Abstract
Diabetic peripheral neuropathy (DPN) is the most common chronic complication of diabetes. It poses a significant challenge for clinicians as it is often diagnosed late when patients present with advanced consequences such as foot ulceration. Autonomic neuropathy (AN) is also a frequent and under-diagnosed complication unless it is overtly symptomatic. Both somatic and autonomic neuropathy are associated with increased mortality. Multiple clinical trials have failed because of limited efficacy in advanced disease, inadequate trial duration, lack of effective surrogate end-points and a lack of deterioration in the placebo arm in clinical trials of DPN. Multifactorial risk factor reduction, targeting glycaemia, blood pressure and lipids can reduce the progression of DPN and AN. Treatment of painful DPN reduces painful symptoms by about 50% at best, but there is limited efficacy with any single agent. This reflects the complex aetiology of painful DPN and argues for improved clinical phenotyping with the use of targeted therapy, taking into account co-morbid conditions such as anxiety, depression and sleep disturbance.
Keywords: diabetes mellitus, peripheral neuropathy, autonomic neuropathy
Diagnosing diabetic peripheral neuropathy: too little too late
The early diagnosis and monitoring of diabetic peripheral neuropathy (DPN) are recommended by both the Toronto consensus 1 and the more recent American Diabetes Association (ADA) position statement on DPN 2. They recommend the presence of at least one symptom or sign of neuropathy and abnormal neurophysiology for the diagnosis of DPN. Symptom questionnaires, composite neurological scores and quantitative sensory testing (QST) may be used to diagnose DPN ( Table 1). The neuropathy disability score 3, a composite measure of neurological deficits (based on Achilles tendon reflexes, 120-Hz vibration, temperature and pin-prick sensation); Toronto Clinical Neuropathy Score 4; and the Michigan Neuropathy Screening Instrument 5 are validated neurological scores of clinical DPN. Although these tests are adequate tools to screen for DPN, they lack the sensitivity to assess change in clinical trials of relatively short duration (12–24 months). Yet they continue to be advocated as measures of efficacy, despite serial failure to show benefits in clinical trials of DPN.
Table 1. Common tests for the assessment of neuropathy.
| Type of nerve | Investigation | Advantages and disadvantages |
|---|---|---|
| Large fibre | Nerve conduction studies | Gold standard
Sensitive, specific, and reproducible and easily standardised Must be done by trained professional |
| Large and small fibres | Neuropathy disability score | Good predictor for risk of ulceration
Subjective Does not detect sub-clinical large fibre damage |
| Small fibre | Quantitative sensory testing | Reproducible and reliable
Subjective |
| Skin biopsy | Gold standard for small fibre testing
Reliable and reproducible Invasive procedure which needs specialised laboratory service |
|
| Corneal confocal
microscopy |
Rapid, reproducible, non-invasive
Detects small fibre damage and tracks worsening and improvement in small phase 2b clinical trials Requires training to perform |
QST 6 is a painless, non-invasive means to diagnose small and large fibre dysfunction and is based on impaired thermal, pain and vibration perception, respectively ( Table 1). Elevated vibration perception threshold is a risk factor for foot ulceration and lower-extremity amputation 7 but is a subjective test 8. Light touch can be assessed by using the 10-g monofilament and is commonly advocated as a screening tool for DPN, although it can only detect advanced neuropathy 9 and those at increased risk of amputation 10. Diagnosing established DPN is akin to ‘closing the stable door after the horse has bolted’.
Nerve conduction studies assess large fibre function and are currently advocated as the gold standard for a definite diagnosis of DPN 11. The typical electrophysiological findings in DPN are reduced amplitude of the compound muscle action potential, slower nerve conduction velocity, prolonged F-wave latency and an altered H-reflex. They are particularly useful for differentiating from other or concomitant neuropathies such as chronic inflammatory demyelinating polyneuropathy (CIDP) 12. They are also advocated as a primary end-point to measure therapeutic effect but have equally failed in the majority of clinical trials of DPN 13.
Thus, several caveats ought to be carefully considered when these tests are employed to assess change over time and the response to therapies. Although composite scores and QST are resourceful methods to assess neuropathy, they have poor sensitivity and reproducibility 14 and low histopathological specificity 15. They may be of value in large longitudinal cohort studies as opposed to individual patients or relatively small phase III clinical trials of short duration 14. Nerve conduction studies cannot assess small fibre neuropathy and have poor inter-rater reproducibility 16, making multi-centre trials difficult 17. Indeed, these measures have consistently failed to show meaningful improvements in clinical trials of neuropathy 18– 20.
Intra-epidermal nerve density (IENFD) evaluation in skin biopsy offers an objective and more reproducible means to assess small nerve fibre pathology 21. IENFD is reduced in pre-diabetes 22, predicts incident neuropathy 23 and improves with lifestyle intervention 24. Unlike neurophysiology, skin biopsy is not confounded by height and weight, although a gender- and age-dependent decline has been reported 21. There are published guidelines on the use of skin biopsy to diagnose DPN 25. However, wider adoption of skin biopsy is limited by cost, the need for a dedicated processing and assessment facility, and the risk of bleeding and infection following the procedure. Corneal confocal microscopy (CCM) is a powerful, non-invasive ophthalmic imaging end-point for DPN and other neuropathies 26. CCM can be used to quantify small fibre pathology with high reproducibility 27. Corneal c-fibres form the sub-basal nerve plexus and are highly metabolically demanding 28 and hence vulnerable even to transient and minor metabolic perturbation 22. Recent studies have established CCM values for the diagnosis 29– 31 and prediction 32, 33 of DPN on the basis of corneal nerve fibre density and length 34– 37. CCM has also shown corneal nerve regeneration in clinical trials before change in other US Food and Drug Administration (FDA)-accepted end-points (for example, neurophysiology and skin biopsy), a finding which has important implications for clinical trial design 13.
Disease modification for diabetic neuropathy
In the 2017 ADA position statement on diabetic neuropathy, early recognition of DPN even in pre-diabetes is recommended 2, 38, 39. There are no FDA-approved disease-modifying treatments for the management of DPN, and improved glycaemic control to prevent progression forms the mainstay of treatment. In type 1 diabetes mellitus (T1DM), improved glycaemic control can prevent the development and delay the progression of neuropathy 40. However, in T2DM, there is limited evidence that improved glycaemic control can slow down the progression of neuropathy 41– 43. The EURODIAB IDDM (European Diabetes Centers Study of Complications in Patients with Insulin-dependent Diabetes Mellitus), which evaluated over 3000 patients with T1DM for a duration of 7 years, identified poor glycaemic control, elevated low-density lipoprotein (LDL) cholesterol and triglycerides, hypertension, obesity and smoking as risk factors for incident DPN 44.
In patients with T1DM, treatment with an angiotensin-converting enzyme (ACE) inhibitor 45 or in combination with a calcium channel blocker 46 has been shown to improve DPN in randomised placebo-controlled trials 47, 48. Statins and fibrates have also been shown to prevent the development of DPN 49, 50 and have been associated with reduced diabetic foot infection 51 and lower-extremity amputation 52, 53 and increased rate of foot ulcer healing 54. Recent experimental studies advocate the use of combination therapies targeting several pathogenetic pathways as the most effective approach to the treatment of DPN 55– 57.
