Abstract
Background
Preliminary microarray data in our laboratory indicated that the novel long noncoding RNA (lncRNA), GASL1, was downregulated in patients with intracranial aneurysms. This study aimed to investigate the expression of lncRNA GASL1 in patients with intracranial aneurysms and its role in the regulation of vascular smooth muscle cell (VSMC) proliferation by transforming growth factor-β1 (TGF-β1).
Material/Methods
The study included 68 patients with unruptured intracranial aneurysms and 56 healthy volunteers. In both groups, serum levels of TGF-β1 were measured using an enzyme-linked immunoassay (ELISA) and Western blot. Human VSMCs in vitro underwent lncRNA GASL1 overexpression using the insertion of an EcoRI-EcoRI fragment into the pIRSE2 vector. Cell viability and proliferation were measured by a cell counting kit-8 (CCK-8) assay. RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR) determined GASL1 expression.
Results
The lncRNA, GASL1, was significantly downregulated, while TGF-β1 was significantly upregulated in the serum of patients with an intracranial aneurysm compared with healthy controls, which was confirmed by receiver operating characteristic (ROC) curve analysis. In human VSMCs, lncRNA GASL1 overexpression increased cell proliferation and downregulated TGF-β1 expression, while treatment with TGF-β1 reduced VSMC proliferation but showed no effects on GASL1 expression.
Conclusions
Expression of the novel lncRNA, GASL1, was downregulated in patients with intracranial aneurysms and regulated the proliferation of VSMCs in vitro by targeting TGF-β1.
MeSH Keywords: Cell Proliferation; Intracranial Aneurysm; Muscle, Smooth, Vascular
Background
Pathological changes in cerebral arteries or veins can be associated with apoptosis of vascular smooth muscle cells (VSMCs) that may weaken the vascular, leading to the development of an intracranial aneurysm [1]. Intracranial aneurysms were previously considered to be a rare cause of intracranial hemorrhage, until the development of imaging methods, including computed tomography (CT) and magnetic resonance imaging (MRI). Worldwide, intracranial aneurysms have an estimated prevalence of 3% [2,3]. Intracranial aneurysms mainly affect the junction of the anterior communicating artery and anterior cerebral artery, the internal carotid artery, the origin of the ophthalmic artery, the middle cerebral artery branch points, and the internal carotid artery bifurcation [4]. Rupture of an intracranial aneurysm is a major cause of life-threatening subarachnoid hemorrhage or stroke [5]. Currently, the pathogenesis of intracranial aneurysm remains unknown, and patient outcome following rupture of an intracranial aneurysm remains poor.
Loss of VSMCs participates in the pathogenesis of intracranial aneurysm [6]. Activation of the transforming growth factor-β1 (TGF-β1) pathway has been shown to have key roles in the regulation of biological behaviors of VSMCs, including as calcification [7], cell proliferation [8] and apoptosis [9]. GASL1 is a newly identified long noncoding RNA (lncRNA) that has been shown to have a role in tumor suppression in liver cancer [10]. Expression of the novel lncRNA, GASL1, has been reported to be downregulated in liver cancer, and overexpression of lncRNA GASL1 inhibited cancer cell proliferation in liver cancer in vitro by restricting the activity of the E2F1 transcription factor, which induces cell proliferation and apoptosis [10].
Preliminary microarray data in our laboratory indicated that the novel lncRNA, GASL1, was downregulated in patients with intracranial aneurysms. Therefore, the aims of this study were to investigate the expression of lncRNA GASL1, in patients with intracranial aneurysms and its role in the regulation of vascular smooth muscle cell (VSMC) proliferation in vitro by transforming growth factor-β1 (TGF-β1).
Material and Methods
Patients enrolment and study inclusion and exclusion criteria
A total of 144 patients with unruptured intracranial aneurysm were diagnosed and treated at the Centre Hospital of Weihai Hospital from March 2015 to March 2017. Among these patients, 68 cases were enrolled into this study according to strict inclusion and exclusion criteria.
