Abstract
Background
This study investigated the effect and mechanism of notoginsenoside R1 (NGR1) on chronic atrophic gastritis (CAG) in a rat model.
Material/Methods
To perform our investigation, a rat model of CAG was established, and then rats were treated with various doses of NGR1. After treatment, hematoxylin-eosin (HE) staining was used for histopathological observation and further scoring. Enzyme-linked immunosorbent assay (ELISA) was used to determine the contents of gastrointestinal hormones, inflammatory factors, gastric mucosal destruction factors, and gastric mucosal-protective factors. Gene and protein expressions were measured using quantitative real-time PCR and Western blot assay, respectively.
Results
Results indicated that NGR1 relieved rat CAG. NGR1 treatment significantly increased the levels of gastrin (GAS) and somatostatin (SS) and reduced motilin (MTL) in the serum of CAG rats. The serum levels of interleukin (IL)-1β and IL-6 were significantly reduced by NGR1 treatment in CAG rats in a dose-dependent manner. Additionally, the increased levels of prostaglandin (PG)E2, nitric oxide synthase (NOS), and endothelin (ET) in CAG rats were significantly decreased by NGR1 administration. Moreover, the decreased level of secretory IgA (sIgA) and glutathione (GSH) in rats caused by MNNG was notably increased by NGR1 treatment. No significant changes were found in glutathione disulfide (GSSG) secretion. Finally, we found that the increased Bcl-2 expression and reduced Bax expression in the stomach tissues of rats caused by MNNG were eliminated by NGR1 treatment.
Conclusions
NGR1 exerts a protective effect on CAG, and it is a multi-target, multi-linked, comprehensive process.
MeSH Keywords: Gastritis, Atrophic; Panax notoginseng; Protective Agents
Background
Chronic atrophic gastritis (CAG) is one of the most common digestive diseases in the world, and it is characterized by atrophy of gastric mucosal epithelium and glands, reduced number of glands, thinning of gastric mucosa, and thickening of mucosal basal layer, or is accompanied by pyloric adenosis and intestinal metaplasia, as well as dysplasia [1]. CAG is often clinically manifested as fullness, belching, abdominal pain, loss of appetite, and weight loss. CAG is a well-known sign of precancerous lesions of gastric cancer, which is ranked fourth in cancer incidence and is currently the second most common cause of cancer-related deaths worldwide [2,3). In recent years, the incidence of CAG has been rising year by year and the average age of patients is tending to be younger [4]. Therefore, active treatment of CAG is an effective means of reducing cancer and preventing gastric cancer. The pathogenesis of CAG is not clear, but it is generally believed that it is caused by repeated damage to the gastric mucosa caused by a variety of endogenous and exogenous factors (such as emotional stress, physicochemical factors, and microbial infection) [5]. In-depth studies on the pathogenesis of CAG have focused increased attention on the treatment of CAG. However, there is no effective treatment for CAG other than drug treatments such as H. pylori eradication, acid suppression, and non-steroidal anti-inflammatory drugs [2,6–8]. Therefore, it is of great importance to develop more effective CAG treatments.
Notoginsenoside R1 (NGR1) is the major phytoestrogen extracted from the plant P. notoginseng; it exhibits anti-apoptotic, anti-oxidant, and anti-inflammatory properties [9]. Recent studies have shown that NGR1 plays an important role in the treatment of cardiac dysfunction [10,11], acute liver failure [12], and diabetic nephropathy [13]. However, to the best of our knowledge, the effect of NGR1 on CAG is still unclear. Therefore, we performed the present study.
N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) is a carcinogen that has been widely used in recent years, and it can directly act on epithelial cells of the stomach without affecting enzymes [14]. MNNG simulates improper intake of nitrate, which can transform into carcinogenic substances such as nitrite in the stomach, which lead to chronic atrophic gastritis and precancerous lesions or even gastric cancer [14–17]. MNNG has a strong mutagenic ability, and can cause mutations in bases in the original DNA chain, leading to cancer [18]. At present, MNNG is widely used in the establishment of animal models of CAG [15–17]. Therefore, in the present study, a CAG rat model was established by administration of MNNG.
The purpose of our study was to investigate the effect of NGR1 on CAG in a rat model and to explore the underlying mechanism. We hope to provide a stronger theoretical basis for the treatment of CAG.
