Abstract
Background
Recent studies have shown that Escherichia coli induced digestive tract diseases may be related to outer membrane vesicles (OMVs) induced intestinal double-strand breaks (DSBs) in intestinal epithelial cells. This study aimed to compare the impact of OMVs forces on DSBs in intestinal epithelial Caco-2 cells, and provide a new treatment for digestive diseases caused by E. coli.
Material/Methods
E.coli OMVs were prepared and co-cultured with Caco-2 cells. The uptake of OMVs by Caco-2 cells was observed by confocal microscopy. The γ-H2AX protein was detected by western-blots. The DSBs caused by OMVs was detected by single cell gel electrophoresis.
Results
The particle size analyzer showed that the average diameters of OMVs centrifuged at 20 000×g and 50 000×g were 217.5±7.29 nm and 186.3±6.59 nm (P<0.05), respectively. Transmission electron microscopy of the OMVs revealed a lipid bilayer structure with a variety of different sizes. Confocal fluorescence microscopy revealed that OMVs almost completely entered Caco-2 cells after 24 hours. The ratio of γ-H2AX protein band gray value normalized data in the OMVs centrifuged at 20 000×g and 50 000×g, and the control group (without OMVs) were 2.23±0.18, 1.58±0.20, 1±0.30 (P<0.05), respectively, while DNA levels of the comet tail (TailDNA%, TDNA%) were 72.21±14.61%, 23.11±4.98%, and 1.02±1.41% (P<0.05), respectively. The corresponding DNA damage was categorized as high (grade 3), moderate (grade 2), and no damage (grade 0).
Conclusions
Different sizes of OMVs induced different degrees of DNA damage in intestinal epithelial Caco-2 cells.
MeSH Keywords: DNA Damage, E. coli OMV, Intestinal Epithelial Caco-2 Cells
Background
Outer membrane vesicles (OMVs) are characterized by a phospholipid bilayer membrane structure and are released from the outer membrane of bacterial cell walls [1,2]. Almost all bacterial cells secrete OMVs. Bacteria release OMVs by disrupting a connection between the outer membrane and peptidoglycan, inducing local membrane curvature, and changing specific protein levels [3–5]. OMVs from different sources have different functions, including regulating host immune response [6], performing vaccine function [7–9], transporting biomolecules [10,11], protecting bacterial cells [12,13], assisting biofilm formation [14,15], and responding to physical and chemical stresses [16].
Escherichia coli is closely associated with a number of digestive diseases and is harmful to human health if intestinal flora becomes disordered or unbalanced. E. coli can adhere to intestinal epithelial cells and then release substances that have negative impact on digestive tract, including diarrhea, gastrointestinal discomfort, intestinal bleeding, and intestinal adhesions [17–22], which in turn may cause inflammatory bowel disease, irritable bowel syndrome, or even intestinal cancer.
Recent studies have shown that one possible cause of E. coli-induced digestive tract diseases may be related to OMV-induced intestinal double-strand breaks (DSBs) in intestinal epithelial cells. E. coli secrete OMVs, which are induced by intestinal epithelial receptor cells to cause DNA DSBs in intestinal epithelial cells [23]. Tyrer et al. found that E. coli OMVs enter intestinal epithelial Caco-2 or HT-29 cells and release virulence factors, such as virulence proteins, heat labile endotoxins (LTs), and enterotoxins which can cause DNA DSBs in intestinal epithelial cells [24] and trigger a corresponding digestive tract disease. DSB induces H2AX phosphorylation in the conserved region of serine 139 at the C-terminus to form γ-H2AX [25–28]. Therefore, detection of γ-H2AX has become the gold standard for DSB detection. A variety of physical, chemical, and biological factors that can induce the formation of γ-H2AX have been identified to date. Ivashkevich et al. [29] used a γ-H2AX kit to detect DNA damage, while Janaki et al. [30] used single cell gel electrophoresis (comet assay) to detect DNA DSBs.
In this study, E. coli OMVs were prepared using intestinal epithelial Caco-2 cells and 2 centrifugal forces. The sizes of the 2 OMVs were compared to determine whether they caused the same damage to the Caco-2 cells. Using this OMV data, the pathogenicity of E. coli can be explained, which provides a new treatment for digestive diseases caused by E. coli.
