; not indicated by exclusively exercise-induced hypoxemia without increasing dyspnoea and resting PaO2 >55 mm Hg |
| 5. | Nocturnal oxygen therapy | Only for severe congestive heart failure and in combination with NIV for other diseases | No clear statement—further evaluation by sleep laboratory recommended |
| 6. | Titration of O2 flow rates | Based on SpO2 (followed by ABG) | Based on CBG |
| 7. | Follow-up/re-assessment of LTOT |
Home visit within 4 weeks, with first re-assessment after 3 months, and thereafter every 6–12 months | Every 3 months (outpatient setting) |
| 8. | Patients who smoke | No contraindications | Conflicting information: LTOT is indicated after avoidance of all inhalative noxae; an overview table however states that there are no contraindications |
COPD, chronic obstructive pulmonary disease; LTOT, long-term oxygen therapy; NIV, non-invasive ventilation; PaO2, arterial partial pressure of oxygen;
SpO2, blood oxygen saturation; 6MWT, 6-minute walk test
It should be noted that the 2008 guideline for Germany (which has a supplementary statement from 2014 [21]) needs to be updated in several points; this is currently ongoing.
The diagnosis of hypoxemic respiratory failure
Hypoxemia is diagnosed by arterial blood gas (ABG) or capillary blood gas (CBG) analysis or blood oxygen saturation (SpO2) measurement by pulse oximetry. ABG is considered the gold standard for measuring hypoxemia. The German guideline considers CBG and ABG analyses to be equivalent (5), although recent data suggest that CBG analysis underestimates the actual oxygenation level in the blood (22). Depending on whether one sets a limit value for LTOT at 55 mm Hg or 60 mm Hg, about 21% or 30% of patients, respectively, would have been prescribed non-indicated LTOT, thus representing overprescription (22). After confirmation of hypoxemia at rest, during exercise, and/or at night, the necessary oxygen flow rates should be titrated to achieve PaO2 =60 mm Hg. Blood gas analysis should be carried out after a rest period of =15 minutes (5). If the PaO2 level is =55 mm Hg, an initial delivery by nasal cannula of oxygen at 2 L/min for =5 min should be made, followed by a CBG analysis to check for effectiveness (5). If the PaO2 level does not sufficiently increase, the flow rate should be further increased (4, 5). A lack of increase requires further diagnostic investigation (5). For mobile patients, oxygen titration should also be carried out under a standardized exercise situation (for example, the 6-minute walk test) (5) with a portable oxygen device.
The prescription of LTOT
Several factors should be taken into account when prescribing LTOT, including the diagnosis, the extent of hypoxemia and hypercapnia, the oxygen flow rate required to achieve PaO2 =60 mm Hg (at rest/during exercise/at night), the patient’s mobility, and the desired delivery system (5). Patients who still smoke should be informed about the risk of explosion/burns (61 cases per 100000 person-years [23]) for smoking during LTOT (19). In addition, nose bleeds, dizziness, and reduced sense of taste and smell are potential side effects of LTOT (24).
Delivery systems
Oxygen source
In the home environment, the oxygen source for LTOT can be concentrators, steel gas cylinders, or liquid oxygen. All forms are available in both static and portable versions. The choice of oxygen source should ideally be made together with patients, taking into account their needs (such as their mobility range and general condition) as well as the device characteristics (e.g., weight, levels of delivery, and oxygen tank capacity).
Concentrators
Concentrators (both static and portable) are the most commonly used devices for oxygen delivery. Concentrators filter nitrogen out of room air and produce a gas with an oxygen purity of 85%–95% (4). The device characteristics, such as battery life, weight, level of noise, and oxygen output of portable concentrators, vary considerably between different manufacturers (4).
Liquid oxygen
Using liquid oxygen is the second most common form of LTOT. The biggest disadvantage as compared to concentrators is the limited oxygen tank capacity of portable oxygen devices and the need to have access to a liquid oxygen refill station.
Steel gas cylinders
Steel gas cylinders with gaseous compressed oxygen are no longer commonly used because of their bulky size and weight. They are considered to be less effective and to be restrictive for patient mobility.
Types of oxygen delivery
Oxygen delivery by nasal cannula is by far the most common LTOT delivery form (4). In rare cases, an oxygen mask or a trans-tracheal cannula is indicated for oxygen delivery.