Material and Methods
Animals
We obtained 60 Sprague-Dawley (SD) rats (age: 8 weeks; body weight: 200±20 g) from the Vital River Company (Beijing, China). All rats were kept under specific pathogen-free (SPF) conditions with a 12 h light/dark cycle and free access to water and food. Our study was performed according to the guidelines of the National Institutes of Health for the Care and Use of Laboratory Animals and was approved by the Ethics Committee of the Second Affiliated Hospital of Nanjing University of Chinese Medicine (Second Hospital of Jiangsu Province).
CAG model establishment and TNR1 treatment
After 1 week of adaptation, the rats were randomly divided into 6 groups (n=10 per group): 1) control group; 2) CAG model group; 3) CAG+Vehicle group; 4) CAG+TGR1–5 group; 5) CAG+TGR1–10 group; and 6) CAG+TGR1–20 group. The CAG rat model was established by treatment with N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) combined with irregular diet for 12 weeks according to previous studies (15, 16). Rats in the CAG+Vehicle and CAG+NGR1 groups were administrated vehicle (sterile distilled water, by gavage) and various doses of NGR1 (5, 10, and 20 mg/kg/day, by gavage) [19] for 60 consecutive days. From week 12, all rats in each group were fed normally and sterile water was given ad libitum. Rats were weighed weekly and their weight changes were recorded. After NGR1 treatment was finished, all rats were anesthetized and killed and their stomachs were quickly removed, then abdominal aortic blood was collected for experiments. NGR1 (purity >98%) was from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO).
Histopathological observation
Gastric tissues were collected from rats of each group, then the tissue samples were fixed with 10% formalin (Invitrogen) and embedded in paraffin, followed by sectioning (5-μm thickness) and hematoxylin and eosin (HE) staining. Finally, we observed and analyzed the sections by using light microscopy. The histological score was assessed according to the diagnostic criteria of gastritis in Houston in 1996 [20]. We calculated the inflammatory score in 10 microscopic fields of each section and scored them on a 4-point scale [16,21] (0=normal; 1=mild, small amount of inflammatory cells infiltrating into the depression or basal area of gastric glands; 2=moderate, moderate amount of inflammatory cell infiltration, which is located in two-thirds of the gastric gland; and 3=significant, massive infiltration of inflammatory cells throughout the gastric gland). The atrophy score of gastric glands was also calculated in 10 microscopic fields of each section and scored on a 4-point scale (2) (0=normal; 1=mild, atrophy less than one-third of the stomach gland; 2=moderate, atrophy is limited to two-thirds of the stomach glands; and 3=significant, atrophy more than two-thirds of the stomach glands). The histological score in this study included the inflammation score and the atrophy score.
Enzyme-linked immunosorbent assay (ELISA)
All rats in each group were anesthetized and abdominal aortic blood were collected. Blood was centrifuged (3000 rpm, 10 min) to collect the serum. Then, the level of GAS (Cat no. D730506, Abcam), SS (Cat no. D730406, Sangon Biotechnology), MTL (Shanghai Jun Yu Biotechnology), IL-1β (Cat no. 100768, Abcam), IL-6 (Cat no. 100772, Abcam), PGE2 (Cat no. 133021, Abcam), NOS (Cat no. D730315, Sangon Biotechnology), ET (Cat no. D730150, Sangon Biotechnology), sIgA (Cat no. D730381, Sangon Biotechnology), GSSG (Cat no. D730505, Sangon Biotechnology), and GSH (Cat no. D730507, Sangon Biotechnology) were detected using ELISA assay according to the manufacturer’s instructions for each kit.
Western blot assay
Total proteins from stomach tissues of rats from each group were extracted by using RIPA buffer (Beyotime Institute of Biotechnology) with a protease/phosphatase inhibitor cocktail (Cell Signaling Technology). We used the bicinchoninic acid assay kit (BCA; Pierce Biotechnology, Rockford, IL, USA) to detect the protein concentrations according to the manufacturer’s instructions. Equal amounts of protein samples (25 μg/lane) were separated on 12% SDS-PAGE and transferred onto polyvinylidene fluoride (PVDF) membranes (Merck Millipore, Billerica, MA). After blocking with 5% non-fat dry milk at room temperature for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. The primary antibodies used were: anti-Bax (cat no. 5023; dilution: 1: 1,000; Cell Signaling Technology, Inc.), anti-Bcl-2 (cat no. 3498; dilution: 1: 1,000; Cell Signaling Technology, Inc.), and anti-β-actin (cat no. 49703; dilution: 1: 1,000; Cell Signaling Technology, Inc.). Subsequently, the membranes were incubated with a second anti-body (cat no. 7074; dilution: 1: 1,000; Cell Signaling Technology, Inc.) at room temperature for 3 h. Finally, the protein bands were visualized using ECL Western blotting detection kits (Millipore) following the manufacturer’s instructions.