Material and Methods
Cell suspension
Intestinal epithelial Caco-2 cells were purchased from the cell bank of the Chinese Academy of Sciences, and E. coli (ATCC 25922) was acquired from Qingdao Rishui Biotechnology Co., Ltd. Caco-2 cells were resuspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% FBS, penicillin (100 μg/mL), and streptomycin (100 μg/mL) and were subsequently inoculated in 25-cm2 culture dishes and incubated at 37°C and 5% CO2. The cells were allowed to grow to an approximate confluence of 80% before passaging.
Extraction of OMVs by ultracentrifugation
The LB broth medium was autoclaved for 20 minutes (120°C, 100 Kpa) and then cooled to room temperature. A single colony on the E. coli culture plate was inoculated and cultured at 37°C and 180 rpm for 10 days. The supernatant was collected by centrifugation at 1500×g for 15 minutes at 4°C using benchtop centrifuge 5810R (Eppendorf, USA). The supernatant was then filtered through a 0.45-μm filter (Merck Millipore), followed by a 0.22-μm filter (Merck Millipore), to remove residual bacteria. The OMVs were obtained by centrifugation at 20 000×g and 50 000×g for 1.5 hours at 4°C in an ultra-speed refrigerated centrifuge (HITACHI 55P-72, Hitachi, Japan), washed, resuspended in 1 mL of HEPES buffer, and finally stored at 4°C for future use.
Particle size analysis
Samples of OMVs (15 μg) obtained using the 2 different centrifugal forces were each dissolved in 1 mL of HEPES buffer and vortexed for 1 minute to allow OMVs to distribute evenly. The size distribution of E.coli OMVs was then analyzed using the Malvern particle size analyzer (Zeta SIZER 3000HS, Malvern, UK).
Transmission electron microscopy
The OMVs obtained by centrifugation at 20 000×g and 50 000×g for 1.5 hours were gently mixed with 1 mL of 4% glutaraldehyde, fixed for 2 hours (4°C), then washed 3 times. The OMVs were then fixed with 1% osmium tetroxide for 2 hours. OMVs were dehydrated using conventional ethanol and acetone gradient, followed by impregnation, embedding, and polymerization with epoxy resin to prepare semi-thin sections (0.5 μm) for subsequent imaging using a light microscope. Ultra-thin sample sections (60 nm) were then prepared and stained using uranium acetate and lead citrate for electron microscopy observation.
Observation of OMV uptake by Caco-2 cells using confocal microscopy
Dio dye (6 μL, 10 mg/mL) was mixed with 20 μg of the OMV suspension and stained in a 37°C incubator for 30 minutes. This was followed by addition of phosphate-buffered saline (PBS) and washing at 50 000×g for 90 minutes. Dio-traced OMVs and Caco-2 cells were co-cultured for 0 hours, 12 hours, and 24 hours and then fixed with 4% paraformaldehyde. The cells were then stained with 6 μL of DAPI dye (10 mg/mL) for 10 minutes at room temperature. One drop of anti-fluorescent culturing agent and appropriate amount of PBS were then added for observation of OMV uptake by the Caco-2 cells using confocal fluorescence microscope TCS-SP2 (Leica, Germany).
Western blot detection of γ-H2AX protein expression
Caco-2 cell were plated into 6-well plates, incubated with DMEM containing 10% FBS, penicillin (100 μg/mL), and streptomycin (100 μg/mL) for 12 hours, and treated with 20 μg OMVs from the 20 000×g group, the 50 000×g group, and the control group for 24 hours, respectively. The total protein from each group was extracted and the loading buffer was boiled for 90 seconds to denature the protein. A 20-μL protein sample was obtained for SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis). The protein was then fixed under a constant electric field of 250 mA followed by transfer to a nitrocellulose membrane. Skim milk powder (5%) was used for blocking overnight at 4°C, to which the corresponding primary antibody was added to incubate the proteins at room temperature overnight. The membrane was washed 3 times with Tris-buffered saline with Tween 20 (TBST) buffer for 10 minutes each time. Horseradish peroxidase-labeled secondary antibody was then added and incubated for 1 hour at room temperature and washed three times with TBST buffer for 10 minutes each time. The experiment was repeated 3 times and results were analyzed after color development by ECL.