Inclusion criteria were patients with unruptured intracranial aneurysms who had complete medical records, who fully understood the experimental protocol, and signed informed consents. The exclusion criteria were patients with ruptured intracranial aneurysm, and with significant comorbidity including chronic diseases, and patients who failed to comply with the study protocol.
Patients in the study group and the control group
Clinical data of the 68 participating patients were obtained from their medical records and by questionnaire. The study group included 35 cases of intracranial aneurysm of the anterior communicating artery, 20 cases of intracranial aneurysm of the posterior communicating artery, and 13 cases of intracranial aneurysm of the middle cerebral artery bifurcation. The diameter of the intracranial aneurysms ranged from 9.26–23.44 mm, with a mean diameter of 14.2±3.8 mm. The study patients included 36 men and 28 women, with an age range of 36–60 years and a mean age of 46.1±5.7 years.
During the same period, 56 healthy volunteers were also enrolled from the Centre Hospital of Weihai as the control group. The control group included 29 men and 27 women, with an age range of 34–62 years and a mean age of 45.6±7.2 years. No significant differences in basic clinical data were found between the two groups, including age, gender, smoking and drinking habits, and body mass index (BMI). About 10 ml of blood was extracted from the antecubital vein of each participant on the day of admission. This study was approved by the Ethics Committee of Centre Hospital of Weihai before the patient enrolment began. All patients and healthy controls signed an informed consent to participate in the study.
Enzyme-linked immunosorbent assay (ELISA) for transforming growth factor-β1 (TGF-β1)
Serum levels of transforming growth factor-β1 TGF-β1 were measured using the human TGF-β1 Quantikine ELISA Kit (DB100B) (R&D Systems, Minneapolis MN, USA). All procedures were performed out according to the manufacturer’s instructions. Serum levels of TGF-β1 were normalized to ng/ml.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA extraction was performed using a TRIzol® reagent kit (Thermo Fisher Scientific Inc., Waltham MA, USA). SuperScript III reverse transcriptase kit (Thermo Fisher Scientific Inc., Waltham MA, USA) was used to synthesize cDNA with total RNA as the template according to following thermal conditions: 55°C for 30 min and 75°C for 15 min. SYBR® Green Real-Time PCR Master Mix (Thermo Fisher Scientific Inc., Waltham MA, USA) was used to prepare the PCR reaction system. Reaction conditions were 95°C for 1 min 20s, followed by 40 cycles of 95°C for 30s and 59°C for 25s.
Primers used in the PCR reactions were:
GASL1: 5′-CTGAGGCCAAAGTTTCCAAC-3′ (forward) and
GASL1: 5′-CAGCCTGACTTTCCCT CTTCT-3′(reverse).
GAPDH: 5′-CCCACTCCTCCACCTTTGAC-3′ (forward) and
GAPDH: 5′-ATGAGGTCCACCACCCTGTT-3′ (reverse). Data normalization was performed using the 2−ΔΔCt method.
Cell culture and transfection of human vascular smooth muscle cells (VSMCs)
Human vascular smooth muscle cells (VSMCs) were purchased from Clonetics (San Diego, CA, USA). Medium 231 (containing smooth muscle growth supplements) was used to culture cells in an incubator at 37°C in an atmosphere containing 5% CO2. Full GASL1 cDNA in an EcoRI-EcoRI fragment was inserted into the pIRSE2 vector (Clontech, Palo Alto, CA, USA). Lipofectamine 2000 (11668-019) (Invitrogen, Carlsbad, CA, USA) was used for transfection. Control cells were without transfection. Negative control cells were cells transfected with the empty pIRSE2 vector.
Cell counting kit-8 (CCK-8) assay of human VSMCs
Cultured VSMCs were collected after transfection. An overexpression rate >200% compared with control cells was achieved through the detection of lncRNA GASL1 expression by qRT-PCR. Cell suspensions (4×104 cells/1 ml) were prepared and each well of a 96-well plate had 0.1 ml of cell suspension added. Cells were cultured under normal conditions and CCK-8 solution (10 μl) was added 24, 48, 72 and 96 h after the initiation of cell culture. Cell culture was performed for a further 4 h. The optical density (OD) values at 450 nm were then measured using a Fisherbrand™ AccuSkan™ GO UV/Vis Microplate Spectrophotometer (Thermo Fisher Scientific Inc., Waltham MA, USA).