qRT-PCR
Total RNA was extracted from the stomach tissues of rats using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Total RNA was reversely transcribed into cDNA by using a PrimeScript RT Reagent Kit (Takara Bio) as per the manufacturer’s instructions. Then, qPCR analysis was conducted using the SYBR® Premix Ex Taq™ II (Takara Bio Inc.) with an ABI 7900 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) to analyze the synthesized cDNAs. GAPDH expression was used as an internal control. Primer sequences were the following:
Bcl-2-Forward: 5′TTGGATCAGGGAGTTGGAAG3′,
Bcl-2-Reverse: 5′TGTCCCTACCAACCAGAAGG3′;
Bax-Forward: 5′CGTCCACCAAGAAGCTGAGCG3′,
Bax-Reverse: 5′CGTCCACCAAGAAGCTGAGCG3′;
GAPDH-Forward: 5′CTTTGGTATCGTGGAAGGACTC3′,
GAPDH-Reverse: 5′GTAGAGGCAGGGATGATGTTCT3′.
The relative mRNA expression level was calculated using the 2−ΔΔCT method [22].
Statistical analysis
Data are displayed as mean ±SD. SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses. We used t tests or one-way ANOVA followed by NSK tests to analyze differences between groups. A value of P<0.05 was considered statistically significant.
Results
Alleviating effects of NGR1 on rat CAG
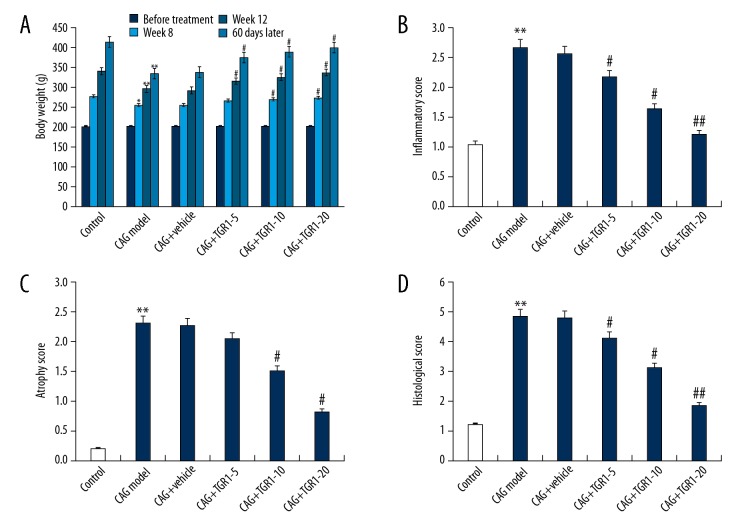
As shown in Figure 1A, compared with the control group, the body weights in the CAG model group were significantly lower at week 8, week 12, and 60 days after treatment. However, after 60 days of NGR1 treatment, the body weights in the NGR1 treatment groups were significantly higher than that in the CAG model group. As shown in Figure 1B–1D, compared with the control group, the inflammatory score, atrophy score, and histological score in the CAG model group were significantly increased, and these increases were eliminated by NGR1 treatment. These results indicate that NGR1 treatment had a significant effect in protecting against CAG in rats.
Figure 1.
Effect of NGR1 on rat CAG. (A) Body weight of rats in different groups at various time points; (B) Inflammatory scores of gastric glands in different groups; (C) Atrophy scores of gastric glands in different groups; (D) Histological scores of gastric glands in different groups. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. *, ** p<0.05, 0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
Effects of NGR1 on gastrointestinal hormones (GAS, SS, MTL) in MNNG-induced CAG Rats
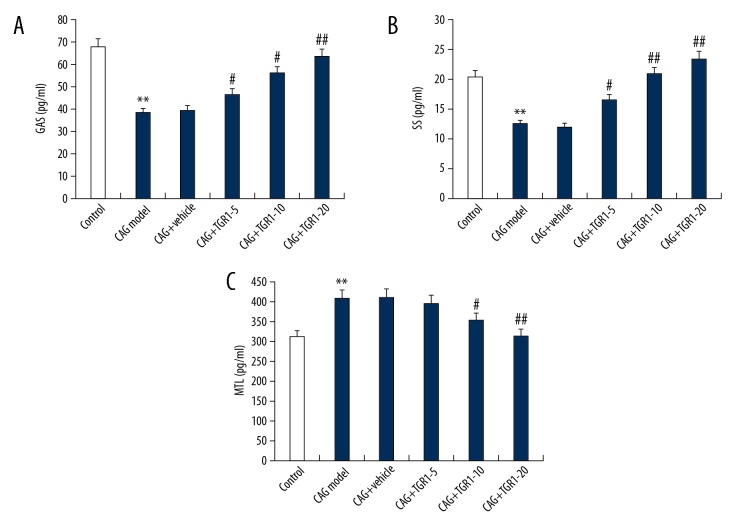
We assessed the levels of gastrointestinal hormones (GAS, SS, MTL) in serum of rats by using ELISA. As shown in Figure 2, compared with the control group, the levels of GAS and SS were significantly reduced and MTL was significantly increased in rats in the MNNG treatment alone group. However, NGR1 treatment significantly increased the levels of GAS and SS and reduced MTL level in the serum of CAG rats in a dose-dependent way.