Single cell gel electrophoresis
A total of 1×105 Caco-2 cells were inoculated in 25-cm2 culture flasks and 20-μg OMV samples were added to the 20 000×g OMV group and the 50 000×g OMV group after adhering for 4 hours. OMVs were not added to the control group. The 3 groups were cultured for 24 hours. The cells were digested and washed once with PBS and the concentration of the cells was adjusted to 103–104 cells/mL after centrifugation. Three layers of rubber sheets were prepared by sequentially using 150 μL of 1% normal-melting agarose, 10 μL of PBS containing 1000 cells, 100 μL of 0.8% low-melting agarose, and 100 μL of 0.5% low-melting agarose. The coverslips were removed, and the slides were immersed in fresh cell lysate and incubated for at least 1 hour at 4°C. The slides were then rinsed twice with PBS, placed in a horizontal electrophoresis tank, and immersed in alkaline running buffer for 20 minutes. Electrophoresis was carried out for 20 minutes at 25 V and 300 mA. The slides were then neutralized with Tris-HCl (pH 7.5) for 15 minutes. Then, 50 μL of the 30-μg/mL ethidium bromide solution was added dropwise to each slide, which was subsequently covered with a cover glass, and stained for 20 minutes in the dark. The electropherogram was observed and photographed using a fluorescence microscope and the experiment was repeated 3 times. The Comet Assay Software Pect (CASP 1.2.3 beta 1) image analysis software was used to analyze each comet image, where X±SE was used to represent the full length (CL), tail length (TL), tail moment of the comet (TM), and olive tail (OTM) for further calculating the tailing factor. According to the tail DNA concentration (TailDNA%) in tail cells, the degree of DNA damage was divided into 5 levels: grade 0, no damage (normal cells) and cell damage rate <5%; grade 1, low DNA damage with cell damage between 5% and 20%; grade 2, moderate damage with cell damage between 20% and 40%; grade 3, high damage with cell damage between 40% and 90%; and grade 4, severe damage with cell damage >95%.
Statistical analysis
The experimental data were processed by SPSS10.0 and t-test analysis was performed between independent samples. The results were expressed as mean ± standard deviation. P<0.05 indicated statistical significance.
Results
Impact of centrifugal force on OMV size
E. coli OMVs collected using 2 centrifugal forces were detected by Malvern particle size analyzer. The average particle sizes for the 20 000×g group, the 50 000×g OMV groups were 217.5±7.29 nm and 186.3±6.59 nm, respectively (Figure 1A). The 2 groups were significantly different from each other (P<0.05), as shown in the corresponding histograms (Figure 1B, Table 1). Transmission electron microscopy clearly showed E. coli OMVs’ lipid bilayer with spherical structures of different sizes and diameters <1 μm (Figure 1C).
Figure 1.
Escherichia coli outer membrane vesicles (OMV) size and morphology. (A) OMV obtained by 20 000×g and 50 000×g centrifugation (n=3). (B) Size comparison between 20 000×g and 50 000×g OMV groups (n=3, per group). (C) 20 000×g and 50 000×g OMV group morphology.
Table 1.
OMV size analysis.
| Centrifugal force/g | Mean/nm | SD |
|---|---|---|
| 20 000×g | 217.5 | 7.29 |
| 50 000×g | 186.3 | 6.59 |
SD – standard deviation.
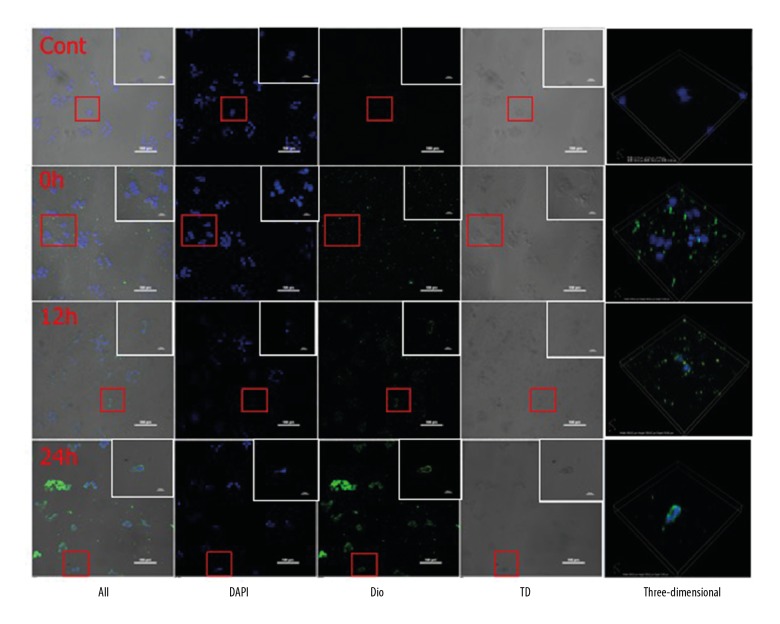
Confocal fluorescence observation of OMV in Caco-2 cells
Confocal fluorescence microscopy showed that the control group without OMVs had only blue nuclei. When Dio-OMVs were added to the Caco-2 cells, OMVs were initially scattered around the cells and located far from the nuclei. After 12 hours of co-culture, green fluorescence of Dio-OMVs was observed around nuclei, indicating that some Dio-OMVs entered the Caco-2 cells. After 24 hours of co-culture, the uptake of Dio-OMVs by Caco-2 cells was significant (Figure 2).