Painful diabetic peripheral neuropathy
Diagnosis of painful diabetic peripheral neuropathy
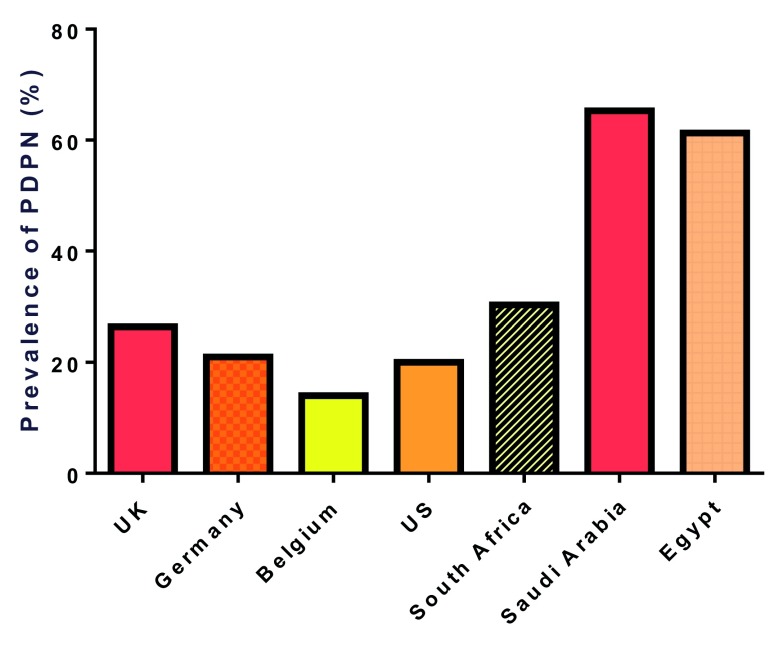
Painful diabetic peripheral neuropathy (PDPN) is a manifestation of small fibre damage 58– 60, characterised by burning pain and tingling with nocturnal exacerbation. It has a significant impact on the patient’s quality of life 61– 63 and can result in depression, anxiety and sleep disturbance 62. Estimates of the prevalence of PDPN range from 14.0 to 65.3% 61, 64– 69 ( Figure 1). This difference can be attributed to different populations studied and different diagnostic methods. The prevalence of PDPN is higher in secondary compared with primary care 70 and in patients with T2DM compared with T1DM 61, 64, 71. It is important to note that for a large proportion (12.5–61.5%) of patients, PDPN remains undiagnosed 71, 72. They are often unaware that the pain is related to diabetes and do not report it to their clinician 72. Older age, longer duration of diabetes, and the presence of DPN increase the risk for PDPN 61, 64– 66, 68; and obesity 61, 65, 70, 71, low physical activity 24, 67, smoking 64, 70, poor glycaemic control 73, 74, low high-density lipoprotein (HDL) cholesterol 61, and raised LDL cholesterol, triglycerides and creatinine 67 are independent risk factors for PDPN. Early diagnosis and intervention may be the key, as a study of subjects with pre-diabetes showed that lifestyle intervention reduced neuropathic symptoms and improved small fibre function and structure 24. Therefore, actively screening patients for PDPN, particularly those at high risk, should allow early identification and symptom relief but may also enable timely disease modification.
Figure 1. Prevalence of painful diabetic peripheral neuropathy (PDPN) reported from different countries ranges from 14.0 to 65.3% 61, 64– 69.
There are several screening tests that distinguish nociceptive and neuropathic pain. The most common screening tests for PDPN are the Douleur Neuropathique 4 (DN4) questionnaire 75, the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) pain scale 76 and the Neuropathic Pain Questionnaire (NPQ) 77. The DN4 is composed of 10 questions (seven symptoms and three neurological deficits), and a score of at least 4 has a high sensitivity (83%) and specificity (90%) for PDPN 78. The LANSS pain scale is composed of five questions on symptoms and two on neurological deficits, and a score at least 12 has a 85% sensitivity and 80% specificity for PDPN 76. The NPQ contains 10 questions on quality of pain and two on change in sensation 77 and detects PDPN with 74.7% sensitivity and 77.6% specificity.
Treatment of painful diabetic peripheral neuropathy
There is no evidence that improvement in glycaemic control improves PDPN; indeed, where rapid and large reductions in HbA1c may precipitate an acute painful neuropathy, the opposite is true 79. The traditional approach to managing PDPN is to try different therapies until one works (with minimal side effects). However, improved clinical phenotyping and targeting of therapies based on underlying mechanism(s) may result in better outcomes 80. Detailed phenotyping using QST suggests that in patients with an irritable nociceptor compared with a non-irritable nociceptor phenotype, there is a better response to oxcarbazepine, with a number needed to treat (NNT) of 3.9 compared with 6.9 in those with the non-irritable nociceptor 81. Identifying abnormalities in rate-dependent depression (RDD), a marker of altered descending inhibitory modulation of pain, may also help to identify patients who will respond optimally to selective norepinephrine reuptake inhibitors for example, duloxetine 82.
Tricyclic anti-depressants (TCAs) modulate pain and have analgesic efficacy by indirectly modifying the opioid system in the brain and via serotonergic and noradrenaline neuromodulation, amongst other mechanisms 83– 85. Amitriptyline is the most commonly used TCA in PDPN, despite not having a label for its treatment. In a systematic review, Moore et al. 86 evaluated 17 studies with 1342 participants and concluded that study quality was modest, and most studies had a high risk of bias due to the small participant numbers 86. Two serotonin and norepinephrine reuptake inhibitors (SNRIs) are recommended for PDPN: duloxetine and venlafaxine. They exert their effect via inhibiting serotonin and noradrenaline reuptake with potentiation of descending inhibitory pathways 87. A Cochrane review including eight randomised controlled trials (n = 2728) showed that duloxetine 60 mg daily was superior to placebo, and the NNT was 5 88. Pregabalin also has FDA approval for PDPN on the basis of a number of randomised controlled trials (RCTs) 89– 91. Snedecor et al. undertook a comparative meta-analysis of a number of agents to treat PDPN and found pregabalin to be the most efficacious in reducing Visual Analogue Scale (VAS) pain scores 92. Duloxetine and pregabalin are both considered first-line therapy by National Institute for Clinical Excellence (NICE) and the 2017 ADA position statement 2, 93. Mirogabalin has also recently shown efficacy and good tolerability in a phase II and two phase III clinical trials in PDPN 94– 96. Tramadol has an opioid plus SNRI effect. A Cochrane Collaboration review found that the efficacy of tramadol in neuropathic pain was determined in small, inadequately sized studies with a risk of bias 97, although a meta-analysis showed an NNT of 4.4. Tapentadol extended-release has also shown efficacy in a number of randomised clinical trials and is recommended by the FDA in PDPN 98– 101.
The COMBO-DN (Combination versus Monotherapy of pregabalin and duloxetine in Diabetic Neuropathy) study compared monotherapy with a combination of duloxetine and pregabalin 102. The pain outcomes between combination (duloxetine 60 mg daily plus pregabalin 300 mg daily) and high-dose monotherapy (duloxetine 120 mg daily or pregabalin 600 mg daily) were comparable 102. In an exploratory post-hoc analysis, high-dose monotherapy was more favourable in patients with severe pain, whereas combination therapy was more beneficial in patients with mild to moderate pain 103. In a double-blind RCT with a parallel-group design, analgesic efficacy was found to be comparable between amitriptyline, duloxetine and pregabalin 104.
In patients presenting with PDPN, the key is to provide symptom relief, but we would argue that this also represents a window of opportunity for risk factor reduction to limit DPN progression. For symptom relief, pregabalin, gabapentin, duloxetine or amitriptyline can be used first line, and if they are not working or have limited effectiveness because of side effects, then a second-line agent or tramadol can be added in combination ( Table 2). Topical therapies such as a glyceryl trinitrate (GTN) spray 105 or patch 106 applied to the feet can be considered and give a favourable NNT of about 4 107, 108. There is an evolving argument that the future management of PDPN will follow a more personalised approach using markers such as RDD 109, CCM 110 and genomics 111– 113 to identify specific mechanisms in patients who will respond better to targeted therapies.
Table 2. Commonly used therapy for painful diabetic peripheral neuropathy.
| Drug class | Agent | Initial dose | Maintenance dose | Comments and common adverse reactions |
|---|---|---|---|---|
| Anticonvulsants | Pregabalin 89– 92 | 25–75 mg three times
a day |
300–600 mg daily | Adverse events (AEs): dizziness, somnolence, headache and weight gain
Approved for the treatment of painful diabetic peripheral neuropathy (DPN) Psychological dependence |
| Gabapentin 128, 129 | 100–300 mg three
times a day |
900–3600 mg daily | AEs: dizziness, somnolence, ataxia and fatigue
Reduce dose if estimated glomerular filtration rate is less than 60 mL/min. |
|
| Antidepressants | Duloxetine 88, 130– 132 | 20–30 mg once daily | 60–120 mg once daily | Approved for the treatment of painful DPN
AEs: somnolence, dizziness, headache, nausea, dry mouth and reduced appetite Avoid in hepatic impairment; avoid with creatinine clearance of less than 30 mL/min. |
| Venlafaxine 133, 134 | 37.5 mg once daily | 75–225 mg once daily | AEs: nausea, dizziness, constipation, dry mouth, weight loss and constipation | |
| Amitriptyline 83, 86 | 10–25 mg once daily | 25–100 mg once daily | AEs: abdominal pain, headaches, dizziness, insomnia, orthostatic hypotension, anorexia,
nausea, urinary retention, constipation, blurred vision, mydriasis, weight gain, xerostomia and somnolence Avoid use in patients older than 60 years of age. |
|
| Opioid-like agonists | Tramadol 97 | 50 mg four times a
day |
200–400 mg four
times a day |
AEs: constipation, somnolence, nausea, headache and dizziness |
| Tapentadol 98– 101 | 50–100 mg four to six
times per day; can take 700 mg on first day |
600 mg daily | AEs: nausea, dizziness, somnolence, constipation, vomiting and headache
Potential for addiction, abuse and misuse |
|
| Topical therapies | Capsaicin 0.0075%
cream 135– 138 |
Applied three or four
times per day |
Can be used as an adjunct to oral therapies
Use is limited by the frequency of application. Cause denervation, hence may increase risk of foot ulceration |
|
| Lidocaine 5%
plaster 92, 139 |
5% for up to 18 hours
per day |
AEs: application site reactions; otherwise, has fewer side effects than systemic agents
Can be used as an adjunct to oral therapies |
||
| Isosorbide
dinitrate 106 |
Patch (5 mg) applied
at bedtime to the bottom of the feet |
AEs: headache. The dose can be halved if this occurs. |
Cardiac autonomic neuropathy
The Toronto consensus panel defined cardiac autonomic neuropathy (CAN) as the impairment of cardiovascular autonomic control in patients with diabetes mellitus after the exclusion of other causes 114. CAN occurs in subjects with impaired glucose tolerance (IGT), and abnormal cardiovascular autonomic reflex tests (CARTs) have been reported in up to 7% of patients at diagnosis of T1DM and T2DM 115– 117 with the prevalence increasing to 30% (Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications, or DCCT/EDIC) after 14 years of T1DM 118, 119 and in other studies to 70% after 15 years 120. CAN is an independent risk factor for mortality 114 and was the strongest risk factor for all-cause mortality in the EURODIAB study (T1DM) and an independent risk factor for mortality in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (T2DM) 121, 122. A meta-analysis of 15 longitudinal studies reported an association between CAN and increased mortality 123. A meta-analysis of 12 studies identified silent myocardial ischaemia (SMI) in 20% of patients with CAN compared with 10% in those without CAN 117. CAN is also associated with left ventricular dysfunction 124, 125 and independently predicts the progression of diabetic nephropathy 126.