Figure 2.
Effect of NGR1 on GAS, SS, and MTL expression in serum of rats with or without CAG. After specific treatment, the levels of GAS (A), SS (B), and MTL (C) expression in serum of rats with or without CAG were detected using ELISA. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. ** p<0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
Effects of NGR1 on inflammatory factors (IL-1β, IL-6) in MNNG-induced CAG Rats
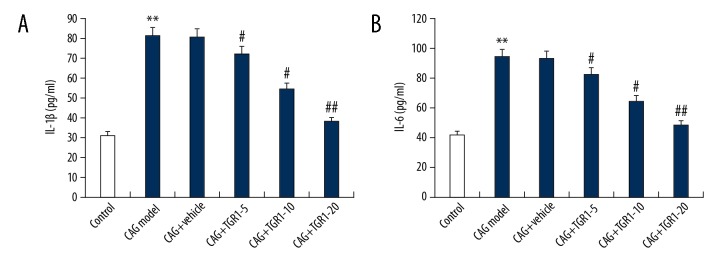
CAG is a process of chronic inflammation of the stomach mucosa; thus, inflammation plays critical roles in the development of CAG. Therefore, we assessed the effect of NGR1 on the expression of inflammatory factors. As shown in Figure 3, the serum levels of IL-1β and IL-6 in rats were significantly enhanced by MNNG treatment, and these enhancements were notably reduced by NGR1 treatment in a dose-dependent manner.
Figure 3.
Effect of NGR1 on IL-1β and IL-6 expression in serum of rats with or without CAG. After specific treatment, the levels of IL-1β (A) and IL-6 (B) expression in serum of rats with or without CAG were detected using ELISA. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. ** p<0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
Effects of NGR1 on gastric mucosal destruction factors (PGE2, NOS, ET) in MNNG-induced CAG Rats
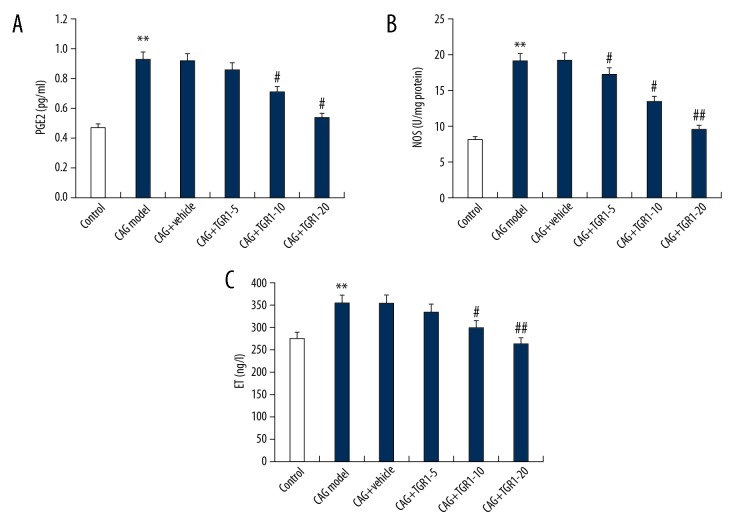
The expression levels of gastric mucosal destruction factors including PGE2, NOS, and ET were analyzed in the present study. The findings suggested that compared with the control group, the serum levels of PGE2, NOS, and ET were significantly increased in the CAG model group. As expected, NGR1 treatment dose-dependently reduced the serum levels of PGE2, NOS, and ET in CAG rats (Figure 4).
Figure 4.