Western blot
Cells were collected after transfection. Overexpression rate above 200% compared with control cells was achieved through the detection of lncRNA GASL1 expression by qRT-PCR. Total RNA extractions from in vitro cultured cells were performed using RIPA buffer (Thermo Fisher Scientific Inc., Waltham MA, USA). After measurement of protein concentrations by BCA assay, protein samples were denatured at 95°C for 5 min, followed by 12% SDS-PAGE gel electrophoresis with 22 μg of protein in each well. Gel transfer was performed to VDF membranes (Thermo Fisher Scientific Inc., Waltham MA, USA). Membranes were incubated with 5% dried skimmed milk powder for 2h at room temperature. Then, the membranes were cultured with rabbit anti-human TGF-β1 primary antibody (1: 2000) (ab92486) (Abcam, Cambridge, MA, USA) and rabbit anti-human GAPDH primary antibody (1: 2000) (ab181602) (Abcam, Cambridge, MA, USA) overnight at 4°C. The membranes were further incubated with goat anti-rabbit IgG-horseradish peroxidase (HRP)-conjugated secondary antibody (1: 1,000) (MBS435036) (MyBioSource, San Diego, CA, USA) for 2 h at 25°C. Signals were developed by Pierce enhanced chemiluminescence (ECL) Western blotting substrate (Thermo Fisher Scientific Inc., Waltham MA, USA). Data normalization was performed using Image J 1.48 software National Institutes of Health (NIH) (Bethesda, MD, USA).
Statistical analysis
Data were analyzed using GraphPad Prism version 6 software (GraphPad, LaJolla, CA, USA). Data were expressed as the mean ± standard deviation (SD). An unpaired t-test and one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test were used for comparisons between two groups and among multiple groups, respectively. The Pearson correlation coefficient was used for correlation analysis. Receiver operating characteristic (ROC) curve and area under the curve (AUC) was performed to evaluate the diagnostic values of serum levels of lncRNA GASL1 and TGF-β1 for intracranial aneurysm. A p-value of <0.05 was used as the cutoff value for statistical significance.
Results
The novel long noncoding RNA (lncRNA) GASL1 was significantly downregulated and transforming growth factor-β1 (TGF-β1) was significantly upregulated in patients with an intracranial aneurysm compared with healthy controls
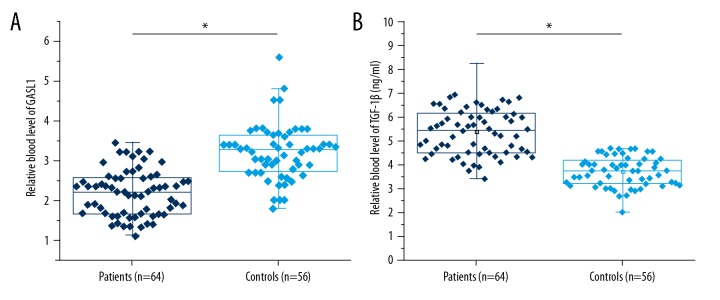
The results showed that when the serum measurements from patients with an intracranial aneurysm were compared with healthy controls, the novel long noncoding RNA (lncRNA) GASL1 was significantly downregulated and transforming growth factor-β1 (TGF-β1) was significantly upregulated in patients with an intracranial aneurysm compared with healthy controls (p<0.05) (Figure 1). These data indicated the potential role of the lncRNA GASL1 and TGF-β1 in intracranial aneurysm.
Figure 1.
The novel long noncoding RNA (lncRNA) GASL1 was significantly downregulated, and transforming growth factor-β1 (TGF-β1) was significantly upregulated, in patients with intracranial aneurysm compared with healthy controls. This figure shows the comparison of serum long noncoding RNA (lncRNA) GASL1 (A) and transforming growth factor-β1 (TGF-β1) (B) between patients with intracranial aneurysm and healthy controls. * p<0.05.