Diagnosis of cardiac autonomic neuropathy
Screening for CAN is recommended at diagnosis in patients with T2DM and after 5 years for those with T1DM. Signs and symptoms of CAN should be assessed in patients with microvascular complications and in patients with hypoglycaemia unawareness 2, 127. The diagnosis of CAN includes the documentation of the symptoms and signs ( Table 3), but there is a weak correlation between symptoms and autonomic deficits 120, 140 and symptoms may be subtle like intermittent palpitations and exercise intolerance. CAN may initially be asymptomatic with the only sign being decreased heart rate variability with deep breathing, which can progress to a resting tachycardia (>100 bpm). In patients who are symptomatic (resting tachycardia with a history of poor glucose control) or where the diagnosis of CAN is very likely, the ADA position statement advises no additional testing 2. It is important to exclude other causes such as idiopathic orthostatic hypotension (OH), syncope and postural orthostatic tachycardia syndrome (POTS) 141. CARTs includes heart rate response to deep breathing, standing and the Valsalva manoeuvre.
Table 3. Symptoms and signs of diabetic autonomic neuropathy.
|
Cardiac autonomic neuropathy
• Resting tachycardia or fixed heart rate or both • Loss of circadian rhythm of blood pressure • Increase in nocturnal systolic blood pressure compared with daytime • Orthostatic hypotension • Exercise intolerance • Syncope and light headedness • Intra-operative cardiovascular lability • ‘Silent ischemia’ and ‘painless’ myocardial infarction • Arrhythmias • Urogenital autonomic neuropathy Bladder dysfunction • Increase in frequency and urgency particularly during the night • Urinary hesitancy and weak stream • Dribbling of urine and involuntary urination • Urinary incontinence Sexual dysfunction • Male: erectile dysfunction, decreased libido and abnormal ejaculation • Female: decreased sexual desire and arousal, increased pain during intercourse and inadequate lubrication Gastrointestinal autonomic neuropathy • Nausea/vomiting • Bloating • Inability to eat a full meal • Profuse and watery diarrhoea (nocturnal) • Constipation |
Management of autonomic neuropathy
Poor glycaemic control and longer diabetes duration are established risk factors for CAN 42, 118, 119. The DCCT showed that intensive glycaemic control in patients with T1DM reduced the development of CAN by 45% 118. Hypertension, obesity, hyperlipidemia and smoking have also been implicated in the development of CAN 42, 117, 120, 142– 144, and the Steno-2 trial showed that intensified multifactorial treatment in patients with T2DM reduced the risk of CAN progression by 68% 145, 146. There are no FDA-approved disease-modifying treatments to reverse CAN. A small early study found favourable effects of alpha-lipoic acid (ALA) on CAN 147, however, more recently a study to evaluate triple anti-oxidant therapy (allopurinol (300 mg once daily), ALA (600 mg twice daily) and nicotinamide (750 mg twice daily)) in patients with mild to moderate CAN found no benefit 148.
Orthostatic hypotension
Symptoms of OH occur on standing and include light-headedness, weakness, faintness and syncope. OH is defined by a blood pressure decrease on standing of greater than 20/10 mm Hg (a decrease of greater than 30/15 for those with blood pressure of greater than 150/90) without an appropriate increase in heart rate (<15 bpm) 149. Treatment of OH involves a review of medication, fluid and salt repletion and encouragement of physical activity and exercise to avoid deconditioning 150, 151. Fludrocortisone is not FDA-approved for OH. It works through sodium retention and constriction of partially denervated blood vessels, but there are concerns over supine hypertension, hypokalaemia, congestive cardiac failure and peripheral oedema 152. Both midodrine and droxidopa are approved by the FDA for the treatment of symptomatic neurogenic OH 153.
Gastroparesis
Gastroparesis is defined as the delayed removal of stomach contents in the absence of a physical obstruction 154. Gastric emptying should be assessed with scintigraphy 4 hours after food intake of digestible solids at 15-min intervals. Dietary modification with frequent small meals and prokinetics are recommended to increase gastric motility. Metoclopramide is the only FDA-approved drug for the treatment of gastroparesis. However, limited efficacy and the risk of tardive dyskinesia have led the FDA and European Medicines Agency to advise use for a maximum of 5 days. New therapies are being investigated and include motilin receptor agonists, ghrelin receptor agonists, and neurokinin receptor antagonists. Mechanical options for intervention include transpyloric stenting, gastric electrical stimulation, and gastric per-oral endoscopic myotomy; in severe intractable gastroparesis, laparoscopic pyloroplasty or gastrectomy may be options 155.
Diabetic diarrhoea
Diabetic diarrhoea is a troublesome gastrointestinal complication which is characterised by watery painless diarrhoea, particularly at night. Other causes of diarrhoea must be excluded, especially therapy with metformin which is often overlooked, and pancreatic exocrine insufficiency (faecal fat of greater than 6 g/72 hours). Pharmacological therapies include antidiarrhoeal agents (for example, Lomotil or Imodium), antibiotics (tetracycline or metronidazole) to eradicate bacterial overgrowth, somatostatin analogues (octreotide), and selective serotonin 5-hydroxy tryptamine type 3 (HT3) receptor antagonists (Ramosetron) 156, 157.
Bladder disturbance
Bladder dysfunction may occur in up to 50% of patients with diabetes due to urogenital autonomic neuropathy 141. The earliest manifestation includes increased initiating threshold for the micturition reflex followed by decreased detrusor activity and incomplete bladder emptying. Treatment includes suprapubic pressure, antimuscarinic medication (oxybutynin 5–30 mg 3 times a day; tolterodine 2–8 mg twice a day) for detrusor hyperreflexia and parasympathomimetic medication to reduce detrusor contractility, and intermittent self-catheterisation 2. It is important to note that none of the medications are FDA-approved for neurogenic bladder and they have approval only for non-neurogenic overactive bladder or other lower urinary tract symptoms.