Effect of NGR1 on PGE2, NOS, and ET expression in serum of rats with or without CAG. After specific treatment, the levels of PGE2 (A), NOS (B), and ET (C) expression in serum of rats with or without CAG were detected using ELISA. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. ** p<0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
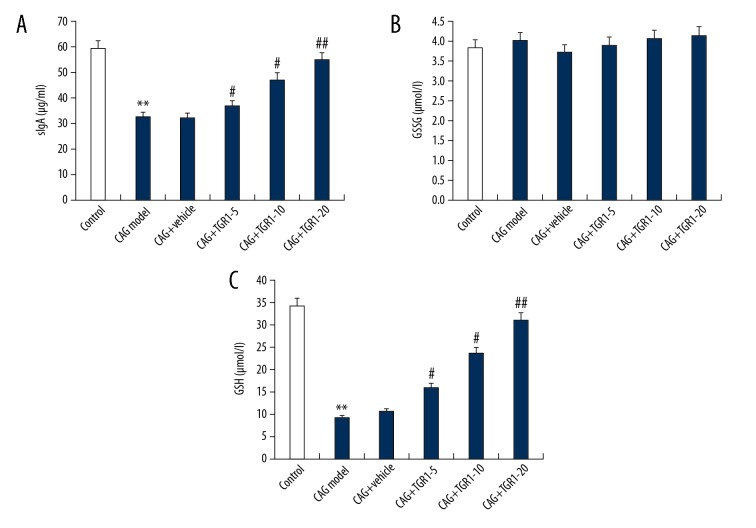
Effects of NGR1 on gastric mucosa protective factors (sIgA, GSSG, and GSH) in MNNG-induced CAG Rats
As shown in Figure 5, our results showed that MNNG administration significantly decreased the levels of sIgA and GSH in the serum of rats, and these changes were notably inhibited by NGR1 treatment in a dose-dependent manner. No significant changes were found in the level of GSSG in different groups.
Figure 5.
Effect of NGR1 on sIgA, GSSG, and GSH expression in serum of rats with or without CAG. After specific treatment, the levels of sIgA (A), GSSG (B), and GSH (C) expression in serum of rats with or without CAG were detected using ELISA. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. ** p<0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
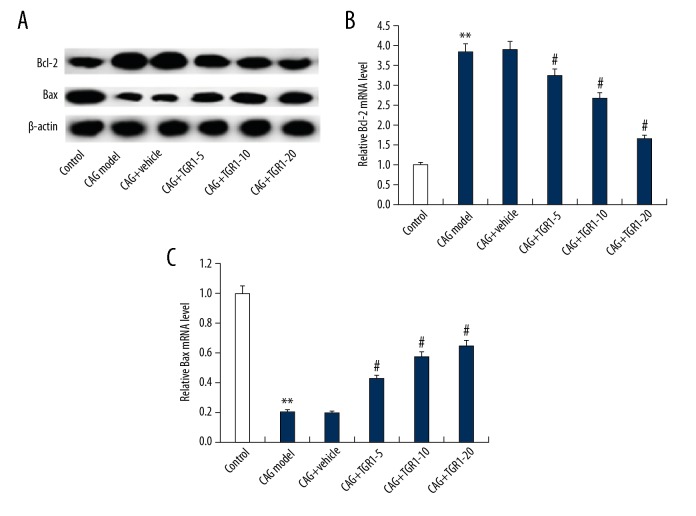
Effects of NGR1 on the expression of Bcl-2 and Bax in MNNG-induced CAG Rats
Finally, the anti-apoptotic gene Bcl-2 and pro-apoptotic gene Bax were analyzed. We found that the increased Bcl-2 expression and reduced Bax expression in the stomach tissues of rats caused by MNNG were dose-dependently eliminated by NGR1 treatment (Figure 6).
Figure 6.
Effect of NGR1 on Bcl-2 and Bax expression the stomach tissues of rats with or without CAG. After specific treatment, the protein (A) and mRNA (B, C) level of Bcl-2 and Bax expression the stomach tissues of rats with or without CAG was detected using Western blot and qRT-PCR assay, respectively. Control: rats without any treatment; CAG Model: rats were treated with MNNG; CAG+Vehicle: rats were treated with MNNG and administered sterile distilled water; CAG+TGR1–5: rats were treated with MNNG and administered 5 mg/kg/day NGR1; CAG+TGR1–10: rats were treated with MNNG and administered 10 mg/kg/day NGR1; CAG+TGR1–20: rats were treated with MNNG and administered 20 mg/kg/day NGR1. Data are displayed as mean ±SD. ** p<0.01 vs. Control group; #, ## p<0.05, 0.01 vs. CAG model group.