In addition to devices with a continuous flow of oxygen, some devices release an oxygen bolus when negative pressure occurs in the oxygen nasal cannula during inspiration (the “demand” systems). This delivery form can make the oxygen supply last significantly longer (25). However, not all patients are suitable for such demand devices. Demand devices may provide only limited or insufficient oxygenation to some patient groups, as oxygen release might not always be triggered, especially for patients during exercise or for patients who are mouth breathers (26). Thus, individual oxygenation testing (both at rest and during exercise) should always be performed before a patient can use a demand system (27).
The follow-up of LTOT
Patients receiving LTOT should receive regular follow-up care. For patients in stable condition, the German guideline recommends specialist check-ups every three months (5). In addition to documenting the clinical condition of the patient, these appointments serve to ensure that LTOT is still indicated, as well as to control the efficiency of the prescribed oxygen flow rates and to assess treatment compliance (4, 5).
Especially for patients with COPD who started LTOT during an exacerbation-related hospitalization, the indications for LTOT may disappear as their condition improves (28). As oversupplying oxygen is costly, and as this type of therapy has known psychosocial consequences (e. g., social isolation, depression, fear of addiction), the indications for LTOT should be reviewed after 8 weeks (4). The practice-relevant aspects for implementing LTOT in day-to-day clinical practice are outlined in the Box.
BOX. Relevant day-to-day aspects for clinical practices.
Prescribe LTOT only during the stable disease phase
Re-evaluate LTOT every three months (need /efficiency/adherence). For unstable disease states, control more frequently
Titrate the required oxygen flow rate individually (at rest, during physical activity, and at night)
Prescribe an adequate delivery system with device instructions
Ensure that demand oxygen delivery systems provide sufficient oxygen saturation levels in the individual patient
Inform patients receiving LTOT who continue to smoke about potential dangers
Discussion
LTOT is a well-established treatment option for patients with hypoxemia. Nonetheless, it is still supported by insufficient evidence, which mainly comes from two randomized controlled trials of patients with COPD that were carried out in the early 1980s—a time at which COPD was treated quite differently than it is today, both in terms of underlying disease and comorbidities. Ultimately, therefore, we cannot assume that these results in the same form are still currently valid. In addition, the evidence for the recommendations in the guidelines is still weak for patients who do not have COPD.
A recent study from the USA that was published in a high-ranking journal (8) has contributed less than expected to clarifying this matter; at best, its conclusions may be useful for patients with mild hypoxemia. The selected inclusion criteria (SpO2: 89% to 93%, with an exercise-induced desaturation for 10 seconds to <90%) are not consensus criteria for LTOT. In addition, this study has several methodological weaknesses (including changes in the primary endpoint during the study, no blood gas analyses, no objective control over the actually-used LTOT duration, and others).
Convincing data are available to demonstrate the short-term effects of LTOT. These include improved exercise capacity, improved oxygenation, and lower exercise-induced dyspnoea (15). In order to investigate the importance of oxygen supplementation for patients with purely exercise-induced hypoxemia, standardized exercise protocols are useful.
Several issues should be considered when evaluating indications for LTOT; however, there is still not a structured or consensus method for addressing these issues.
In addition, it is important to evaluate which flow rates are required for each individual (at rest, at night, and during exercise). Frequently, set flow rates over 24 hours are prescribed; however, this does not take into account the actual requirements of everyday life. Additionally, the most suitable delivery form that best meets patient’s needs must be determined. Manufacturers should make it possible to measure compliance in terms of device use (which is technically possible). In addition, modified delivery systems could increase the efficiency of LTOT during increased oxygen demand (e. g., by using nasal cannulae with larger luminal diameter and an oxygen reservoir) (29).
Oxygen supplementation during exercise cannot be recommended for normoxemic patients with COPD, given its questionable effectiveness, the substantial resources required, and the potential for psychological stress.
Several issues remain unanswered, such as whether guideline-compliant, targeted therapy for nocturnal hypoxemia improves prognosis, or which long-term effects LTOT has on purely exercised-induced oxygen deficiency. With respect to the latter question, the British Thoracic Society recommends that oxygen therapy should be provided during exercise only after proof of improvement in exercise endurance (evidence level B) (4). This approach makes sense (15). However, until the guideline for Germany is updated (which is currently in progress), the 2008 guideline remains valid.