Erectile dysfunction
Erectile dysfunction (ED) is a common manifestation in men with diabetes 141. It may be three times more prevalent, occur 10 to 15 years earlier and is more severe and less responsive to treatment compared with those without diabetes 158. ED is associated with a higher HbA1c, presence of metabolic syndrome, hypertension, atherogenic dyslipidemia, lower estimated glomerular filtration rate, higher albumin/creatinine ratio and more severe small fibre neuropathy in men with T1DM 159– 161. Sexual dysfunction is also more common in women with diabetes, and 47% of women with diabetic neuropathy had sexual dysfunction 162. Reduced sexual arousal, decreased lubrication and painful intercourse are the most common symptoms of sexual dysfunction in females with diabetes. Recent recommendations include active smoking cessation (improves ED by about 30%), treatment of those with testosterone deficiency and treatment with statins. 5-phosphodiesterase inhibitors, intra-cavernosal and transurethral prostaglandins, and penile implants can be used for more severe cases 163– 165. There is about a 50% non-responder rate in patients with diabetes as they are less likely to respond to PDE5 inhibitors 166. Additional modalities such as low-intensity extracorporeal shock wave therapy show promise 167– 169 but require larger and better designed clinical trials. There are several explanations for the lack of response 166, but it may also reflect a more severe neurogenic component to ED in these patients 159. Indeed, in a recent study, the assessment of nocturnal penile tumescence and rigidity, which reflects predominantly neurogenic abnormalities, had an area under the curve (AUC) of 0.860 in differentiating sildenafil responders from non-responders 170.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Zheng Gang Zhang, Department of Neurology, Henry Ford Hospital, Detroit, MI, USA
Simone E. Baltrusch, Institute of Medical Biochemistry and Molecular Biology, University of Rostock, Rostock, Germany
Mark Yorek, Department of Internal Medicine, University of Iowa, Iowa City, IA, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 3 approved]
References
- 1. Tesfaye S, Boulton AJ, Dyck PJ, et al. : Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–93. 10.2337/dc10-1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pop-Busui R, Boulton AJ, Feldman EL, et al. : Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care. 2017;40(1):136–54. 10.2337/dc16-2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Young MJ, Boulton AJ, Macleod AF, et al. : A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993;36(2):150–4. 10.1007/BF00400697 [DOI] [PubMed] [Google Scholar]
- 4. Perkins BA, Olaleye D, Zinman B, et al. : Simple screening tests for peripheral neuropathy in the diabetes clinic. Diabetes Care. 2001;24(2):250–6. 10.2337/diacare.24.2.250 [DOI] [PubMed] [Google Scholar]
- 5. Herman WH, Pop-Busui R, Braffett BH, et al. : Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–44. 10.1111/j.1464-5491.2012.03644.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maier C, Baron R, Tölle TR, et al. : Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150(3):439–50. 10.1016/j.pain.2010.05.002 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 7. Young MJ, Breddy JL, Veves A, et al. : The prediction of diabetic neuropathic foot ulceration using vibration perception thresholds. A prospective study. Diabetes Care. 1994;17(6):557–60. 10.2337/diacare.17.6.557 [DOI] [PubMed] [Google Scholar]
- 8. Martin CL, Waberski BH, Pop-Busui R, et al. : Vibration perception threshold as a measure of distal symmetrical peripheral neuropathy in type 1 diabetes: results from the DCCT/EDIC study. Diabetes Care. 2010;33(12):2635–41. 10.2337/dc10-0616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan LS: The clinical use of the 10g monofilament and its limitations: a review. Diabetes Res Clin Pract. 2010;90(1):1–7. 10.1016/j.diabres.2010.06.021 [DOI] [PubMed] [Google Scholar]
- 10. Rith-Najarian SJ, Stolusky T, Gohdes DM: Identifying diabetic patients at high risk for lower-extremity amputation in a primary health care setting. A prospective evaluation of simple screening criteria. Diabetes Care. 1992;15(10):1386–9. 10.2337/diacare.15.10.1386 [DOI] [PubMed] [Google Scholar]
- 11. Dyck PJ, Overland CJ, Low PA, et al. : Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42(2):157–64. 10.1002/mus.21661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajabally YA, Stettner M, Kieseier BC, et al. : CIDP and other inflammatory neuropathies in diabetes - diagnosis and management. Nat Rev Neurol. 2017;13(10):599–611. 10.1038/nrneurol.2017.123 [DOI] [PubMed] [Google Scholar]
- 13. Malik RA: Wherefore Art Thou, O Treatment for Diabetic Neuropathy? Int Rev Neurobiol. 2016;127:287–317. 10.1016/bs.irn.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 14. Shy ME, Frohman EM, So YT, et al. : Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2003;60(6):898–904. 10.1212/01.WNL.0000058546.16985.11 [DOI] [PubMed] [Google Scholar]
- 15. Løseth S, Stålberg E, Jorde R, et al. : Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255(8):1197–202. 10.1007/s00415-008-0872-0 [DOI] [PubMed] [Google Scholar]
- 16. Dyck PJ, Norell JE, Tritschler H, et al. : Challenges in design of multicenter trials: end points assessed longitudinally for change and monotonicity. Diabetes Care. 2007;30(10):2619–25. 10.2337/dc06-2479 [DOI] [PubMed] [Google Scholar]
- 17. Malik RA: Which test for diagnosing early human diabetic neuropathy? Diabetes. 2014;63(7):2206–8. 10.2337/db14-0492 [DOI] [PubMed] [Google Scholar]
- 18. Apfel SC: Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. 10.1016/S0074-7742(02)50083-0 [DOI] [PubMed] [Google Scholar]
- 19. Kennedy WR, Navarro X, Goetz FC, et al. : Effects of pancreatic transplantation on diabetic neuropathy. N Engl J Med. 1990;322(15):1031–7. 10.1056/NEJM199004123221503 [DOI] [PubMed] [Google Scholar]
- 20. Wahren J, Foyt H, Daniels M, et al. : Long-Acting C-Peptide and Neuropathy in Type 1 Diabetes: A 12-Month Clinical Trial. Diabetes Care. 2016;39(4):596–602. 10.2337/dc15-2068 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Bakkers M, Merkies IS, Lauria G, et al. : Intraepidermal nerve fiber density and its application in sarcoidosis. Neurology. 2009;73(14):1142–8. 10.1212/WNL.0b013e3181bacf05 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Asghar O, Petropoulos IN, Alam U, et al. : Corneal confocal microscopy detects neuropathy in subjects with impaired glucose tolerance. Diabetes Care. 2014;37(9):2643–6. 10.2337/dc14-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Løseth S, Stålberg EV, Lindal S, et al. : Small and large fiber neuropathy in those with type 1 and type 2 diabetes: a 5-year follow-up study. J Peripher Nerv Syst. 2016;21(1):15–21. 10.1111/jns.12154 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 24. Smith AG, Russell J, Feldman EL, et al. : Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29(6):1294–9. 10.2337/dc06-0224 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Lauria G, Cornblath DR, Johansson O, et al. : EFNS guidelines on the use of skin biopsy in the diagnosis of peripheral neuropathy. Eur J Neurol. 2005;12(10):747–58. 10.1111/j.1468-1331.2005.01260.x [DOI] [PubMed] [Google Scholar]
- 26. Malik RA, Kallinikos P, Abbott CA, et al. : Corneal confocal microscopy: a non-invasive surrogate of nerve fibre damage and repair in diabetic patients. Diabetologia. 2003;46(5):683–8. 10.1007/s00125-003-1086-8 [DOI] [PubMed] [Google Scholar]
- 27. Hertz P, Bril V, Orszag A, et al. : Reproducibility of in vivo corneal confocal microscopy as a novel screening test for early diabetic sensorimotor polyneuropathy. Diabet Med. 2011;28(10):1253–60. 10.1111/j.1464-5491.2011.03299.x [DOI] [PubMed] [Google Scholar]
- 28. Kawashima W, Hatake K, Kudo R, et al. : Estimating the Time after Death on the Basis of Corneal Opacity. J Forensic Res. 2014;6:269 10.4172/2157-7145.1000269 [DOI] [Google Scholar]