Discussion
We found that NGR1 administration relieved MNNG-induced pathological changes and symptoms of CAG in rats. Our findings suggest that NGR1 treatment affected the expression of gastrointestinal hormones (GAS, SS, MTL), inflammatory factors (IL-1β, IL-6), gastric mucosal destruction factors (PGE2, NOS, ET), and gastric mucosa-protective factors (sIgA and GSH) in the serum of CAG rats. Moreover, we found that Bcl-2 and Bax were involved in the influences of NGR1 on CAG. Our data show that NGR1 treatment mitigated the CAG induced by MNNG administration.
CAG is one of the most common digestive diseases worldwide [1]. In recent years, the incidence of CAG has been rising year by year and the average of patients is becoming younger [4]. CAG severely affects the health and quality of life of patients [23,24]. However, there is still no effective treatment method for CAG. Finding new effective CAG treatments is clinically significant. NGR1 is the major phytoestrogen extracted from P. notoginseng, and increasing evidence indicates that NGR1 has a variety of pharmacological effects, including anti-apoptotic, anti-oxidant, and anti-inflammatory properties [9]. More and more studies indicate that NGR1 has an important influence on the development of many diseases. Fang et al. reported that NGR1 prevented vascular smooth muscle cell growth, metastasis, and neointimal hyperplasia via regulating the PI3K/Akt pathway [25]. Tu et al. suggested that NGR1 plays a protective role in neonatal cerebral hypoxic-ischemic brain injury [26]. Zhai et al. indicated that NGR1 can relieve diabetic encephalopathy [27]. Zhao et al. reported that NGR1 prevented wear particle-induced periprosthetic osteolysis [28]. NGR1 was also found to ameliorate podocyte injury in diabetic nephropathy rats [19]. Another study indicated that NGR1 plays a protective role in myocardial inflammation through inhibiting cardiomyocyte inflammatory response and apoptosis [29]. However, the effect of NGR1 on CAG is still unclear. Therefore, we conducted the present study.
Firstly, to perform our investigation, a rat model of CAG was established by administration of MNNG. After treatment with various doses of NGR1, we found that NGR1 significantly increased the body weights of rats, which were reduced by MNNG treatment. NGR1 dose-dependently reduced the inflammation score, atrophy score, and histological score in the stomach tissues of CAG rats. These data show the protective role of NGR1 in CAG.
GAS is produced by gastric antral G cells and is a major nutrient of gastric mucosa. It exerts nutrition and protective effects on gastric mucosa directly or indirectly. SS is mainly secreted by endocrine cells (D cells) and has a wide inhibitory effect on the gastrointestinal system. With the development of CAG, gastric antrum tissue atrophy is aggravated, G cells gradually decrease, GAS content decreases, and SS also decreases. The dysfunction of GAS-SS-gastric acid secretion axis can accelerate atrophic lesions of gastric mucosal glands. MTL has a drastic effect on gastrointestinal motility and gastrointestinal electrical activity, aiding gastric emptying and food digestion. Our present study showed that the levels of GAS and SS were significantly reduced, and MTL level was significantly increased in rats in the MNNG treatment alone group. However, NGR1 treatment significantly increased the levels of GAS and SS, and reduced MTL level, indicating that NGR1 has a good regulatory effect on rat gastrointestinal hormones and is an important mechanism for NGR1 in the treatment of CAG.
Studies have shown that the secretion of pro-inflammatory cytokines (IL-1β and IL-6) plays an important role in the development of CAG [30]. The expression of IL-1β and IL-6 in the serum of CAG rats was analyzed in our study, and we found that the increased serum levels of IL-1β and IL-6 in CAG rats were significantly reduced by NGR1 treatment. These data suggest that NGR1 improves the degree of inflammation of the gastric mucosa by reducing IL-1β and IL-6 expression.
Next, we studied whether NGR1 has inhibitory effects on the factors causing or aggravating CAG, and the serum levels of PEG2, NOS, and ET were determined after NGR1 treatment. The results indicated that the serum levels of PEG2, NOS, and ET were significantly increased in the CAG rats, and NGR1 treatment dose-dependently reduced the serum levels of PEG2, NOS, and ET. This experiment confirmed that NGR1 inhibits the destruction of gastric mucosal destruction factor in CAG rats, reduces the degree of inflammation, ensures the blood supply to the gastric mucosa, maintains the stability of the internal environment of the body, and may be effective in treating CAG. Then, we explored whether NGR1 also plays a role in gastric mucosal protective factors (sIgA, GSH, and GSSG). Our findings show that MNNG administration significantly decreased the levels of sIgA and GSH in the serum of rats, and these changes were notably inhibited by NGR1 treatment in a dose-dependent manner. However, no significant changes were found in the level of GSSG in rats from different groups. NGR1 enhanced GSH expression, increased the ratio of GSH/GSSG, scavenged oxygen free radicals, regulated immunity, and treated CAG.