Diagnostic values of serum levels of lncRNA GASL1 and TGF-β1 for intracranial aneurysm
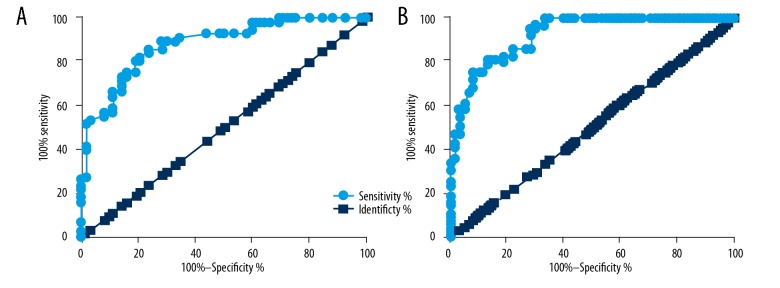
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic values of serum lncRNA GASL1 and TGF-β1 levels for intracranial aneurysm. ROC curve analysis showed that for serum lncRNA GASL1, the area under the curve (AUC) was 0.8820 (standard error: 0.03011; 95% CI, 0.8229–0.9410), for serum TGF-β1, the AUC was 0.9420 (standard error: 0.02246; 95% CI, 0.8799–0.9680) (Figure 2). Therefore, the altered expression of lncRNA GASL1 and TGF-β1 distinguished between patients with intracranial aneurysm and healthy controls in this study.
Figure 2.
The diagnostic values of serum levels of long noncoding RNA (lncRNA) GASL1 and transforming growth factor-β1 (TGF-β1) for intracranial aneurysm. This figure shows the diagnostic values of serum levels of (A) long noncoding RNA (lncRNA), GASL1, and (B) transforming growth factor-β1 (TGF-β1) for intracranial aneurysm.
Serum levels of lncRNA GASL1 and TGF-β1 were negatively correlated in intracranial aneurysm patients but not in healthy controls
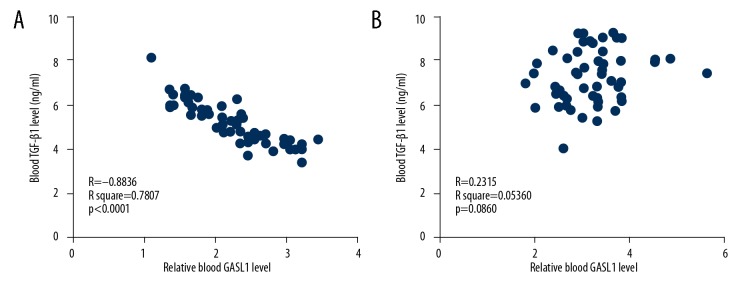
Pearson correlation coefficient was used for correlation analysis of serum levels of lncRNA GASL1 and TGF-β1. Serum levels of lncRNA GASL1 and TGF-β1 were negatively correlated with the presence of intracranial aneurysm (Figure 3A), while no significant correlations between serum levels of lncRNA GASL1 and TGF-β1 were found in healthy controls (Figure 3B). Therefore, lncRNA GASL1 and TGF-β1 may interact in patients with intracranial aneurysm patients but not in healthy controls.
Figure 3.
Serum levels of long noncoding RNA (lncRNA) GASL1 and transforming growth factor-β1 (TGF-β1) were negatively correlated in intracranial aneurysm patients but not in healthy controls. This figure shows the correlation between long noncoding RNA (lncRNA), GASL1, and transforming growth factor-β1 (TGF-β1) in intracranial aneurysm patients (A) and healthy controls (B).