- 29. Perkins BA, Lovblom LE, Bril V, et al. : Corneal confocal microscopy for identification of diabetic sensorimotor polyneuropathy: a pooled multinational consortium study. Diabetologia. 2018;61(8):1856–61. 10.1007/s00125-018-4653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petropoulos IN, Alam U, Fadavi H, et al. : Rapid automated diagnosis of diabetic peripheral neuropathy with in vivo corneal confocal microscopy. Invest Ophthalmol Vis Sci. 2014;55(4):2071–8. 10.1167/iovs.13-13787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pritchard N, Edwards K, Dehghani C, et al. : Longitudinal assessment of neuropathy in type 1 diabetes using novel ophthalmic markers (LANDMark): study design and baseline characteristics. Diabetes Res Clin Pract. 2014;104(2):248–56. 10.1016/j.diabres.2014.02.011 [DOI] [PubMed] [Google Scholar]
- 32. Azmi S, Ferdousi M, Petropoulos IN, et al. : Corneal Confocal Microscopy Identifies Small-Fiber Neuropathy in Subjects With Impaired Glucose Tolerance Who Develop Type 2 Diabetes. Diabetes Care. 2015;38(8):1502–8. 10.2337/dc14-2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pritchard N, Edwards K, Russell AW, et al. : Corneal confocal microscopy predicts 4-year incident peripheral neuropathy in type 1 diabetes. Diabetes Care. 2015;38(4):671–5. 10.2337/dc14-2114 [DOI] [PubMed] [Google Scholar]
- 34. Tavakoli M, Mitu-Pretorian M, Petropoulos IN, et al. : Corneal confocal microscopy detects early nerve regeneration in diabetic neuropathy after simultaneous pancreas and kidney transplantation. Diabetes. 2013;62(1):254–60. 10.2337/db12-0574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Azmi S, Ferdousi M, Petropoulos IN, et al. : Corneal confocal microscopy shows an improvement in small-fiber neuropathy in subjects with type 1 diabetes on continuous subcutaneous insulin infusion compared with multiple daily injection. Diabetes Care. 2015;38(1):e3–4. 10.2337/dc14-1698 [DOI] [PubMed] [Google Scholar]
- 36. Culver DA, Dahan A, Bajorunas D, et al. : Cibinetide Improves Corneal Nerve Fiber Abundance in Patients With Sarcoidosis-Associated Small Nerve Fiber Loss and Neuropathic Pain. Invest Ophthalmol Vis Sci. 2017;58(6):BIO52–BIO60. 10.1167/iovs.16-21291 [DOI] [PubMed] [Google Scholar]
- 37. Lewis EJH, Perkins BA, Lovblom LE, et al. : Effect of omega-3 supplementation on neuropathy in type 1 diabetes: A 12-month pilot trial. Neurology. 2017;88(24):2294–301. 10.1212/WNL.0000000000004033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee CC, Perkins BA, Kayaniyil S, et al. : Peripheral Neuropathy and Nerve Dysfunction in Individuals at High Risk for Type 2 Diabetes: The PROMISE Cohort. Diabetes Care. 2015;38(5):793–800. 10.2337/dc14-2585 [DOI] [PubMed] [Google Scholar]
- 39. Ferdousi M, Azmi S, Petropoulos IN, et al. : Corneal Confocal Microscopy Detects Small Fibre Neuropathy in Patients with Upper Gastrointestinal Cancer and Nerve Regeneration in Chemotherapy Induced Peripheral Neuropathy. PLoS One. 2015;10(10):e0139394. 10.1371/journal.pone.0139394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ang L, Jaiswal M, Martin C, et al. : Glucose control and diabetic neuropathy: lessons from recent large clinical trials. Curr Diab Rep. 2014;14(9):528. 10.1007/s11892-014-0528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohkubo Y, Kishikawa H, Araki E, et al. : Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28(2):103–17. 10.1016/0168-8227(95)01064-K [DOI] [PubMed] [Google Scholar]
- 42. Ismail-Beigi F, Craven T, Banerji MA, et al. : Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376(9739):419–30. 10.1016/S0140-6736(10)60576-4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 43. Callaghan BC, Little AA, Feldman EL, et al. : Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012; (6):CD007543. 10.1002/14651858.CD007543.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tesfaye S, Chaturvedi N, Eaton SE, et al. : Vascular risk factors and diabetic neuropathy. N Engl J Med. 2005;352(4):341–50. 10.1056/NEJMoa032782 [DOI] [PubMed] [Google Scholar]
- 45. Malik RA, Williamson S, Abbott C, et al. : Effect of angiotensin-converting-enzyme (ACE) inhibitor trandolapril on human diabetic neuropathy: randomised double-blind controlled trial. Lancet. 1998;352(9145):1978–81. 10.1016/S0140-6736(98)02478-7 [DOI] [PubMed] [Google Scholar]
- 46. Ruggenenti P, Lauria G, Iliev IP, et al. : Effects of manidipine and delapril in hypertensive patients with type 2 diabetes mellitus: the delapril and manidipine for nephroprotection in diabetes (DEMAND) randomized clinical trial. Hypertension. 2011;58(5):776–83. 10.1161/HYPERTENSIONAHA.111.174474 [DOI] [PubMed] [Google Scholar]
- 47. Malik RA: Can diabetic neuropathy be prevented by angiotensin-converting enzyme inhibitors? Ann Med. 2000;32(1):1–5. 10.3109/07853890008995903 [DOI] [PubMed] [Google Scholar]
- 48. Malik RA, Tomlinson DR: Angiotensin-converting enzyme inhibitors: are there credible mechanisms for beneficial effects in diabetic neuropathy? Int Rev Neurobiol. 2002;50:415–30. 10.1016/S0074-7742(02)50084-2 [DOI] [PubMed] [Google Scholar]
- 49. Nielsen SF, Nordestgaard BG: Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol. 2014;2(11):894–900. 10.1016/S2213-8587(14)70173-1 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Davis TM, Yeap BB, Davis WA, et al. : Lipid-lowering therapy and peripheral sensory neuropathy in type 2 diabetes: the Fremantle Diabetes Study. Diabetologia. 2008;51(4):562–6. 10.1007/s00125-007-0919-2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Nassaji M, Ghorbani R, Saboori Shkofte H: Previous Atorvastatin Treatment and Risk of Diabetic Foot Infection in Adult Patients: A Case-control Study. Wounds. 2017;29(7):196–201. [PubMed] [Google Scholar]
- 52. Rajamani K, Colman PG, Li LP, et al. : Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373(9677):1780–8. 10.1016/S0140-6736(09)60698-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sohn MW, Meadows JL, Oh EH, et al. : Statin use and lower extremity amputation risk in nonelderly diabetic patients. J Vasc Surg. 2013;58(6):1578–1585.e1. 10.1016/j.jvs.2013.06.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fox JD, Baquerizo-Nole KL, Macquhae F, et al. : Statins may be associated with six-week diabetic foot ulcer healing. Wound Repair Regen. 2016;24(2):454–7. 10.1111/wrr.12400 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 55. Davidson EP, Coppey LJ, Shevalye H, et al. : Effect of Dietary Content of Menhaden Oil with or without Salsalate on Neuropathic Endpoints in High-Fat-Fed/Low-Dose Streptozotocin-Treated Sprague Dawley Rats. J Diabetes Res. 2018;2018:2967127. 10.1155/2018/2967127 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 56. Davidson EP, Coppey LJ, Shevalye H, et al. : Impaired Corneal Sensation and Nerve Loss in a Type 2 Rat Model of Chronic Diabetes Is Reversible With Combination Therapy of Menhaden Oil, α-Lipoic Acid, and Enalapril. Cornea. 2017;36(6):725–31. 10.1097/ICO.0000000000001182 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Davidson EP, Coppey LJ, Shevalye H, et al. : Vascular and Neural Complications in Type 2 Diabetic Rats: Improvement by Sacubitril/Valsartan Greater Than Valsartan Alone. Diabetes. 2018;67(8):1616–26. 10.2337/db18-0062 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Sorensen L, Molyneaux L, Yue DK: The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. 2006;29(4):883–7. 10.2337/diacare.29.04.06.dc05-2180 [DOI] [PubMed] [Google Scholar]
- 59. Vlckova-Moravcova E, Bednarik J, Belobradkova J, et al. : Small-fibre involvement in diabetic patients with neuropathic foot pain. Diabet Med. 2008;25(6):692–9. 10.1111/j.1464-5491.2008.02446.x [DOI] [PubMed] [Google Scholar]
- 60. Quattrini C, Tavakoli M, Jeziorska M, et al. : Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56(8):2148–54. 10.2337/db07-0285 [DOI] [PubMed] [Google Scholar]
- 61. van Acker K, Bouhassira D, de Bacquer D, et al. : Prevalence and impact on quality of life of peripheral neuropathy with or without neuropathic pain in type 1 and type 2 diabetic patients attending hospital outpatients clinics. Diabetes Metab. 2009;35(3):206–13. 10.1016/j.diabet.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 62. Bohlega S, Alsaadi T, Amir A, et al. : Guidelines for the pharmacological treatment of peripheral neuropathic pain: expert panel recommendations for the middle East region. J Int Med Res. 2010;38(2):295–317. 10.1177/147323001003800201 [DOI] [PubMed] [Google Scholar]
- 63. daCosta DiBonaventura M, Cappelleri JC, Joshi AV: A longitudinal assessment of painful diabetic peripheral neuropathy on health status, productivity, and health care utilization and cost. Pain Med. 2011;12(1):118–26. 10.1111/j.1526-4637.2010.01012.x [DOI] [PubMed] [Google Scholar]