Figure 2.
Confocal microscopy imaging of Dio-labeled outer membrane vesicles (OMVs) taken up by Caco-2 cells.
Impact of different OMV sizes on the expression of γ-H2AX protein in Caco-2 cells
E. coli OMVs collected using 2 different centrifugal forces were co-cultured with Caco-2 cells for 24 hours. Western blot was used to analyze the ratio of γ-H2AX protein band gray value normalized data in Caco-2 cells and the results showed that expression of γ-H2AX protein in the 20 000×g group, the 50 000×g group, and the control groups were 2.23±0.18, 1.58±0.20, and 1±0.30 (P<0.05), respectively (Figure 3A). The expression of γ-H2AX protein statistical analysis of the relative expression levels of γ-H2AX protein revealed significant differences among the 3 groups (P<0.05) (Figure 3B).
Figure 3.
γ-H2AX protein levels. (A) Expression of γ-H2AX protein. (B) Expression of γ-H2AX protein in Escherichia coli outer membrane vesicles (OMVs) obtained with different centrifugal forces.
Impact of OMV size on DNA damage in Caco-2 cells
Caco-2 cells were co-cultured with 20 000×g OMVs and 50 000×g OMVs for 24 hours. Fluorescence imaging was performed using single-cell gel electrophoresis. The results showed that the tail of 20 000×g OMV group was longer than that of and 50 000×g OMV group, while the control group exhibited almost no tail (Figure 4A). The Comet Assay Software Pect (CASP 1.2.3 beta 1) (Table 2) was used to perform statistical analysis of TailDNA% for the 3 groups (Figure 4B). The results showed that TailDNA% of the 20 000×g OMVs group, the 50 000×g OMVs group, and the control groups were 72.21±14.61%, 23.11±4.98%, and 1.02±1.41%, respectively. Significant differences were observed among the 3 groups (P<0.05). The degree of DNA damage for the 3 groups were high (grade 3), moderate (grade 2), and no damage (grade 0), respectively.
Figure 4.
Impact of outer membrane vesicle (OMV) size on DNA damage in Caco-2 cells. (A) Single-cell gel electrophoresis; (B) TailDNA% in Caco-2 cells for Escherichia coli OMVs obtained with different centrifugal forces.
Table 2.
Software analysis of tail length (TL), comet full length (CL), tail moment (TM), and olive tail length (OTM) to calculate tailing DNA rate.