For other chronic diseases that are associated with hypoxemia, there are no reliable data for indicating LTOT. Despite the lack of evidence, prescriptions are currently being recommended analogous to those for COPD.
In general, and especially for borderline indications, consideration should be given as to whether oxygen therapy brings more clinical and prognostic benefits than negative effects from feelings of shame, fear of social exclusion, and complications due to the devices themselves. Here, regular follow-up evaluations and discussions with patients are recommended.
Ultimately, the central questions about whether LTOT provides valid long-term positive effects on quality of life and mortality still have not been sufficiently clarified.
Outlook
The evidence presented here clearly shows that high-quality studies are urgently needed in the future to address the open questions surrounding LTOT. However, this will be difficult to achieve in light of the enormous scope of the studies required and the associated high costs. Questions about survival benefits due to oxygen supplementation will be difficult to clarify in a scientifically and ethically acceptable manner. It is therefore all the more important to emphasize patient-relevant success criteria, such as the improvement of subjective well-being, exercise capacity, social inclusion, and thus the overall quality of life, for the affected patients when considering indication for LTOT.
Key Messages.
Long-term oxygen therapy (LTOT) is an established therapeutic measure for patients with chronic hypoxemia (PaO2 = 55 mm Hg).
From the numerous devices available, it is important to prescribe a system that is suitable for the day-to-day life of those affected.
The aims of LTOT are to improve quality of life and performance as well as to reduce morbidity and mortality.
The scientific evidence for LTOT is insufficient, especially with regard to long-term effects.
The German guideline for oxygen therapy from the year 2008 is partially outdated and is currently under revision.
Acknowledgments
Translated from the original German by Dr. Veronica A. Raker
Footnotes
Conflict of interest statement Prof. Kenn has received consultant honoraria, registration fee reimbursement, and reimbursement for travel and accomodation costs from RESMED, and honoraria for preparation of scientific training courses and study support (third-party funds) for self-initiated research from Linde, RESMED, and Heinen + Löwenstein.
The remaining authors declare that no conflict of interest exists.
References
- 1.Levine BE, Bigelow DB, Hamstra RD, et al. The role of long-term continuous oxygen administration in patients with chronic airway obstruction with hypoxemia. Ann Intern Med. 1967;66:639–650. doi: 10.7326/0003-4819-66-4-639. [DOI] [PubMed] [Google Scholar]
- 2.Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med. 1980;93:391–398. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 3.Report of the Medical Research Council Working Party. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Lancet. 1981;1:681–686. [PubMed] [Google Scholar]
- 4.Hardinge M, Annandale J, Bourne S, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax. 2015;70(Suppl 1):i1–i43. doi: 10.1136/thoraxjnl-2015-206865. [DOI] [PubMed] [Google Scholar]
- 5.Magnussen H, Kirsten AM, Kohler D, et al. Leitlinien zur Langzeit-Sauerstofftherapie. Pneumologie. 2008;62:748–756. doi: 10.1055/s-2008-1038290. [DOI] [PubMed] [Google Scholar]
- 6.Cooper CB, Waterhouse J, Howard P. Twelve year clinical study of patients with hypoxic cor pulmonale given long term domiciliary oxygen therapy. Thorax. 1987;42:105–110. doi: 10.1136/thx.42.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorecka D, Gorzelak K, Sliwinski P, Tobiasz M, Zielinski J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax. 1997;52:674–679. doi: 10.1136/thx.52.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Albert RK, Au DH, Blackford AL, et al. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med. 2016;375:1617–1627. doi: 10.1056/NEJMoa1604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fletcher EC, Luckett RA, Goodnight-White S, et al. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis. 1992;145:1070–1076. doi: 10.1164/ajrccm/145.5.1070. [DOI] [PubMed] [Google Scholar]