lncRNA GASL1 as an upstream negative regulator of TGF-β1
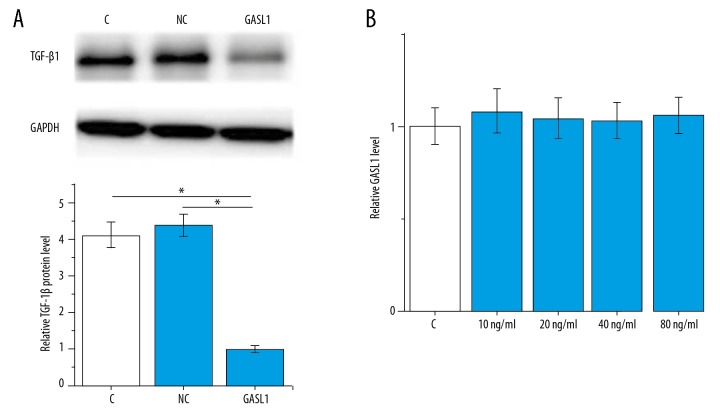
Correlation analysis indicated a potential interaction between lncRNA GASL1 and TGF-β1. To further investigate this interaction, lncRNA GASL1 overexpression in human vascular smooth muscle cells (VSMCs) were constructed and the effects on TGF-β1 were explored by Western blot. As shown in Figure 4A, lncRNA GASL1 overexpression significantly downregulated the expression of TGF-β1 in VSMCs (p<0.05). In contrast, exogenous TGF-β1 treatment at concentrations of 10, 20, 40 and 80 ng/ml showed no significant effects on lncRNA GASL1 expression (p>0.05) (Figure 4B). Therefore, lncRNA GASL1 is likely to be an upstream negative regulator of TGF-β1.
Figure 4.
Long noncoding RNA (lncRNA) GASL1 as a possible upstream negative regulator of transforming growth factor-β1 (TGF-β1). This figure shows the effects of long noncoding RNA (lncRNA), GASL1 overexpression on transforming growth factor-β1 (TGF-β1) expression (A) and exogenous TGF-β1 treatment on lncRNA GASL1 expression (B). p<0.05; C – control cells without transfection; NC – negative control cells transfected with empty pIRSE2 vector; GASL1 – cells with GASL1 overexpression.
lncRNA GASL1 overexpression promoted proliferation of VSMCs
Loss of VSMCs contributes to the pathogenesis of intracranial aneurysm [6]. In this study, proliferation of VSMCs following lncRNA GASL1 overexpression was detected by the cell counting kit-8 (CCK-8) assay. As shown in Figure 5, the proliferation of VSMCs was significantly increased following lncRNA GASL1 overexpression (p<0.05). Also, exogenous TGF-β1 treatment at 10 ng/ml significantly inhibited the proliferation of VSMCs (p<0.05) and significantly reduced the enhancing effects of lncRNA GASL1 overexpression on cell proliferation (p<0.05). Therefore, lncRNA GASL1 overexpression may promote proliferation of VSMCs by inhibiting TGF-β1.
Figure 5.
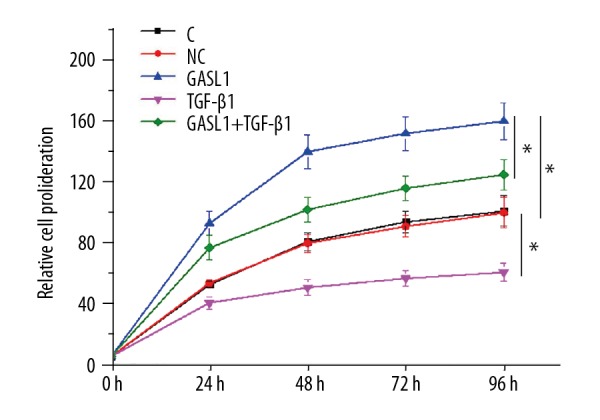
Long noncoding RNA (lncRNA) GASL1 overexpression promoted proliferation of human vascular smooth muscle cells (VSMCs) in vitro. p<0.05; C – control cells without transfection; NC – negative control cells transfected with empty pIRSE2 vector; GASL1 – cells with GASL1 overexpression.
Discussion
The novel long noncoding RNA (lncRNA), GASL1, has previously been shown to have functionality in human liver cancer [10]. The findings of the present study showed that downregulation of lncRNA GASL1 might have a role in the pathogenesis of intracranial aneurysm through its effects on vascular smooth muscle cells (VSMCs). The present study showed that the actions of lncRNA GASL1 in intracranial aneurysm might be achieved through the interactions with TGF-β1 and its regulatory roles on the proliferation of VSMCs.