Finally, as abnormalities of apoptosis and proliferation of gastric epithelial cells are involved in the progression of CAG, we analyzed whether the anti-apoptotic gene Bcl-2 and the pro-apoptotic gene Bax are involved in the effect of NGR1 on CAG. We found that the increased Bcl-2 expression and reduced Bax expression in the stomach tissues of rats caused by MNNG treatment were dose-dependently eliminated by NGR1 treatment. These data indicate that NGR1 promotes the normal apoptosis of gastric epithelial cells to accelerate its normal renewal and repair and prevent cancer damage.
Taken together, our results show that NGR1 relieved rat CAG through regulating secretion of gastrointestinal hormones, inhibiting production of inflammatory factors, reducing expression of gastric mucosal destruction factors, increasing expression of gastric mucosa protective factors, and regulating Bcl-2 and Bax expression. However, this study is only a preliminary study of the effect of NGR1 on atrophic gastritis. The effects and precise mechanisms of NGR1 in chronic atrophic gastritis need to be further explored, and we will conduct in-depth research on these issues in the future.
Conclusions
NGR1 can treat CAG, and the process is a multi-target and multi-linked process. Therefore, NGR1 is a promising agent for the treatment of CAG.
Footnotes
Source of support: This study was supported by the Second Affiliated Hospital of Nanjing University of Chinese Medicine (Second Hospital of Jiangsu Province) (No. SEZJJP201603), National Natural Science Foundation of China (No.81673795) and the Jiangsu Provincial Natural Science Foundation Project (No. BK20151567)
Conflict of interests
None.
References
- 1.de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–52. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- 2.Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015;20:25–40. doi: 10.15430/JCP.2015.20.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Adamu MA, Weck MN, Rothenbacher D, Brenner H. Incidence and risk factors for the development of chronic atrophic gastritis: A five-year follow-up of a population-based cohort study. Int J Cancer. 2011;128:1652–58. doi: 10.1002/ijc.25476. [DOI] [PubMed] [Google Scholar]
- 5.Giannakis M, Chen SL, Karam SM, et al. Helicobacter pylori evolution during progression from chronic atrophic gastritis to gastric cancer and its impact on gastric stem cells. Proc Natl Acad Sci USA. 2008;105:4358–63. doi: 10.1073/pnas.0800668105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pellicano R, Ribaldone DG, Fagoonee S, et al. A 2016 panorama of Helicobacter pylori infection: Key messages for clinicians. Panminerva Med. 2016;58:304–17. [PubMed] [Google Scholar]
- 7.Gao X, Yuan J, Li H, Ren S. Clinical research on acupuncture and moxibustion treatment of chronic atrophic gastritis. J Tradit Chin Med. 2007;27:87–91. [PubMed] [Google Scholar]
- 8.den Hollander WJ, Kuipers EJ. Current pharmacotherapy options for gastritis. Expert Opin Pharmacother. 2012;13:2625–36. doi: 10.1517/14656566.2012.747510. [DOI] [PubMed] [Google Scholar]
- 9.Sun B, Xiao J, Sun XB, Wu Y. Notoginsenoside R1 attenuates cardiac dysfunction in endotoxemic mice: An insight into oestrogen receptor activation and PI3K/Akt signalling. Br J Pharmacol. 2013;168:1758–70. doi: 10.1111/bph.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge ZR, Xu MC, Huang YU, et al. Cardioprotective effect of notoginsenoside R1 in a rabbit lung remote ischemic postconditioning model via activation of the TGF-β1/TAK1 signaling pathway. Exp Ther Med. 2016;11:2341–48. doi: 10.3892/etm.2016.3222. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Xia KP, Ca HM, Shao CZ. Protective effect of notoginsenoside R1 in a rat model of myocardial ischemia reperfusion injury by regulation of Vitamin D3 upregulated protein 1/NF-κB pathway. Pharmazie. 2015;70:740–44. [PubMed] [Google Scholar]