| Name | Group | Tail DNA% | Tail length | Comet length | Tail moment | Olive tail moment |
|---|---|---|---|---|---|---|
| 20 000×g | 1 | 67.1583 | 110 | 197 | 0.00663 | 43.9357 |
| 2 | 73.1448 | 118 | 169 | 0.0796036 | 52.8612 | |
| 3 | 86.3233 | 124 | 171 | 0.00576123 | 61.7247 | |
| 50 000×g | 1 | 19.4544 | 53 | 126 | 10.3108 | 8.58909 |
| 2 | 28.7827 | 65 | 140 | 18.7 | 14.0588 | |
| 3 | 21.0904 | 56 | 139 | 11.8106 | 10.5368 | |
| Control | 1 | 0.999067 | 99.8 | 0.22104 | 73.8742 | 3 |
| 2 | 9.13098 | 97.3465 | 2.65345 | 86.3 | 3 | |
| 3 | 0.903067 | 99.808 | 0.192041 | 107.041 | 3 |
Discussion
This study revealed that OMVs prepared by different centrifugal forces are significantly different from each other. Transmission electron microscopy imaging showed that although the morphology of OMVs was similar between the 20 000×g OMVs group and the 50 000×g OMVs group, the particle size distribution of the 2 OMV groups was different, as detected by particle size analyzer. Furthermore, OMVs of different sizes showed distinct capability for DNA damage in Caco-2 cells, which was likely due to distinct OMV inclusions. Currently, differential centrifugation combined with filtration, ultrafiltration, and ExoQuick kits, among others, is the main method for preparation of vesicles of different sizes [31–34]. Each procedure has its advantages and disadvantages and there are no methods that can generate vesicles of a precise size. Instead, vesicles that fall within a certain size range can be created. Vesicles with larger particle sizes can be obtained by low-speed centrifugal force. The vesicle particle size decreases with increasing centrifugal force. High speed centrifugal force impacts the extraction of small and high-purity vesicles. In summary, OMVs prepared by using distinct centrifugal forces combined with other methods have different properties, thus carrying distinct information and performing distinct biological functions.
Single cell gel electrophoresis (comet assay) is commonly used to detect DNA DSBs. In this study, cell lysis was performed based on the principle of degeneration, which further unwinds DNA. Nuclei are then electrophoresed, and damaged DNA fragments migrate out influenced by the electric field. The undamaged DNA remains in the nuclei and migrates slowly. Eventually, staining with fluorescent DNA dye allows to observe the DNA shape and migration patterns and to analyze DNA damage via comet tails and the percentage of tail DNA. The degree of DNA DSB induced by OMVs can then be determined. Duthie et al. [35] used Caco-2 cells as an in vitro model of human colon cells and employed alkaline single-cell gel electrophoresis to determine DNA damage. Follmann et al. [36] used alkaline gel electrophoresis to determine DNA DSBs in bovine colon cells. In our study, alkaline double-cell gel electrophoresis was used to analyze the DNA DSBs of Caco-2 cells. The results showed that E. coli OMVs induced significant DNA DSBs (DNA tailing) in Caco-2 cells, which was consistent with results reported by Robichová and Anderson et al. [37,38]. Further studies have shown that both types of OMVs can induce DNA DSBs in Caco-2 cells with different degree of DNA damage.
DNA DSB is an important type of DNA damage. It is first signaled by the phosphorylation of serine residue of histone H2AX at the C-terminus near breakpoint, forming γ-H2AX [39–41]. Phosphorylated γ-H2AX can transfer DNA damage signals fast, leading to activation of phosphorylation of downstream molecules and triggering a series of biological cascades and cytological responses. The results of this study showed that E. coli OMVs induced higher levels of γ-H2AX protein in Caco-2 cells. Different levels of OMVs induced Caco-2 cells to express distinct levels of γ-H2AX protein. E. coli OMVs also induced changes in γ-H2AX protein levels expressed in Caco-2 cells, which may be the result of DNA damage in Caco-2 cells.
In conclusion, E. coli OMV-induced DNA damage in intestinal epithelial Caco-2 cells may be one of the causes for E. coli-induced digestive diseases. This study revealed that OMVs centrifuges at 20 000×g were larger than OMVs centrifuged at 50 000×g, and that 20 000×g OMVs more severely damage Caco-2 cell DNA, which may be related to OMV inclusions.
Conclusions
Our study revealed that E. coli could secrete OMVs, and that different sizes of OMVs induced different degrees of DNA damage in intestinal epithelial Caco-2 cells.
Acknowledgements
The authors would like to thank Yang Jieshun and Dou Xiaoyun from the School of Life Sciences at Chongqing Medical University for their support and assistance with transmission electron microscopy and laser confocal microscopy, respectively.