A recent study reported 1,518 differentially expressed lncRNAs between intracranial aneurysm and healthy people [11], indicating the involvement of lncRNAs in the pathogenesis of this disease. However, previous studies on the expression pattern and functionality of a specific lncRNA in intracranial aneurysm are rare. VSMCs are critical for the integrity of vascular wall and loss of VSMCs is a known contributor to the progression of intracranial aneurysm [6]. The lncRNA HIF1A-AS1 has been shown to be involved in regulating the proliferation and apoptosis of VSMCs in patients with thoracic aortic aneurysms [12]. In another study, the regulatory role of lncRNA-p21 on cell proliferation, apoptosis, and atherosis of VSMCs has also been demonstrated [13]. However, expression patterns and functionalities of these lncRNAs in patients with intracranial aneurysm remain unknown. In our laboratory, preliminary microarray data showed that lncRNA GASL1 was a downregulated in patients with intracranial aneurysm when compared with healthy controls (unpublished data). Therefore, this study was undertaken to investigate the expression of the lncRNA, GASL1, in patients with intracranial aneurysms and its role in the regulation of VSMC proliferation by TGF-β1.
The role of TGF-β in the regulation of proliferation of VSMCs is controversial. It has been reported that increased concentrations of TGF-β were closely correlated with the inhibition of proliferation of VSMCs [14,15]. However, in another study, the interactions between TGF-β/Smad3 and Wnt/β-catenin pathways were shown to promote the proliferation of VSMCs [8]. In the present study, significantly upregulated serum levels of TGF-β1 were found in patients with intracranial aneurysm. Also in vitro cell experiments showed that treatment with exogenous TGF-β1 inhibited the proliferation of VSMCs. The results of the present study and data from previously published studies have shown the complexity of the regulatory role of TGF-β1 on the proliferation of VSMCs.
TGF-β1 participates in the pathogenesis of multiple human diseases [16], and the functionality of TGF-β1 signaling under certain conditions is achieved through the interactions with several different lncRNAs [17–19]. In this study, a significantly negative correlation was found between serum TGF-β1 levels and lncRNA GASL1 levels in patients with intracranial aneurysm. The in vitro cell experiments showed that lncRNA GASL1 showed features of an upstream inhibitor of TGF-β1 in the regulation of proliferation of VSMCs, as the proliferation of VSMCs increased following lncRNA GASL1 overexpression. However, a recent study showed that lncRNA GASL1 was acting as a tumor suppressor in liver cancer, and overexpression of lncRNA GASL1 inhibited cancer cell proliferation by restricting the activity of the E2F1 transcription factor [10]. Therefore, lncRNA GASL1 may play opposite roles in the proliferation of different types of human cells.
The regulatory role of lncRNA GASL1 on the expression of TGF-β1 in the human body appears to be disease-specific due to the lack of significant correlation between serum levels of TGF-β1 and lncRNA GASL1 in healthy controls. Further controlled clinical studies are required to confirm the diagnostic potential of measuring serum lncRNA GASL1 levels and to determine whether lncRNA GASL1 may serve as a potential therapeutic target for the treatment of intracranial aneurysm. However, data from the present preliminary study demonstrated the presence of sequential GASL1-TGF-β1 signaling in the proliferation of VSMCs. Currently, there is no supporting data to confirm whether this interaction between lncRNA GASL1 and TGF-β1 is direct or indirect, and future studies are required to explore this interaction.
Conclusions
The novel long noncoding RNA (lncRNA), GASL1, was downregulated in patients with intracranial aneurysm. The effects of lncRNA GASL1 overexpression on the development of intracranial aneurysm might be mediated by downregulation of the expression of transforming growth factor-β1 (TGF-β1) and by promoting the proliferation of vascular smooth muscle cells (VSMCs).