- 12.Zhao J, Shi Z, Liu S, et al. Ginsenosides Rg1 from Panax ginseng: A potential therapy for acute liver failure patients? Evid Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/538059. 538059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gui D, Wei L, Jian G, et al. Notoginsenoside R1 ameliorates podocyte adhesion under diabetic condition through α3β1 integrin upregulation in vitro and in vivo. Cell Physiol Biochem. 2014;34:1849–62. doi: 10.1159/000366384. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Liang M, Yao W, et al. Functional role of lncRNA LOC101927497 in N-methyl-N′-nitro-N-nitrosoguanidine-induced malignantly transformed human gastric epithelial cells. Life Sci. 2018;193:93–103. doi: 10.1016/j.lfs.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhu X, Liu S, Zhou J, et al. Effect of Astragalus polysaccharides on chronic atrophic gastritis induced by N-methyl-N′-nitro-N-nitrosoguanidine in rats. Drug Res (Stuttg) 2013;63:597–602. doi: 10.1055/s-0033-1341518. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Huang K, Zhong G, et al. Acupuncture decreases NF-κB p65, miR-155, and miR-21 and Increases miR-146a expression in chronic atrophic gastritis rats. Evid Based Complement Alternat Med. 2016;2016 doi: 10.1155/2016/9404629. 9404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo Y, Matsui H, Ninomiya T, et al. Non-invasive approach for diagnosing atrophic gastritis using the 13C-bicarbonate breath test. Int J Mol Med. 2001;7:381–84. doi: 10.3892/ijmm.7.4.381. [DOI] [PubMed] [Google Scholar]
- 18.Pithva SP, Ambalam PS, Ramoliya JM, et al. Antigenotoxic and antimutagenic activities of probiotic Lactobacillus rhamnosus Vc against N-methyl-N′-nitro-N-nitrosoguanidine. Nutr Cancer. 2015;67:1142–50. doi: 10.1080/01635581.2015.1073751. [DOI] [PubMed] [Google Scholar]
- 19.Huang G, Lv J, Li T, et al. Notoginsenoside R1 ameliorates podocyte injury in rats with diabetic nephropathy by activating the PI3K/Akt signaling pathway. Int J Mol Med. 2016;38:1179–89. doi: 10.3892/ijmm.2016.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Zhong J, Zhou Q, et al. The regenerating gene iα is overexpressed in atrophic gastritis rats with hypergastrinemia. Gastroenterol Res Pract. 2011;2011 doi: 10.1155/2011/403956. 403956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔt method. Methods. 2011;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Liu Y, You P, Feng G. Occurrence of gastric cancer in patients with atrophic gastritis during long-term follow-up. Scand J Gastroenterol. 2018;53:843–48. doi: 10.1080/00365521.2018.1477987. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Wang S, Qi M, et al. Psychological distress in patients with chronic atrophic gastritis: The risk factors, protection factors, and cumulative effect. Psychol Health Med. 2018;23:797–803. doi: 10.1080/13548506.2018.1428756. [DOI] [PubMed] [Google Scholar]
- 25.Fang H, Yang S, Luo Y, et al. Notoginsenoside R1 inhibits vascular smooth muscle cell proliferation, migration and neointimal hyperplasia through PI3K/Akt signaling. Sci Rep. 2018;8:7595. doi: 10.1038/s41598-018-25874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu L, Wang Y, Chen D, et al. Protective effects of notoginsenoside R1 via regulation of the PI3K-Akt-mTOR/JNK pathway in neonatal cerebral hypoxic-ischemic brain injury. Neurochem Res. 2018;43(6):1210–26. doi: 10.1007/s11064-018-2538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai Y, Meng X, Luo Y, et al. Notoginsenoside R1 ameliorates diabetic encephalopathy by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Oncotarget. 2018;9:9344–63. doi: 10.18632/oncotarget.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S, Yan L, Li X, et al. Notoginsenoside R1 suppresses wear particle-induced osteolysis and RANKL mediated osteoclastogenesis in vivo and in vitro. Int Immunopharmacol. 2017;47:118–25. doi: 10.1016/j.intimp.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhong L, Zhou XL, Liu YS, et al. Estrogen receptor α mediates the effects of notoginsenoside R1 on endotoxin-induced inflammatory and apoptotic responses in H9c2 cardiomyocytes. Mol Med Rep. 2015;12:119–26. doi: 10.3892/mmr.2015.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Wang W, Zhang T, et al. Differential expression of phospholipase C and epsilon 1 in gastritis and gastric cancer is associated with chronic atrophic. PLoS One. 2012;7:e47563. doi: 10.1371/journal.pone.0047563. [DOI] [PMC free article] [PubMed] [Google Scholar]