Footnotes
Source of support: This study was supported by the National Natural Science Foundation of the China(No. 31571453), National Major Scientific Instruments and Equipment Development Project (2013YQ03062906), Chongqing Natural Science Foundation (cstc2016jcyjA0098, cstc2017jcyjAX0432)
References
- 1.Livshits MA, Khomyakova E, Evtushenko EG, et al. Corrigendum: Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2016;6:21447. doi: 10.1038/srep21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mcbroom AJ, Johnson AS, Kuehn MJ. Outer membrane vesicle production by Escherichia coli is independent of membrane instability. J Bacteriol. 2006;188(15):5385–92. doi: 10.1128/JB.00498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuehn MJ, Kesty NC. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19(22):2645–55. doi: 10.1101/gad.1299905. [DOI] [PubMed] [Google Scholar]
- 4.Alves NJ, Turner KB, Medintz IL, et al. Emerging therapeutic delivery capabilities and challenges utilizing enzyme/protein packaged bacterial vesicles. Ther Deliv. 2015;6(7):873–87. doi: 10.4155/tde.15.40. [DOI] [PubMed] [Google Scholar]
- 5.Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64(2):e783–89. doi: 10.1146/annurev.micro.091208.073413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez Vidakovics MLA, Jendholm J, Mörgelin M, et al. B cell activation by outer membrane vesicles – a novel virulence mechanism. PLoS Pathogens. 2010;6(1):543–47. doi: 10.1371/journal.ppat.1000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pol LD, Stork M, Ley PVD. Outer membrane vesicles as platform vaccine technology. Biotechnol J. 2015;10(11):1689–706. doi: 10.1002/biot.201400395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim OY, Hong BS, Park KS, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2011;190(8):4092–102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- 9.Jagannadham MV, Chattopadhyay MK. Role of outer membrane vesicles of bacteria. Resonance. 2015;20(8):711–25. [Google Scholar]
- 10.Bomberger JM, Maceachran DP, Coutermarsh BA, et al. Long-distance delivery of bacterial virulence factors by pseudomonas aeruginosa outer membrane vesicles. PLoS Pathogens. 2009;5(4):e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cañas MA, Giménez R, Fábrega MJ, et al. Outer membrane vesicles from the probiotic Escherichia coli Nissle 1917 and the commensal ECOR12 enterintestinal epithelial cells via clathrin-dependent endocytosis and elicit differential effects on DNA damage. PLoS One. 2016;11(8):e0160374. doi: 10.1371/journal.pone.0160374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vella BD, Schertzer JW. Understanding and exploiting bacterial outer membrane vesicles. Pseudomonas. 2015:217–50. [Google Scholar]
- 13.Kulkarni HM, Jagannadham MV. Biogenesis and multifaceted roles of outer membrane vesicles from Gram-negative bacteria. Microbiology. 2014;160(10):2109–21. doi: 10.1099/mic.0.079400-0. [DOI] [PubMed] [Google Scholar]
- 14.Kouokam JC, Sun NW. Outer membrane vesicle-mediated export of a pore-forming cytotoxin from Escherichia coli. Toxin Reviews. 2008;25(1):31–46. [Google Scholar]
- 15.Lee EY, Bang JY, Park GW, et al. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli. Proteomics. 2007;7(17):3143–53. doi: 10.1002/pmic.200700196. [DOI] [PubMed] [Google Scholar]
- 16.Baumgarten T, Sperling S, Seifert J, et al. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-t1e cell surface hydrophobicity and biofilm formation. Appl Environ Microbiol. 2012;78(17):6217–24. doi: 10.1128/AEM.01525-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine MM. Escherichia coli that cause diarrhea: Enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155(3):377–89. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 18.Besser RE, Lett SM, Weber JT, et al. An outbreak of diarrhea and hemolytic uremic syndrome from Escherichia coli O157: H7 in fresh-pressed apple cider. JAMA. 1993;269(17):2217–20. [PubMed] [Google Scholar]
- 19.Roubos-van den Hil PJ, Nout MJ, Beumer RR, et al. Fermented soya bean (tempe) extracts reduce adhesion of enterotoxigenic Escherichia coli to intestinal epithelial cells. J Appl Microbiol. 2010;106(3):1013–21. doi: 10.1111/j.1365-2672.2008.04068.x. [DOI] [PubMed] [Google Scholar]
- 20.Roberts LC. The interaction of adherent invasive Escherichia coli with intestinal epithelial cells, in vitro derived M-cells and macrophages. Acta Pharmacol Sin. 2008;31(3):361–66. [Google Scholar]