- 64. Abbott CA, Malik RA, van Ross ER, et al. : Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care. 2011;34(10):2220–4. 10.2337/dc11-1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jambart S, Ammache Z, Haddad F, et al. : Prevalence of painful diabetic peripheral neuropathy among patients with diabetes mellitus in the Middle East region. J Int Med Res. 2011;39(2):366–77. 10.1177/147323001103900204 [DOI] [PubMed] [Google Scholar]
- 66. Davies M, Brophy S, Williams R, et al. : The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518–22. 10.2337/dc05-2228 [DOI] [PubMed] [Google Scholar]
- 67. Ziegler D, Rathmann W, Meisinger C, et al. : Prevalence and risk factors of neuropathic pain in survivors of myocardial infarction with pre-diabetes and diabetes. The KORA Myocardial Infarction Registry. Eur J Pain. 2009;13(6):582–7. 10.1016/j.ejpain.2008.07.007 [DOI] [PubMed] [Google Scholar]
- 68. Jacovides A, Bogoshi M, Distiller LA, et al. : An epidemiological study to assess the prevalence of diabetic peripheral neuropathic pain among adults with diabetes attending private and institutional outpatient clinics in South Africa. J Int Med Res. 2014;42(4):1018–28. 10.1177/0300060514525759 [DOI] [PubMed] [Google Scholar]
- 69. Sadosky A, McDermott AM, Brandenburg NA, et al. : A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008;8(1):45–56. 10.1111/j.1533-2500.2007.00164.x [DOI] [PubMed] [Google Scholar]
- 70. Aslam A, Singh J, Rajbhandari S: Prevalence of Painful Diabetic Neuropathy Using the Self-Completed Leeds Assessment of Neuropathic Symptoms and Signs Questionnaire in a Population with Diabetes. Can J Diabetes. 2015;39(4):285–95. 10.1016/j.jcjd.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 71. Ziegler D, Landgraf R, Lobmann R, et al. : Painful and painless neuropathies are distinct and largely undiagnosed entities in subjects participating in an educational initiative (PROTECT study). Diabetes Res Clin Pract. 2018;139:147–54. 10.1016/j.diabres.2018.02.043 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Daousi C, MacFarlane IA, Woodward A, et al. : Chronic painful peripheral neuropathy in an urban community: a controlled comparison of people with and without diabetes. Diabet Med. 2004;21(9):976–82. 10.1111/j.1464-5491.2004.01271.x [DOI] [PubMed] [Google Scholar]
- 73. Harris M, Eastman R, Cowie C: Symptoms of sensory neuropathy in adults with NIDDM in the U.S. population. Diabetes Care. 1993;16(11):1446–52. 10.2337/diacare.16.11.1446 [DOI] [PubMed] [Google Scholar]
- 74. Smith AG, Singleton JR: Impaired glucose tolerance and neuropathy. Neurologist. 2008;14(1):23–9. 10.1097/NRL.0b013e31815a3956 [DOI] [PubMed] [Google Scholar]
- 75. Spallone V, Morganti R, D'Amato C, et al. : Validation of DN4 as a screening tool for neuropathic pain in painful diabetic polyneuropathy. Diabet Med. 2012;29(5):578–85. 10.1111/j.1464-5491.2011.03500.x [DOI] [PubMed] [Google Scholar]
- 76. Bennett M: The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain. 2001;92(1–2):147–57. 10.1016/S0304-3959(00)00482-6 [DOI] [PubMed] [Google Scholar]
- 77. Krause SJ, Backonja MM: Development of a neuropathic pain questionnaire. Clin J Pain. 2003;19(5):306–14. 10.1097/00002508-200309000-00004 [DOI] [PubMed] [Google Scholar]
- 78. Unal-Cevik I, Sarioglu-Ay S, Evcik D: A comparison of the DN4 and LANSS questionnaires in the assessment of neuropathic pain: validity and reliability of the Turkish version of DN4. J Pain. 2010;11(11):1129–35. 10.1016/j.jpain.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 79. Gibbons CH, Freeman R: Treatment-induced neuropathy of diabetes: an acute, iatrogenic complication of diabetes. Brain. 2015;138(Pt 1):43–52. 10.1093/brain/awu307 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 80. Vollert J, Maier C, Attal N, et al. : Stratifying patients with peripheral neuropathic pain based on sensory profiles: algorithm and sample size recommendations. Pain. 2017;158(8):1446–55. 10.1097/j.pain.0000000000000935 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 81. Demant DT, Lund K, Vollert J, et al. : The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain. 2014;155(11):2263–73. 10.1016/j.pain.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 82. Marshall AG, Lee-Kubli C, Azmi S, et al. : Spinal Disinhibition in Experimental and Clinical Painful Diabetic Neuropathy. Diabetes. 2017;66(5):1380–90. 10.2337/db16-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Botney M, Fields HL: Amitriptyline potentiates morphine analgesia by a direct action on the central nervous system. Ann Neurol. 1983;13(2):160–4. 10.1002/ana.410130209 [DOI] [PubMed] [Google Scholar]
- 84. Benbouzid M, Gavériaux-Ruff C, Yalcin I, et al. : Delta-opioid receptors are critical for tricyclic antidepressant treatment of neuropathic allodynia. Biol Psychiatry. 2008;63(6):633–6. 10.1016/j.biopsych.2007.06.016 [DOI] [PubMed] [Google Scholar]
- 85. de Gandarias JM, Echevarria E, Acebes I, et al. : Effects of imipramine administration on mu-opioid receptor immunostaining in the rat forebrain. Arzneimittelforschung. 1998;48(7):717–9. [PubMed] [Google Scholar]
- 86. Moore RA, Derry S, Aldington D, et al. : Amitriptyline for neuropathic pain in adults. Cochrane Database Syst Rev. 2015; (7):CD008242. 10.1002/14651858.CD008242.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 87. Hossain SM, Hussain SM, Ekram AR: Duloxetine in Painful Diabetic Neuropathy: A Systematic Review. Clin J Pain. 2016;32(11):1005–10. 10.1097/AJP.0000000000000343 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 88. Lunn MP, Hughes RA, Wiffen PJ: Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014; (1):CD007115. 10.1002/14651858.CD007115.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lesser H, Sharma U, LaMoreaux L, et al. : Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63(11):2104–10. 10.1212/01.WNL.0000145767.36287.A1 [DOI] [PubMed] [Google Scholar]
- 90. Rosenstock J, Tuchman M, LaMoreaux L, et al. : Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110(3):628–38. 10.1016/j.pain.2004.05.001 [DOI] [PubMed] [Google Scholar]
- 91. Arezzo JC, Rosenstock J, LaMoreaux L, et al. : Efficacy and safety of pregabalin 600 mg/d for treating painful diabetic peripheral neuropathy: a double-blind placebo-controlled trial. BMC Neurol. 2008;8:33. 10.1186/1471-2377-8-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Snedecor SJ, Sudharshan L, Cappelleri JC, et al. : Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Pract. 2014;14(2):167–84. 10.1111/papr.12054 [DOI] [PubMed] [Google Scholar]
- 93. Iqbal Z, Azmi S, Yadav R, et al. : Diabetic Peripheral Neuropathy: Epidemiology, Diagnosis, and Pharmacotherapy. Clin Ther. 2018;40(6):828–49. 10.1016/j.clinthera.2018.04.001 [DOI] [PubMed] [Google Scholar]
- 94. Javed S, Alam U, Malik RA: Mirogabalin and emerging therapies for diabetic neuropathy. J Pain Res. 2018;11:1559–66. 10.2147/JPR.S145999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Merante D, Rosenstock J, Sharma U, et al. : Efficacy of Mirogabalin (DS-5565) on Patient-Reported Pain and Sleep Interference in Patients with Diabetic Neuropathic Pain: Secondary Outcomes of a Phase II Proof-of-Concept Study. Pain Med. 2017;18(11):2198–207. 10.1093/pm/pnw342 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 96. Vinik A, Rosenstock J, Sharma U, et al. : Efficacy and safety of mirogabalin (DS-5565) for the treatment of diabetic peripheral neuropathic pain: a randomized, double-blind, placebo- and active comparator-controlled, adaptive proof-of-concept phase 2 study. Diabetes Care. 2014;37(12):3253–61. 10.2337/dc14-1044 [DOI] [PubMed] [Google Scholar]
- 97. Duehmke RM, Derry S, Wiffen PJ, et al. : Tramadol for neuropathic pain in adults. Cochrane Database Syst Rev. 2017;6:CD003726. 10.1002/14651858.CD003726.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 98. Vinik AI, Shapiro DY, Rauschkolb C, et al. : A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014;37(8):2302–9. 10.2337/dc13-2291 [DOI] [PubMed] [Google Scholar]