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Cebral JR, Duan X, Chung BJ, et al. Wall mechanical properties and hemodynamics of unruptured intracranial aneurysms. Am J Neuroradiol. 2015;36(9):1695–703. doi: 10.3174/ajnr.A4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JR, Thompson WL, Alkattan AK, et al. Three-dimensional printing of anatomically accurate, patient specific intracranial aneurysm models. J Neurointerv Surg. 2016;8(5):517–20. doi: 10.1136/neurintsurg-2015-011686. [DOI] [PubMed] [Google Scholar]
- 3.Vlak MH, Algra A, Brandenburg R, Rinkl GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–36. doi: 10.1016/S1474-4422(11)70109-0. [DOI] [PubMed] [Google Scholar]
- 4.Brown RD, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. 2014;13(4):393–404. doi: 10.1016/S1474-4422(14)70015-8. [DOI] [PubMed] [Google Scholar]
- 5.Korja M, Kaprio J. Controversies in epidemiology of intracranial aneurysms and SAH. Nat Rev Neurol. 2016;12(1):50–55. doi: 10.1038/nrneurol.2015.228. [DOI] [PubMed] [Google Scholar]
- 6.Guo F, Li Z, Song L, et al. Increased apoptosis and cysteinyl aspartate specific protease-3 gene expression in human intracranial aneurysm. J Clin Neurosci. 2007;14(6):550–55. doi: 10.1016/j.jocn.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 7.Liu D, Cui W, Liu B, et al. Atorvastatin protects vascular smooth muscle cells from TGF-β1-stimulated calcification by inducing autophagy via suppression of the β-catenin pathway. Cell Physiol Biochem. 2014;33(1):129–41. doi: 10.1159/000356656. [DOI] [PubMed] [Google Scholar]
- 8.DiRenzo DM, Chaudhary MA, Shi X, et al. A crosstalk between TGF-β/Smad3 and Wnt/β-catenin pathways promotes vascular smooth muscle cell proliferation. Cell Signal. 2016;28(5):498–505. doi: 10.1016/j.cellsig.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang JS, Eun SY, Ham SA, et al. PPARδ modulates oxLDL-induced apoptosis of vascular smooth muscle cells through a TGF-β/FAK signaling axis. Int J Biochem Cell Biol. 2015;62:54–61. doi: 10.1016/j.biocel.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 10.Gasri-Plotnitsky L, Ovadia A, Shamalov K, et al. A novel lncRNA, GASL1, inhibits cell proliferation and restricts E2F1 activity. Oncotarget. 2017;8(14):23775–86. doi: 10.18632/oncotarget.15864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H, Yue H, Hao Y, et al. Expression profile of long noncoding RNAs in human cerebral aneurysms: A microarray analysis. J Neurosurg. 2016;127(5):1055–62. doi: 10.3171/2016.9.JNS16839. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Zhang X, Yuan Y, et al. BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. Eur J Cardiothorac Surg. 2014;47(3):439–46. doi: 10.1093/ejcts/ezu215. [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Cai J, Han Y, et al. LncRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis and atherosclerosis by enhancing p53 activity. Circulation. 2014;130(17):1452–65. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moses HL, Yang EY, Pietenpol JA. TGF-β stimulation and inhibition of cell proliferation: New mechanistic insights. Cell. 1990;63(2):245–47. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- 15.Sakakibara K, Kubota K, Worku B, et al. TGF-beta inhibits vascular smooth muscle cell proliferation through downregulation of cyclin A. J Surg Res. 2003;114(2):283. [Google Scholar]
- 16.Sheng J, Chen W, Zhu HJ. The immune suppressive function of transforming growth factor-β (TGF-β) in human diseases. Growth Factors. 2015;33(2):92–101. doi: 10.3109/08977194.2015.1010645. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Dong M, Fan D, et al. LncRNA ANCR down-regulation promotes TGF-β-induced EMT and metastasis in breast cancer. Oncotarget. 2017;8(40):67329–43. doi: 10.18632/oncotarget.18622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Wan L, Liu Z, et al. Long non-coding RNA XIST promotes TGF-β-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018;418:185–95. doi: 10.1016/j.canlet.2018.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Kurashige J, Nambara S, et al. A long non-coding RNA activated by transforming growth factor-β is an independent prognostic marker of gastric cancer. Ann Surg Oncol. 2015;22(3):915–22. doi: 10.1245/s10434-015-4554-8. [DOI] [PubMed] [Google Scholar]