- 21.Wang CT, Xie YH, Digestion DO. [Research of correlation between intestinal bacteria and digestive system diseases]. Medical Recapitulate. 2015 [in Chinese] [Google Scholar]
- 22.Song M, Zhou L, Liu X, et al. [The relationship between digestive system diseases complicated with osteoporosis and intestinal flora]. Chinese Journal of Osteoporosis. 2018 [in Chinese] [Google Scholar]
- 23.Tyrer PC, Frizelle FA, Keenan JI. Escherichia coli-derived outer membrane vesicles are genotoxic to human enterocyte-like cells. Infect Agent Cancer. 2014;9(1):2. doi: 10.1186/1750-9378-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murase K, Martin P, Porcheron G, et al. Hlyf produced by extraintestinal pathogenic Escherichia coli is a virulence factor that regulates outer membrane vesicle biogenesis. J Infect Dis. 2016;213(5):856–65. doi: 10.1093/infdis/jiv506. [DOI] [PubMed] [Google Scholar]
- 25.Riballo E, Kühne M, Rief N, et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Molecular Cell. 2004;16(5):715–24. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury D, Keogh MC, Ishii H, et al. γ-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20(5):801–9. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Sayed AE, Igarashi K, Watanabeasaka T, et al. Double strand break repair and γ-H2AX formation in erythrocytes of medaka (Oryzias latipes) after γ-irradiation. Environ Pollut. 2017;224:35–43. doi: 10.1016/j.envpol.2016.11.050. [DOI] [PubMed] [Google Scholar]
- 28.Solier S, Sordet O, Kohn KW, et al. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol. 2009;29(1):68–82. doi: 10.1128/MCB.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ivashkevich A, Redon CE, Nakamura AJ, et al. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2011;327(1–2):123–33. doi: 10.1016/j.canlet.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janaki DV, Nagarani N, Yokesh BM, et al. Genotoxic effects of profenofos on the marine fish, Therapon jarbua. Toxicology Mech Methods. 2012;22(2):111–17. doi: 10.3109/15376516.2011.603393. [DOI] [PubMed] [Google Scholar]
- 31.Lobb RJ, Becker M, Wen SW, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Momen-Heravi F, Balaj L, Alian S, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013;394(10):1253–62. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pienimaeki-Roemer A, Kuhlmann K, et al. Lipidomic and proteomic characterization of platelet extracellular vesicle subfractions from senescent platelets. Transfusion. 2015;55(3):507–21. doi: 10.1111/trf.12874. [DOI] [PubMed] [Google Scholar]
- 34.Alvarez-Erviti L, Seow Y, Yin H, et al. Delivery of siRNA to the brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–45. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 35.Duthie SJ, Dobson VL. Dietary flavonoids protect human colonocyte DNA from oxidative attack in vitro. Eur J Nutr. 1999;38(1):28–34. doi: 10.1007/s003940050043. [DOI] [PubMed] [Google Scholar]
- 36.Follmann W, Birkner S. The use of cultured primary bovine colon epithelial cells as a screening model to detect genotoxic effects of heterocyclic aromatic amines in the comet assay. J Toxicol Environ Health A. 2008;71(13–14):947–53. doi: 10.1080/15287390801988962. [DOI] [PubMed] [Google Scholar]
- 37.Robichová S, Slamenová D. Study of N-nitrosomorpholine-induced DNA strand breaks in Caco-2 cells by the classical and modified comet assay: influence of vitamins E and C. Nutr Cancer. 2001;39(2):267–72. doi: 10.1207/S15327914nc392_17. [DOI] [PubMed] [Google Scholar]
- 38.Anderson D, Hambly RJ, Yu TW, et al. The effect of potassium diazoacetate on human peripheral lymphocytes, human adenocarcinoma colon caco-2 cells, and rat primary colon cells in the comet assay. Teratog Carcinog Mutagen. 2015;19(2):137–46. doi: 10.1002/(sici)1520-6866(1999)19:2<137::aid-tcm6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira RF, Souza DR, Souza AS. Factors that induce DNA damage involving histone H2AX phosphorylation. Radiology. 2015;277(1):307–8. doi: 10.1148/radiol.2015150818. [DOI] [PubMed] [Google Scholar]
- 40.Oh KS, Bustin M, Mazur SJ, et al. UV-induced histone H2AX phosphorylation and DNA damage related proteins accumulate and persist in nucleotide excision repair-deficient XP-B cells. DNA Repair. 2011;10(1):5–15. doi: 10.1016/j.dnarep.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solier S, Sordet O, Kohn KW, et al. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol. 2009;29(1):68–82. doi: 10.1128/MCB.00581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]