- 10.Calverley PM, Brezinova V, Douglas NJ, Catterall JR, Flenley DC. The effect of oxygenation on sleep quality in chronic bronchitis and emphysema. Am Rev Respir Dis. 1982;126:206–210. doi: 10.1164/arrd.1982.126.2.206. [DOI] [PubMed] [Google Scholar]
- 11.Casanova C, Cote C, Marin JM, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest. 2008;134:746–752. doi: 10.1378/chest.08-0520. [DOI] [PubMed] [Google Scholar]
- 12.Stoller JK, Panos RJ, Krachman S, Doherty DE, Make B. Oxygen therapy for patients with COPD: current evidence and the long-term oxygen treatment trial. Chest. 2010;138:179–187. doi: 10.1378/chest.09-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drummond MB, Blackford AL, Benditt JO, et al. Continuous oxygen use in nonhypoxemic emphysema patients identifies a high-risk subset of patients: retrospective analysis of the National Emphysema Treatment Trial. Chest. 2008;134:497–506. doi: 10.1378/chest.08-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J. 2001;18:77–84. doi: 10.1183/09031936.01.00082201. [DOI] [PubMed] [Google Scholar]
- 15.Jarosch I, Gloeckl R, Damm E, et al. Short-term effects of supplemental oxygen on 6-min walk test outcomes in patients with COPD: a randomized, placebo-controlled, single-blind, crossover trial. Chest. 2017;151:795–803. doi: 10.1016/j.chest.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med. 2003;168:1034–1042. doi: 10.1164/rccm.200212-1525OC. [DOI] [PubMed] [Google Scholar]
- 17.Spielmanns M, Fuchs-Bergsma C, Winkler A, et al. Effects of oxygen supply during training on subjects with COPD who are normoxemic at rest and during exercise: a blinded randomized controlled trial. Respir Care. 2015;60:540–548. doi: 10.4187/respcare.03647. [DOI] [PubMed] [Google Scholar]
- 18.Neunhauserer D, Steidle-Kloc E, Weiss G, et al. Supplemental oxygen during high-intensity exercise training in nonhypoxemic chronic obstructive pulmonary disease. Am J Med. 2016;129:1185–1193. doi: 10.1016/j.amjmed.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 19.Koehler U, Hildebrandt O, Jerrentrup L, et al. (Long-term oxygen therapy (LTOT)—what should physicians, homecare-providers and health insurance companies know?) Pneumologie. 2014;68:193–198. doi: 10.1055/s-0033-1359198. [DOI] [PubMed] [Google Scholar]
- 20.Magnet FS, Schwarz SB, Callegari J, et al. Long-term oxygen therapy: comparison of the German and British guidelines. Respiration. 2017;93:253–263. doi: 10.1159/000455879. [DOI] [PubMed] [Google Scholar]
- 21.Magnussen H, Hauck RW, Worth H, Herth FJ. Position zur Langzeit-Sauerstofftherapie. Pneumologie. 2014;68:591–593. doi: 10.1055/s-0034-1377329. [DOI] [PubMed] [Google Scholar]
- 22.Magnet FS, Majorski DS, Callegari J, et al. Capillary PO2 does not adequately reflect arterial PO2 in hypoxemic COPD patients. Int J Chron Obstruct Pulmon Dis. 2017;12:2647–2653. doi: 10.2147/COPD.S140843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanash HA, Huss F, Ekstrom M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479–2484. doi: 10.2147/COPD.S91508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katsenos S, Constantopoulos SH. Long-term oxygen therapy in COPD: factors affecting and ways of improving patient compliance. Pulm Med. 2011;2011 doi: 10.1155/2011/325362. 325362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiep B, Carter R. Oxygen conserving devices and methodologies. Chron Respir Dis. 2008;5:109–114. doi: 10.1177/1479972308090691. [DOI] [PubMed] [Google Scholar]
- 26.Roberts CM, Bell J, Wedzicha JA. Comparison of the efficacy of a demand oxygen delivery system with continuous low flow oxygen in subjects with stable COPD and severe oxygen desaturation on walking. Thorax. 1996;51:831–834. doi: 10.1136/thx.51.8.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiep BL, Barnett J, Schiffman G, Sanchez O, Carter R. Maintaining oxygenation via demand oxygen delivery during rest and exercise. Respir Care. 2002;47:887–892. [PubMed] [Google Scholar]
- 28.Magnet FS, Storre JH, Windisch W. Home oxygen therapy: evidence versus reality. Expert Rev Respir Med. 2017;11:425–441. doi: 10.1080/17476348.2017.1325323. [DOI] [PubMed] [Google Scholar]
- 29.Gloeckl R, Heinzelmann I, Matthaei M, et al. Benefits of an oxygen reservoir cannula versus a conventional nasal cannula during exercise in hypoxemic COPD patients: a crossover trial. Respiration. 2014;88:399–405. doi: 10.1159/000368165. [DOI] [PubMed] [Google Scholar]