- 99. Niesters M, Proto PL, Aarts L, et al. : Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113(1):148–56. 10.1093/bja/aeu056 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 100. Schwartz S, Etropolski MS, Shapiro DY, et al. : A pooled analysis evaluating the efficacy and tolerability of tapentadol extended release for chronic, painful diabetic peripheral neuropathy. Clin Drug Investig. 2015;35(2):95–108. 10.1007/s40261-014-0249-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vadivelu N, Kai A, Maslin B, et al. : Tapentadol extended release in the management of peripheral diabetic neuropathic pain. Ther Clin Risk Manag. 2015;11:95–105. 10.2147/TCRM.S32193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tesfaye S, Wilhelm S, Lledo A, et al. : Duloxetine and pregabalin: high-dose monotherapy or their combination? The "COMBO-DN study"--a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013;154(12):2616–25. 10.1016/j.pain.2013.05.043 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 103. Bouhassira D, Wilhelm S, Schacht A, et al. : Neuropathic pain phenotyping as a predictor of treatment response in painful diabetic neuropathy: data from the randomized, double-blind, COMBO-DN study. Pain. 2014;155(10):2171–9. 10.1016/j.pain.2014.08.020 [DOI] [PubMed] [Google Scholar]
- 104. Boyle J, Eriksson ME, Gribble L, et al. : Randomized, placebo-controlled comparison of amitriptyline, duloxetine, and pregabalin in patients with chronic diabetic peripheral neuropathic pain: impact on pain, polysomnographic sleep, daytime functioning, and quality of life. Diabetes Care. 2012;35(12):2451–8. 10.2337/dc12-0656 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 105. Yuen KC, Baker NR, Rayman G: Treatment of Chronic Painful Diabetic Neuropathy With Isosorbide Dinitrate Spray: a double-blind placebo-controlled cross-over study. Diabetes Care. 2002;25(10):1699–703. 10.2337/diacare.25.10.1699 [DOI] [PubMed] [Google Scholar]
- 106. Rayman G, Baker NR, Krishnan ST: Glyceryl trinitrate patches as an alternative to isosorbide dinitrate spray in the treatment of chronic painful diabetic neuropathy. Diabetes Care. 2003;26(9):2697–8. 10.2337/diacare.26.9.2697-a [DOI] [PubMed] [Google Scholar]
- 107. Agrawal RP, Choudhary R, Sharma P, et al. : Glyceryl trinitrate spray in the management of painful diabetic neuropathy: a randomized double blind placebo controlled cross-over study. Diabetes Res Clin Pract. 2007;77(2):161–7. 10.1016/j.diabres.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 108. Agrawal RP, Goswami J, Jain S, et al. : Management of diabetic neuropathy by sodium valproate and glyceryl trinitrate spray: a prospective double-blind randomized placebo-controlled study. Diabetes Res Clin Pract. 2009;83(3):371–8. 10.1016/j.diabres.2008.12.018 [DOI] [PubMed] [Google Scholar]
- 109. Alam U, Jeziorska M, Petropoulos IN, et al. : Diagnostic utility of corneal confocal microscopy and intra-epidermal nerve fibre density in diabetic neuropathy. PLoS One. 2017;12(7):e0180175. 10.1371/journal.pone.0180175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kalteniece A, Ferdousi M, Petropoulos I, et al. : Greater corneal nerve loss at the inferior whorl is related to the presence of diabetic neuropathy and painful diabetic neuropathy. Sci Rep. 2018;8(1):3283. 10.1038/s41598-018-21643-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Eijkenboom I, Sopacua M, Hoeijmakers JGJ, et al. : Yield of peripheral sodium channels gene screening in pure small fibre neuropathy. J Neurol Neurosurg Psychiatr. 2018; pii: jnnp-2018-319042. 10.1136/jnnp-2018-319042 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 112. Huang J, Han C, Estacion M, et al. : Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain. 2014;137(Pt 6):1627–42. 10.1093/brain/awu079 [DOI] [PubMed] [Google Scholar]
- 113. Lauria G, Ziegler D, Malik R, et al. : The role of sodium channels in painful diabetic and idiopathic neuropathy. Curr Diab Rep. 2014;14(10):538. 10.1007/s11892-014-0538-5 [DOI] [PubMed] [Google Scholar]
- 114. Spallone V, Ziegler D, Freeman R, et al. : Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011;27(7):639–53. 10.1002/dmrr.1239 [DOI] [PubMed] [Google Scholar]
- 115. Ziegler D, Voss A, Rathmann W, et al. : Increased prevalence of cardiac autonomic dysfunction at different degrees of glucose intolerance in the general population: the KORA S4 survey. Diabetologia. 2015;58(5):1118–28. 10.1007/s00125-015-3534-7 [DOI] [PubMed] [Google Scholar]
- 116. Carnethon MR, Prineas RJ, Temprosa M, et al. : The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006;29(4):914–9. 10.2337/diacare.29.04.06.dc05-1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Vinik AI, Maser RE, Mitchell BD, et al. : Diabetic Autonomic Neuropathy. Diabetes Care. 2003;26(5):1553–79. 10.2337/diacare.26.5.1553 [DOI] [PubMed] [Google Scholar]
- 118. Martin CL, Albers JW, Pop-Busui R, et al. : Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–8. 10.2337/dc13-2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pop-Busui R, Low PA, Waberski BH, et al. : Effects of Prior Intensive Insulin Therapy on Cardiac Autonomic Nervous System Function in Type 1 Diabetes Mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009;119(22):2886–93. 10.1161/CIRCULATIONAHA.108.837369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Low PA, Benrud-Larson LM, Sletten DM, et al. : Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care. 2004;27(12):2942–7. 10.2337/diacare.27.12.2942 [DOI] [PubMed] [Google Scholar]
- 121. Soedamah-Muthu SS, Chaturvedi N, Witte DR, et al. : Relationship between risk factors and mortality in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study (PCS). Diabetes Care. 2008;31(7):1360–6. 10.2337/dc08-0107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pop-Busui R, Evans GW, Gerstein HC, et al. : Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010;33(7):1578–84. 10.2337/dc10-0125 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 123. Maser RE, Mitchell BD, Vinik AI, et al. : The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26(6):1895–901. 10.2337/diacare.26.6.1895 [DOI] [PubMed] [Google Scholar]
- 124. Vinik AI, Ziegler D: Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115(3):387–97. 10.1161/CIRCULATIONAHA.106.634949 [DOI] [PubMed] [Google Scholar]
- 125. Dinh W, Füth R, Lankisch M, et al. : Cardiovascular autonomic neuropathy contributes to left ventricular diastolic dysfunction in subjects with Type 2 diabetes and impaired glucose tolerance undergoing coronary angiography. Diabet Med. 2011;28(3):311–8. 10.1111/j.1464-5491.2010.03221.x [DOI] [PubMed] [Google Scholar]
- 126. Astrup AS, Tarnow L, Rossing P, et al. : Cardiac autonomic neuropathy predicts cardiovascular morbidity and mortality in type 1 diabetic patients with diabetic nephropathy. Diabetes Care. 2006;29(2):334–9. 10.2337/diacare.29.02.06.dc05-1242 [DOI] [PubMed] [Google Scholar]
- 127. Ziegler D, Keller J, Maier C, et al. : Diabetic neuropathy. Exp Clin Endocrinol Diabetes. 2014;122(7):406–15. 10.1055/s-0034-1366435 [DOI] [PubMed] [Google Scholar]
- 128. Mellegers MA, Furlan AD, Mailis A: Gabapentin for neuropathic pain: systematic review of controlled and uncontrolled literature. Clin J Pain. 2001;17(4):284–95. 10.1097/00002508-200112000-00002 [DOI] [PubMed] [Google Scholar]
- 129. Rudroju N, Bansal D, Talakokkula ST, et al. : Comparative efficacy and safety of six antidepressants and anticonvulsants in painful diabetic neuropathy: a network meta-analysis. Pain Physician. 2013;16(6):E705–14. [PubMed] [Google Scholar]
- 130. Tanenberg RJ, Irving GA, Risser RC, et al. : Duloxetine, pregabalin, and duloxetine plus gabapentin for diabetic peripheral neuropathic pain management in patients with inadequate pain response to gabapentin: an open-label, randomized, noninferiority comparison. Mayo Clin Proc. 2011;86(7):615–26. 10.4065/mcp.2010.0681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Goldstein DJ, Lu Y, Detke MJ, et al. : Duloxetine vs. placebo in patients with painful diabetic neuropathy. Pain. 2005;116(1–2):109–18. 10.1016/j.pain.2005.03.029 [DOI] [PubMed] [Google Scholar]
- 132. Wasan AD, Ossanna MJ, Raskin J, et al. : Safety and efficacy of duloxetine in the treatment of diabetic peripheral neuropathic pain in older patients. Curr Drug Saf. 2009;4(1):22–9. 10.2174/157488609787354404 [DOI] [PubMed] [Google Scholar]
- 133. Trouvin AP, Perrot S, Lloret-Linares C: Efficacy of Venlafaxine in Neuropathic Pain: A Narrative Review of Optimized Treatment. Clin Ther. 2017;39(6):1104–22. 10.1016/j.clinthera.2017.05.347 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 134. Rowbotham MC, Goli V, Kunz NR, et al. : Venlafaxine extended release in the treatment of painful diabetic neuropathy: a double-blind, placebo-controlled study. Pain. 2004;110(3):697–706. 10.1016/j.pain.2004.05.010 [DOI] [PubMed] [Google Scholar]
- 135. Markovits E, Gilhar A: Capsaicin--an effective topical treatment in pain. Int J Dermatol. 1997;36(6):401–4. 10.1046/j.1365-4362.1997.00102.x [DOI] [PubMed] [Google Scholar]
- 136. Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. The Capsaicin Study Group. Arch Intern Med. 1991;151(11):2225–9. 10.1001/archinte.1991.00400110079017 [DOI] [PubMed] [Google Scholar]
- 137. Polydefkis M, Hauer P, Sheth S, et al. : The time course of epidermal nerve fibre regeneration: studies in normal controls and in people with diabetes, with and without neuropathy. Brain. 2004;127(Pt 7):1606–15. 10.1093/brain/awh175 [DOI] [PubMed] [Google Scholar]
- 138. Burness CB, McCormack PL: Capsaicin 8 % Patch: A Review in Peripheral Neuropathic Pain. Drugs. 2016;76(1):123–34. 10.1007/s40265-015-0520-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 139. Wolff RF, Bala MM, Westwood M, et al. : 5% lidocaine medicated plaster in painful diabetic peripheral neuropathy (DPN): a systematic review. Swiss Med Wkly. 2010;140(21–22):297–306. [DOI] [PubMed] [Google Scholar]
- 140. Suarez GA, Opfer-Gehrking TL, Offord KP, et al. : The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52(3):523–8. 10.1212/WNL.52.3.523 [DOI] [PubMed] [Google Scholar]
- 141. Freeman R: Autonomic peripheral neuropathy. Lancet. 2005;365(9466):1259–70. 10.1016/S0140-6736(05)74815-7 [DOI] [PubMed] [Google Scholar]
- 142. Boulton AJM, Vinik AI, Arezzo JC, et al. : Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. 10.2337/diacare.28.4.956 [DOI] [PubMed] [Google Scholar]
- 143. Valensi P, Pariès J, Attali JR, et al. : Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications--the French multicenter study. Metabolism. 2003;52(7):815–20. 10.1016/S0026-0495(03)00095-7 [DOI] [PubMed] [Google Scholar]
- 144. Witte DR, Tesfaye S, Chaturvedi N, et al. : Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48(1):164–71. 10.1007/s00125-004-1617-y [DOI] [PubMed] [Google Scholar]
- 145. Charles M, Fleischer J, Witte DR, et al. : Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013;56(1):101–8. 10.1007/s00125-012-2744-5 [DOI] [PubMed] [Google Scholar]
- 146. Charles M, Ejskjaer N, Witte DR, et al. : Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34(10):2244–9. 10.2337/dc11-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ziegler D, Schatz H, Conrad F, et al. : Effects of treatment with the antioxidant alpha-lipoic acid on cardiac autonomic neuropathy in NIDDM patients. A 4-month randomized controlled multicenter trial (DEKAN Study). Deutsche Kardiale Autonome Neuropathie. Diabetes Care. 1997;20(3):369–73. 10.2337/diacare.20.3.369 [DOI] [PubMed] [Google Scholar]
- 148. Pop-Busui R, Stevens MJ, Raffel DM, et al. : Effects of triple antioxidant therapy on measures of cardiovascular autonomic neuropathy and on myocardial blood flow in type 1 diabetes: a randomised controlled trial. Diabetologia. 2013;56(8):1835–44. 10.1007/s00125-013-2942-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pop-Busui R: Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010;33(2):434–41. 10.2337/dc09-1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Freeman R, Abuzinadah AR, Gibbons C, et al. : Orthostatic Hypotension: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(11):1294–309. 10.1016/j.jacc.2018.05.079 [DOI] [PubMed] [Google Scholar]
- 151. Gibbons CH, Schmidt P, Biaggioni I, et al. : The recommendations of a consensus panel for the screening, diagnosis, and treatment of neurogenic orthostatic hypotension and associated supine hypertension. J Neurol. 2017;264(8):1567–82. 10.1007/s00415-016-8375-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Freeman R: Clinical practice. Neurogenic orthostatic hypotension. N Engl J Med. 2008;358(6):615–24. 10.1056/NEJMcp074189 [DOI] [PubMed] [Google Scholar]
- 153. Kaufmann H: Droxidopa for symptomatic neurogenic orthostatic hypotension: what can we learn? Clin Auton Res. 2017;27(Suppl 1):1–3. 10.1007/s10286-017-0426-6 [DOI] [PubMed] [Google Scholar]
- 154. Parkman HP, Hasler WL, Fisher RS: American Gastroenterological Association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127(5):1592–622. 10.1053/j.gastro.2004.09.055 [DOI] [PubMed] [Google Scholar]
- 155. Kumar M, Chapman A, Javed S, et al. : The Investigation and Treatment of Diabetic Gastroparesis. Clin Ther. 2018;40(6):850–61. 10.1016/j.clinthera.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 156. Murao S, Hosokawa H: Serotonin 5-HT3 receptor antagonist for treatment of severe diabetic diarrhea. Diabetes Care. 2010;33(3):e38. 10.2337/dc09-2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Ogbonnaya KI, Arem R: Diabetic diarrhea. Pathophysiology, diagnosis, and management. Arch Intern Med. 1990;150(2):262–7. 10.1001/archinte.1990.00390140018005 [DOI] [PubMed] [Google Scholar]
- 158. Feldman HA, Goldstein I, Hatzichristou DG, et al. : Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54–61. 10.1016/S0022-5347(17)34871-1 [DOI] [PubMed] [Google Scholar]
- 159. Azmi S, Ferdousi M, Alam U, et al. : Small-fibre neuropathy in men with type 1 diabetes and erectile dysfunction: a cross-sectional study. Diabetologia. 2017;60(6):1094–101. 10.1007/s00125-017-4245-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Giugliano F, Maiorino M, Bellastella G, et al. : Determinants of erectile dysfunction in type 2 diabetes. Int J Impot Res. 2010;22(3):204–9. 10.1038/ijir.2010.1 [DOI] [PubMed] [Google Scholar]
- 161. van den Eeden SK, Sarma AV, Rutledge BN, et al. : Effect of intensive glycemic control and diabetes complications on lower urinary tract symptoms in men with type 1 diabetes: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) study. Diabetes Care. 2009;32(4):664–70. 10.2337/dc07-2375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Enzlin P, Mathieu C, van den Bruel A, et al. : Sexual dysfunction in women with type 1 diabetes: a controlled study. Diabetes Care. 2002;25(4):672–7. 10.2337/diacare.25.4.672 [DOI] [PubMed] [Google Scholar]
- 163. Hackett G, Kirby M, Wylie K, et al. : British Society for Sexual Medicine Guidelines on the Management of Erectile Dysfunction in Men-2017. J Sex Med. 2018;15(4):430–57. 10.1016/j.jsxm.2018.01.023 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 164. Mulhall JP, Giraldi A, Hackett G, et al. : The 2018 Revision to the Process of Care Model for Evaluation of Erectile Dysfunction. J Sex Med. 2018;15(9):1280–92. 10.1016/j.jsxm.2018.06.005 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 165. Mulhall JP, Giraldi A, Hackett G, et al. : The 2018 Revision to the Process of Care Model for Management of Erectile Dysfunction. J Sex Med. 2018;15(10):1434–45. 10.1016/j.jsxm.2018.05.021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 166. Mulhall JP, Carlsson M, Stecher V, et al. : Predictors of Erectile Function Normalization in Men With Erectile Dysfunction Treated With Placebo. J Sex Med. 2018;15(6):866–72. 10.1016/j.jsxm.2018.03.089 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 167. Gruenwald I, Kitrey ND, Appel B, et al. : Low-Intensity Extracorporeal Shock Wave Therapy in Vascular Disease and Erectile Dysfunction: Theory and Outcomes. Sex Med Rev. 2013;1(2):83–90. 10.1002/smrj.9 [DOI] [PubMed] [Google Scholar]
- 168. Kitrey ND, Vardi Y, Appel B, et al. : Low Intensity Shock Wave Treatment for Erectile Dysfunction-How Long Does the Effect Last? J Urol. 2018;200(1):167–70. 10.1016/j.juro.2018.02.070 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 169. Bechara A, Casabé A, De Bonis W, et al. : Twelve-Month Efficacy and Safety of Low-Intensity Shockwave Therapy for Erectile Dysfunction in Patients Who Do Not Respond to Phosphodiesterase Type 5 Inhibitors. Sex Med. 2016;4(4):e225–e232. 10.1016/j.esxm.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 170. Elhanbly SM, Abdel-Gawad MM, Elkholy AA, et al. : Nocturnal penile erections: A retrospective study of the role of RigiScan in predicting the response to sildenafil in erectile dysfunction patients. J Adv Res. 2018;14:93–6. 10.1016/j.jare.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation