Abstract
Background:
Susceptibility to organophosphate (OP) pesticide neurotoxicity may be greatest during the prenatal period; however, previous studies have produced mixed findings concerning in utero OP pesticide exposure and child cognition.
Objectives:
Our objective was to determine whether maternal urinary concentrations of OP pesticide metabolites are inversely associated with child nonverbal IQ at 6 y of age and to examine potential effect measure modification by the PON1 gene.
Methods:
Data came from 708 mother–child pairs participating in the Generation R Study. Maternal urine concentrations of six dialkylphosphates (DAPs), collected at , 18–25, and of gestation, were determined. Child nonverbal IQ was measured at 6 y of age using the Mosaics and Categories subtests from the Snijders-Oomen Nonverbal Intelligence Test–Revised. PON1 was determined in cord blood for 474 infants. Multiple linear regression models were fit to estimate the DAP–IQ associations and PON1 interactions.
Results:
Overall, associations between child nonverbal IQ and maternal DAP concentrations were small and imprecise, and these associations were inconsistent across urine sampling periods. However, for a 10-fold difference in total DAP concentration for the of gestation samples, adjusted child nonverbal IQ was 3.9 points lower (95% CI: , ). Heterogeneity in the DAP–IQ association by PON1 gene allele status was not observed ().
Conclusions:
Consistent evidence of an association between higher maternal urinary DAP concentrations and lower child IQ scores at 6 y of age was not observed. There was some evidence for an inverse relation of child nonverbal IQ and late pregnancy urinary DAPs, but the estimated association was imprecise. https://doi.org/10.1289/EHP3024
Introduction
Organophosphate (OP) pesticides have been used for more than 50 y because they enhance crop yield and degrade rapidly. Some of the active pesticide, however, stays on food crops, and metabolites are often detected in human consumers (Eaton et al. 2008). Most exposure in the general population is from diet (Llop et al. 2017; Sokoloff et al. 2016; van den Dries et al. 2018), though other exposure routes can be important in selected populations (Eskenazi et al. 1999). OP pesticide toxicity at high doses, via inhibition of acetylcholinesterase, has been well described (O’Malley 1997); whether toxicity at lower doses occurs, via other mechanisms (Burke et al. 2017; Terry 2012), is unclear. Because susceptibility to adverse effects on cognition may be greatest during early development, many studies of low-dose exposure have focused on prenatal exposure, and the results have been suggestive in some cases (Bouchard et al. 2011; Engel et al. 2007, 2011; Eskenazi et al. 2007) but inconclusive overall (Engel et al. 2016). This heterogeneity may be explained by underlying genetic factors, specifically the PON1 gene, which may modify the association between organophosphate pesticide exposure and cognition (Engel et al. 2016; Huen et al. 2010). Although the use of OP pesticides has been reduced, recent data show that the levels of metabolites in population biomonitoring studies have been stable for at least the first decade of this millennium (CDC 2009).
Among the dozens of OP pesticides in current use, some yield specific metabolites; most, however, degrade to one or more dialkylphosphates (DAPs), and the measurement of six DAPs in urine is the most-used method of estimating exposure to this class of compounds (Bravo et al. 2002). The concentration of DAPs in urine reflects exposure in the past day or two, and individual exposure varies substantially from day to day, depending on diet (Sokoloff et al. 2016). This intraindividual variability means that estimates of exposure are improved if urine specimens are collected from an individual at more than one point in time. Often, however, epidemiologic studies have had at most two urine specimens per subject, which may have limited their statistical power to detect adverse associations (Perrier et al. 2016).
The present study examines maternal urinary concentrations of OP pesticide metabolites in relation to child nonverbal IQ at 6 y of age, with potential effect measure modification by the PON1 gene. Maternal urinary concentrations of OP pesticide metabolites were measured at three time points during pregnancy in the present study, with the intent to reduce possible exposure misclassification.
Methods
Study Population and Follow-Up
Generation R is a prospective population-based birth cohort designed to identify early environmental and genetic determinants of development throughout life and has been described in detail previously (Kooijman et al. 2016). Briefly, all mothers who resided in the study area in Rotterdam, Netherlands, and had a delivery date between April 2002 and January 2006 were eligible. Mothers were enrolled during pregnancy or in the first months after the birth of their child when newborns visited the routine child health centers. Among the 9,778 mothers who participated in the study, 8,879 (91%) were enrolled during pregnancy (Figure 1). Among the 4,918 women enrolled during pregnancy between February 2004 and January 2006, spot urine specimens during early, middle, and late pregnancy (, 18–25, of gestational age, respectively) were collected at the time of routine ultrasound examinations, which occurred throughout the day. In total, 2,083 women provided a complete set of three urine specimens. Mothers provided written informed consent for themselves and their children at the time of enrollment. The study protocol underwent human subjects review at Erasmus Medical Center, Rotterdam, Netherlands (institutional review board registration no. IRB00001482).
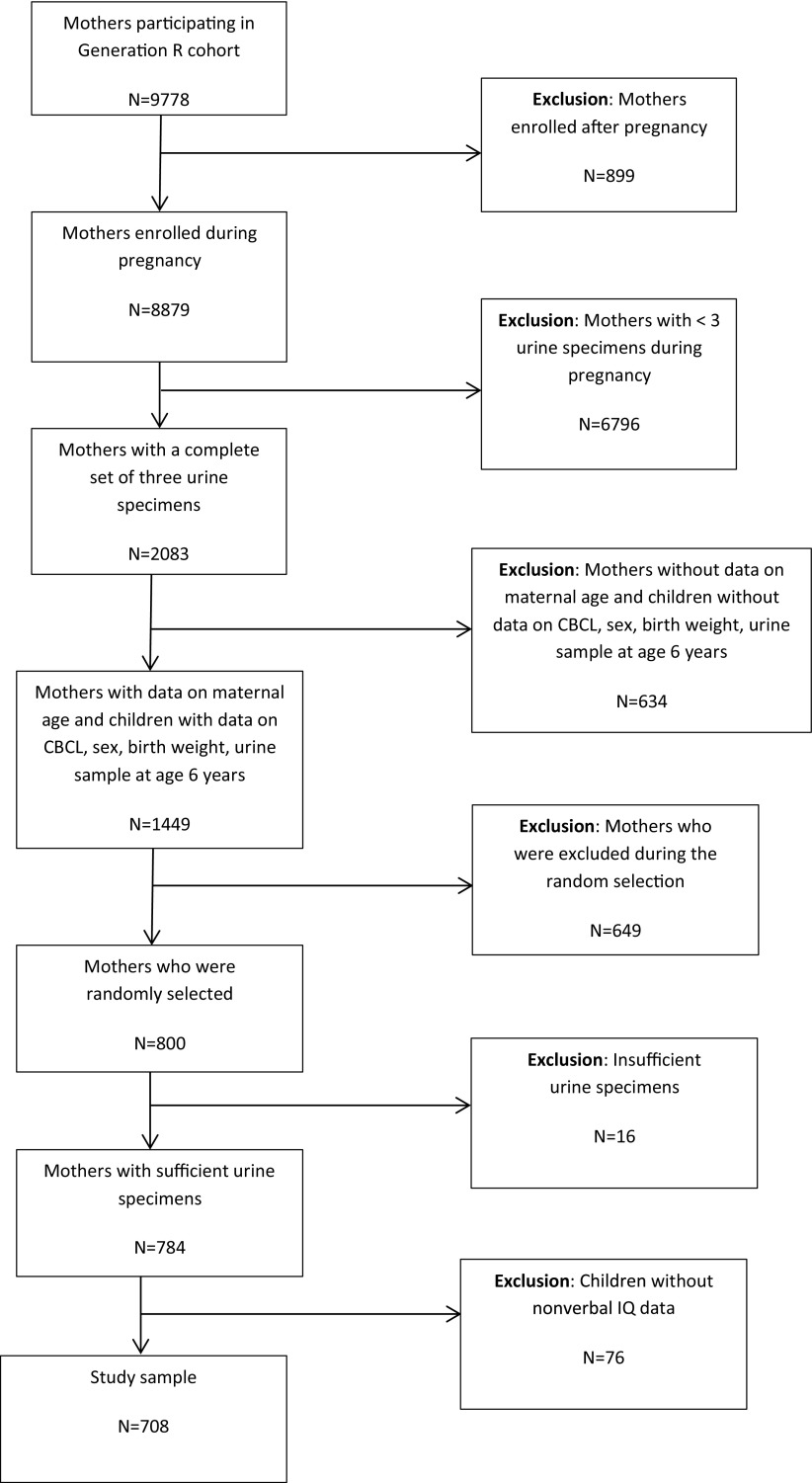
Figure 1.
Study sample selection () from the overall Generation R cohort (). Note: CBCL, Child Behavior Checklist.
When the child was 6 y of age, families were invited to participate in an in-person follow-up to collect cognitive data, additional biospecimens, and sociodemographic and health data. Of the 2,083 mother–child pairs with three pregnancy urine specimens, 1,998 (96%) were followed to 6 y of age. From these 1,998 mother–child pairs with three prenatal urine specimens, women with missing data on maternal age and children with missing data on sex, birth weight, and without Child Behavior Checklist (CBCL) data at 6 y of age were excluded (Figure 1). This resulted in a total of 1,449 mother–child pairs. From these 1,449 pairs, 800 mothers were selected using a random number generator for analyses of DAP metabolites in the maternal urine samples. Given our assumptions, 800 mothers was sufficient to provide 80% power to detect a 2-point decrement in IQ per unit increase in average DAP concentration (calculations not shown). Next, 16 participants were excluded from the lab analyses due to insufficient urine specimens. The final analytic sample included 708 mother–child pairs with exposure and outcome data and who had a sufficient volume of urine for analysis.
Urine Collection and Analysis of DAP Metabolites
Details of maternal and 6-y-old child urine specimen collection have been described elsewhere (Kruithof et al. 2014). All urine samples were collected between 0800 and 2000 hours in polypropylene urine collection containers that were kept for a maximum of 20 h in a cold room (4°C) before being frozen at in portions in polypropylene vials. Measurements of six nonspecific DAP metabolites of OP pesticides were conducted at the Institut National de Santé Publique (INSPQ) in Quebec, Canada, using gas chromatography coupled with tandem mass spectrometry (GC–MS/MS) (Health Canada 2010). Three dimethyl (DM) metabolites [dimethylphosphate (DMP), dimethylthiophosphate (DMTP), and dimethyldithiophosphate (DMDTP)] were determined, as well as three diethyl (DE) metabolites [diethylphosphate (DEP), diethylthiophosphate (DETP), and diethyldithiophosphate (DEDTP)]. The limit of detection (LOD) was for DMP, 0.40 for DMTP, 0.09 for DMDTP, 0.50 for DEP, 0.12 for DETP, and 0.06 for DEDTP. Measured values below the LOD were included in the data analysis. The inter-day precision of the method during this project, expressed as the coefficient of variation percent, varied between 4.2 and 8.8 for DEDTP, 4.1 and 7.2 for DEP, 5.0 and 9.1 for DETP, 5.5 and 7.1 for DMDTP, 5.3 and 8.0 for DMP, and 5.5 and 7.7 for DMTP based on reference materials (clinical check-urine level II 637 E-495 and MRM E-459).
Molar concentrations were used to facilitate comparison of our results with those from other studies, based on the following molecular weights: DMP 126.0, DMTP 142.1, DMDTP 158.2, DEP 154.1, DETP 170.2, and DEDTP . To account for urinary dilution, creatinine concentrations were determined based on the Jaffe reaction (Butler 1975; O’Brien et al. 2016). The limit of detection for creatinine was , and the day-to-day coefficient of variation percent varied between 3.0 and 3.3.
Assessment of Child Nonverbal IQ at 6 y of Age
The children’s nonverbal IQ was assessed by administering the Mosaics and Categories subtests from the Snijders-Oomen Nonverbal Intelligence Test–Revised, a well-validated instrument developed in Netherlands (Tellegen et al. 1998). These two language-independent subtests include items that probe visuospatial and abstract reasoning abilities and were selected because of the multiethnic composition of the Generation R Study. Raw scores were derived for each subtest and standardized to reflect a mean and standard deviation of the Dutch normative population for ages . The sum of the standardized scores of the two subtests was converted into the SON-R IQ score using age-specific reference scores provided in the SON-R manual (, ). These standardized scores, based on the two subtests, correlated well () with those based on the complete instrument (Ghassabian et al. 2014).
Genetic Analyses
In total, 474 children in the study sample had genetic data available from cord blood. Genotyping was performed using Illumina and arrays. Quality control included filters for sample () and single nucleotide polymorphism (SNP) call rates (), minor allele frequencies (MAF; ), and deviations from the Hardy-Weinberg equilibrium (). We additionally checked heterozygosity, sex accuracy, and relatedness. From this data set we extracted information on rs705379 (PON1-108), rs705381 (PON161), rs854560 (PON1-L55M), rs854572 (PON1-909), and rs662 (PON1-Q192). Only the latter was directly genotyped, all others were imputed. We used Mach 1.0 (Li et al. 2010) to impute to the 1000 Genomes Iv3 reference panel (1000 Genomes Project Consortium et al. 2015). All four imputed SNPs had excellent imputation quality (all ) and high MAF (all ). SNPs were included as allele dosages ranging from zero to two copies of the effects allele (Medina-Gomez et al. 2015). See Table S1 for effect allele and SNP descriptive statistics.
Additional Data Collection
Maternal reproductive, sociodemographic, and cognitive data were assessed by multiple questionnaires and instruments throughout the study. During pregnancy, data on maternal height and weight were collected as was information on maternal age, parity (0, 1, or ), smoking (no smoking during pregnancy, smoked until pregnancy was recognized, or continued smoking during pregnancy), alcohol intake during pregnancy [no alcohol consumption during pregnancy, alcohol consumption until pregnancy was recognized, continued occasionally (), or continued frequently ()], marital status (married/partner or single), household total net income [ (i.e., below the Dutch social security level), 1,200–2,000 euros per month, , highest completed education level (low: at general secondary school; intermediate: of secondary education; or high: university degree or higher vocational training), and ethnicity (Dutch national origin, other-Western, or non-Western)]. In addition, maternal dietary intake in the first trimester was assessed using a modified version of a validated semiquantitative food frequency questionnaire (FFQ), and the 293 food items were reduced to 24 predefined food groups (e.g., meat, grains, vegetables, fruits) according to the European Prospective Investigation into Cancer and Nutrition (EPIC)–soft classification, based on origin, culinary usage, and nutrient profiles (Steenweg-de Graaff et al. 2012; van den Dries et al. 2018).
An adapted Infant/Toddler Home Observation for Measurement of the Environment (IT-HOME) inventory (Caldwell and Bradley 1984) was administered during a home visit at approximately 3 months of age (). The validated 29-item version of the IT-HOME was used to measure the events, objects, and social interactions experienced by the child in the family context (Rijlaarsdam et al. 2012). Higher scores on the IT-HOME indicate a more enriched environment.
Maternal nonverbal IQ was measured when mother–child pairs attended the 6-y examination, and was assessed using a computerized Raven’s Advanced Progressive Matrices Test, set I (Prieler 2003). The test is a 12-item reliable and validated short version of the Raven’s Progressive Matrices to assess nonverbal cognitive ability (Chiesi et al. 2012).
Statistical Methods
Exposure.
The three DM metabolites (DMP, DMTP, and DMDTP) were summed as total DM (nmol/L) and the three DE metabolites (DEP, DETP, and DEDTP) were summed as total DE (nmol/L). Total DAP concentrations (nmol/L) were calculated by summing the six metabolites. Urinary concentrations were expressed on a volume and creatinine basis (nmol/g creatinine). Missing DAP metabolite values and missing covariate data were imputed (10 times) with the Multivariate Imputation by Chained Equation (MICE) method in R (version 3.2.3; R Development Core Team) (van Buuren and Groothuis-Oudshoorn 2011). DAP metabolite concentrations were transformed before running the multiple imputation (MI) procedure. Child nonverbal IQ was included as a predictor but was not imputed. Apart from household income (13%) and the IT-HOME score (29%), the percentage of missing values did not exceed 10% before imputation.
Statistical model.
Initial exploratory data analyses suggested that the OP pesticide–nonverbal IQ associations were nonlinear in functional form and that multivariable models with -transformed exposure generally provided a better model fit than models with OP pesticide concentration untransformed (see Table S2). Thus, first, parametric models with DAP concentrations -transformed were fit to estimate the OP pesticide–nonverbal child IQ associations, and second, restricted cubic spline models were fit to describe the functional form of the exposure–outcome association utilizing the rms-package in R, which estimates a p-value for nonlinearity (i.e., statistically significant values indicate a departure from linearity) (Harrell 2018). These associations were graphically depicted by plots. In parametric models, nonverbal IQ associations for each urine collection phase (gestational age , 18–25, and ) were modeled a) separately, b) as a single average of prenatal exposure, and c) for each urine collection phase modeled jointly (i.e., all three DAP concentrations included as three separate terms in each model). The first two regression models (model for each urine collection phase separately and the model for average prenatal exposure) consisted of an unadjusted model and an adjusted model. The third model consisted of a mutually adjusted model in which the three exposures from each time period were jointly estimated. The adjustment variables were maternal age, ethnicity, education, income, marital status, alcohol consumption during pregnancy, nonverbal IQ, body mass index (BMI), height, parity, and smoking during pregnancy; child sex; and the IT-HOME score. Potential adjustment variables were selected a priori based on previous studies of OP pesticides and child cognition and on biologically plausible covariate–exposure and covariate–outcome associations observed in our data (Bouchard et al. 2011; Engel et al. 2007, 2011, 2016; Eskenazi et al. 2007, 2014; London et al. 2012; van den Dries et al. 2018). Finally, to investigate whether the association of DAP concentrations on child IQ differed according to PON1 genotype, the interaction between DAP concentrations and PON1 genotype was formally tested using an a priori criteria for interaction of . Genetic analyses were carried out in both the full sample and in the Dutch national origin sample.
Sensitivity analyses.
First, values below the LOD were substituted with the LOD divided by the square root of 2, instead of using measured values as in the primary analyses. The replacement of values below the LOD with the LOD divided by the square root of 2 is a common substitution method in environmental exposure studies (Baccarelli et al. 2005). Second, models were refit with metabolite concentrations expressed as nanomoles per liter with creatinine concentration added as a separate covariate (O’Brien et al. 2016; Schisterman et al. 2005). Third, models truncating the bottom and top 3% of exposure and child nonverbal IQ values were fit to test data robustness. Fourth, the primary models were adjusted for fruit and vegetable intake (van den Dries et al. 2018). Fifth, a multiple informants model was fit as an alternative strategy to model the OP pesticide concentrations collected at three points in time during pregnancy (Sánchez et al. 2011). Sixth, to examine potential selection effects by maternal education, interaction models to assess effect measure modification by maternal education were fit. Seventh, to examine potential sex-specific associations, OP pesticide × child sex interaction models were fit. Eighth, a “complete case” analysis of the data was conducted, utilizing only observations with complete data for all covariates. Ninth, summed models excluding DEDTP metabolites were fit because of concentration values were . Last, each of the two subtests making up the child nonverbal IQ score (Mosaics and Categories) were modeled as the outcome of interest, in place of the nonverbal IQ score, to assess the specificity of results.
Results
Sample Characteristics
Overall, the Generation R Study mothers were on average 30 y of age at enrollment (SD 5 y), and diverse with respect to ethnicity, education, and income (Table 1). Compared with all women in the Generation R Study, the women in the present analysis were more likely to be older, nulliparous, Dutch, highly educated, married, and to occasionally consume alcoholic beverages during pregnancy. Women were also more likely to have a lower BMI and a higher income. Compared with all Generation R Study children who attended the 6-y examination, the IT-HOME scores of our sample were slightly higher.
Table 1.
Characteristics of all Generation R cohort members and of the participants included in the analysis, and average dialkylphosphates (DAP) concentration and nonverbal IQ score by category of characteristics.
| Characteristic | Generation R cohort (%)a () | Included in the analyses (%)a () | Average DAP exposure [median (25th–75th percentile)]b,c | Child nonverbal IQ score [mean (SD)]c |
|---|---|---|---|---|
| Maternal and infant characteristics at time of enrollment | ||||
| Sex of infant at birth | ||||
| Male | 50.6 | 51.3 | 378 (263–511) | 103 (16) |
| Female | 49.4 | 48.7 | 341 (248–480) | 102 (14) |
| Missing (n) | 153 | — | ||
| p-Valued | 0.064 | 0.289 | ||
| Age (y) | ||||
| 4.2 | 1.7 | 292 (229–477) | 97 (15) | |
| 15.9 | 10.2 | 332 (250–456) | 91 (15) | |
| 26.4 | 26.4 | 324 (245–476) | 103 (15) | |
| 36.9 | 45.8 | 382 (257–519) | 104 (16) | |
| 16.6 | 16.0 | 384 (269–489) | 104 (13) | |
| Missing (n) | — | — | ||
| p-Valued | 0.076 | |||
| BMI () | ||||
| 2.1 | 2.4 | 365 (281–563) | 98 (21) | |
| 57.9 | 65.4 | 378 (269–509) | 103 (15) | |
| 26.3 | 23.4 | 344 (257–460) | 101 (15) | |
| 13.8 | 8.8 | 262 (196–427) | 99 (15) | |
| Missing (n) | 899 | 3 | ||
| p-Valued | 0.047 | |||
| Height in cm (quartiles) | ||||
| 23.6 | 16.4 | 343 (260–505) | 97 (15) | |
| 27.4 | 31.0 | 349 (245–490) | 102 (15) | |
| 24.6 | 25.3 | 343 (245–489) | 103 (15) | |
| 24.4 | 27.3 | 370 (279–511) | 105 (16) | |
| Missing (n) | 934 | 1 | ||
| p-Valued | 0.709 | |||
| Parity (previous births) | ||||
| 0 | 55.1 | 61.5 | 361 (258–502) | 103 (16) |
| 1 | 30.2 | 27.3 | 379 (271–502) | 102 (15) |
| 14.7 | 11.2 | 295 (216–429) | 100 (14) | |
| Missing (n) | 378 | 4 | ||
| p-Valued | 0.017 | 0.468 | ||
| Ethnicity | ||||
| Dutch | 50.0 | 57.9 | 370 (253–487) | 106 (15) |
| Other-Western | 11.6 | 12.4 | 365 (283–514) | 101 (15) |
| Non-Western | 38.4 | 29.7 | 335 (248–516) | 96 (14) |
| Missing (n) | 694 | — | ||
| p-Valued | 0.587 | |||
| Education | ||||
| Low (no education finished, primary education, lower vocational training, intermediate general school or at general secondary school) | 26.5 | 14.8 | 315 (215–459) | 95 (16) |
| Intermediate ( of secondary education, intermediate vocational training or first year of higher vocational training) | 30.7 | 30.0 | 331 (247–478) | 100 (15) |
| High (university degree or higher vocational training) | 42.8 | 55.2 | 384 (280–509) | 106 (15) |
| Missing (n) | 1,221 | 24 | ||
| p-Valued | ||||
| Household income per month (euros) | ||||
| 20.7 | 12.6 | 312 (219–465) | 96 (15) | |
| 1,200–2,000 | 18.5 | 17.0 | 320 (247–473) | 101 (15) |
| 60.8 | 70.4 | 381 (278–501) | 105 (15) | |
| Missing (n) | 3,066 | 90 | ||
| p-Valued | 0.007 | |||
| Marital status | ||||
| Married/living with partner | 85.5 | 90.3 | 371 (267–504) | 103 (15) |
| No partner | 14.5 | 9.7 | 268 (210–415) | 101 (16) |
| Missing (n) | 1,213 | 29 | ||
| p-Valued | 0.211 | |||
| Nonverbal IQ score | ||||
| 16.0 | 18.5 | 321 (219–477) | 96 (14) | |
| 43.1 | 44.1 | 353 (263–507) | 102 (15) | |
| 16.7 | 19.1 | 373 (274–468) | 105 (15) | |
| 14.1 | 18.4 | 382 (252–501) | 107 (17) | |
| Missing (n) | 3,427 | 16 | ||
| p-Valued | 0.263 | |||
| Smoking | ||||
| No smoking during pregnancy | 73.4 | 78.1 | 375 (268–505) | 103 (15) |
| Until pregnancy recognized | 8.6 | 8.5 | 342 (258–500) | 102 (13) |
| Continued during pregnancy | 18.0 | 13.4 | 275 (186–469) | 98 (14) |
| Missing (n) | 1,534 | 61 | ||
| p-Valued | 0.001 | 0.008 | ||
| Alcohol beverage consumption | ||||
| No alcohol consumption during pregnancy | 48.0 | 37.9 | 329 (246–485) | 99 (15) |
| Until pregnancy recognized | 13.2 | 17.3 | 377 (268–516) | 104 (13) |
| Continued occasionally ( glass/week) | 31.6 | 38.5 | 381 (257–509) | 105 (16) |
| Continued frequently ( glass/week for at least two trimesters) | 7.2 | 6.4 | 349 (295–461) | 106 (14) |
| Missing (n) | 1,870 | 37 | 0.285 | |
| p-Valued | ||||
| Child at 6 y of age | ||||
| Total DAP in nmol/g creatinine (quartiles) | ||||
| — | 24.6 | 341 (248–483) | 100 (16) | |
| — | 24.9 | 332 (244–457) | 103 (14) | |
| — | 25.4 | 377 (266–503) | 102 (14) | |
| — | 25.1 | 380 (268–519) | 104 (17) | |
| Missing (n) | — | 28 | ||
| p-Valued | 0.152 | 0.140 | ||
| Household income per month (euros) | ||||
| 16.5 | 15.0 | 320 (223–502) | 96 (16) | |
| 1,600–4,000 | 49.2 | 47.0 | 344 (252–475) | 101 (16) |
| 34.3 | 38.0 | 390 (287–529) | 107 (14) | |
| Missing (n) | 3,953 | 40 | ||
| p-Valued | 0.001 | |||
| Child’s home environments | ||||
| IT-HOME scoree (quartiles) | ||||
| 36.3 | 22.6 | 321 (224–469) | 97 (16) | |
| 24.9 | 26.7 | 362 (258–505) | 101 (15) | |
| 18.2 | 19.4 | 360 (264–491) | 103 (14) | |
| 20.6 | 31.3 | 376 (269–516) | 104 (16) | |
| Missing (n) | 5,301 | 203 | ||
| p-Valued | 0.164 | 0.004 |
Note: BMI, body mass index; DAP, dialkylphosphates; IT-HOME, Infant/Toddler Home Observation for Measurement of the Environment inventory; —, all dashes, except for those for the child DAP concentrations (as noted), mean “0” as in, no missing values.
Values presented are percentages of the study sample unless noted otherwise.
Average DAP exposure represents the average exposure during pregnancy (measured at three time points) in nmol/g creatinine.
Values presented are based on the study sample ().
p-Value calculated with the use of Kruskal-Wallis test for differences in average DAP concentrations across pregnancy and nonverbal IQ scores by characteristic.
IT-HOME inventory is a 29-item validated measure of the events, objects, and social interactions experienced by a child in the family context. IT-HOME was assessed by observation during home visits at average child age of 3.38 months ().
The average DAP concentration in maternal pregnancy urine was higher among those who were older, had a lower BMI, higher income and education, and had partners (Table 1). Child DAP concentrations in urine were weakly associated with those in maternal pregnancy urine (; see also Table S3). The child’s DAP concentrations were also slightly positively associated with the HOME scores (not shown). Child nonverbal IQ scores were most strongly related to maternal ethnicity, education, and income and to their HOME score (Table 1).
DAP Concentrations
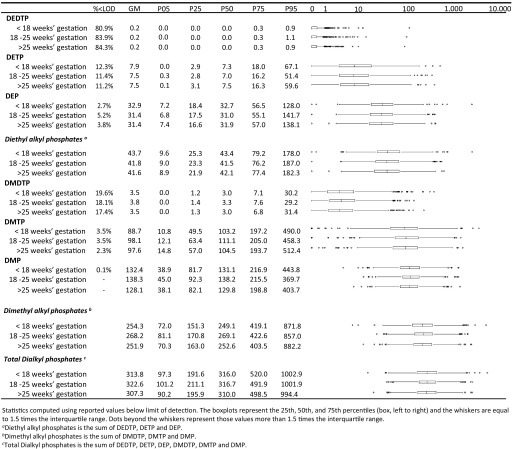
Total DAPs comprised mostly dimethyl alkyl phosphates, and the distribution of concentrations was fairly stable across the three sampling periods (Figure 2). As reported previously, the intraclass correlation coefficient for total DAP concentration across the three phases of pregnancy urine collection in this study was 0.38 (Spaan et al. 2015).
Figure 2.
Dialkylphosphate concentrations on a creatinine basis in maternal urine among Generation R participants included in analyses (). Statistics were computed using reported values below the limit of detection. The outer limits of the boxes (left to right) represent the 25th and 75th percentiles; the vertical bars within the boxes represent the 50th percentiles. The whiskers indicate 1.5 times the interquartile range (IQR), and the values more than 1.5 times the IQR are represented as points. Diethyl alkyl phosphates is the sum of DEDTP, DETP, and DEP. Dimethyl alkyl phosphates is the sum of DMDTP, DMTP, and DMP. Total dialkylphosphates is the sum of DEDTP, DETP, DEP, DMDTP, DMTP, and DMP. Note: DEDTP, diethyldithiophosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DMP, dimethylphosphate; DMTP, dimethylthiophosphate.
DAP–IQ Associations
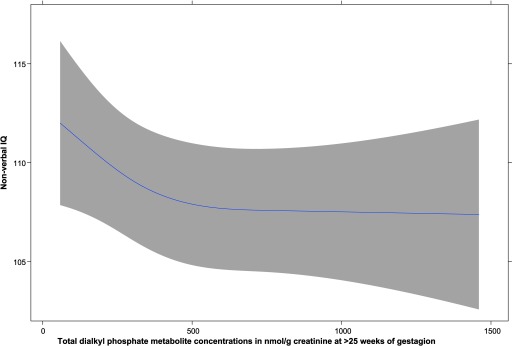
Overall, the estimated differences in child nonverbal IQ for a -unit increase in OP pesticide concentration were inconsistent between adjacent sampling periods (Table 2). The heterogeneity in association across sampling periods was statistically significant for total DAPs and dimethyl alkyl phosphates. For each 10-fold difference in total DAP concentration for the of gestation samples, however, adjusted child nonverbal IQ was 3.9 points lower [95% confidence interval (CI): , ]. The results for dimethyl alkyl phosphate at of gestation showed inverse associations that were slightly stronger than for total DAPs or diethyl alkyl phosphates, but these estimates were essentially similar given the width of the 95% CIs for measures at . A representative spline (mean of 10 restricted spline smooths from the MICE models) for total DAP concentration at of gestation is shown in Figure 3 and indicates a slightly steeper and inverse association between exposure and outcome at lower levels of exposure, and the p value for nonlinearity was 0.11. Restricted cubic splines of all 12 exposure–nonverbal IQ associations were largely consistent with results from parametric models (see Figures S1–S3).
Table 2.
Difference in cognitive test score at 6 y of age (and 95% confidence interval) per nanomoles per gram creatinine increase in maternal urine dialkylphosphate metabolite concentration, by timing of pregnancy urine sampling and degree of adjustment ().
| Dialkylphosphate type | Not adjusted | Adjusteda | Mutually adjustedb | |||
|---|---|---|---|---|---|---|
| (95% CI) | (95% CI) | (95% CI) | ||||
| Dialkylphosphates (total)c | ||||||
| of gestation | , 2.8 | , 1.5 | , 1.6 | |||
| 18–25 weeks of gestation | 3.2 | , 7.0 | 0.9 | , 4.7 | 2.6 | , 6.6 |
| of gestation | , 2.8 | , | , | |||
| Mean of three urines | 0.6 | , 5.5 | , 2.4 | — | ||
| p Homogeneityd | 0.051 | |||||
| Diethyl alkyl phosphatese | ||||||
| of gestation | 0.1 | , 2.6 | , 1.1 | , 1.0 | ||
| 18–25 weeks of gestation | 2.1 | , 4.7 | 0.6 | , 3.1 | 0.9 | , 3.5 |
| of gestation | 2.2 | , 4.7 | , 2.3 | , 2.3 | ||
| Mean of three urines | 4.4 | 0.6, 8.2 | 1.0 | , 4.9 | — | |
| p Homogeneityd | 0.475 | |||||
| Dimethyl alkyl phosphatesf | ||||||
| of gestation | , 1.7 | , 0.7 | , 0.9 | |||
| 18–25 weeks of gestation | 2.6 | , 6.3 | 0.7 | , 4.3 | 2.3 | , 6.1 |
| of gestation | , 1.9 | , | , | |||
| Mean of three urines | , 4.1 | , 1.7 | — | |||
| p Homogeneityd | 0.030 | |||||
Note: BMI, body mass index; CI, confidence interval; DEDTP, diethyldithiophosphate; DEP, diethylphosphate; DETP, diethylthiophosphate; DMDTP, dimethyldithiophosphate; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; IT-HOME, Infant/Toddler Home Observation for Measurement of the Environment inventory; —, not applicable.
Adjusted for age of the mother, sex of child, ethnicity categories (Dutch, other-Western, and non-Western), education (low, intermediate, and high), income (low, middle, and high), marital status, maternal alcohol consumption (no alcohol consumption during pregnancy, alcohol consumption until pregnancy was known, occasional alcohol consumption during pregnancy, and frequent alcohol consumption during pregnancy), maternal nonverbal IQ, BMI categories (, 18.5–25, 25–30, ), height of the mother, parity categories (0, 1, ), IT-HOME quartiles, and smoking categories (no smoking during pregnancy, smoked until pregnancy was known, smoked during pregnancy).
Adjusted model with the inclusion of the three exposures in one model.
Total dialkylphosphates is the sum of DEDTP, DETP, DEP, DMDTP, DMTP, and DMP.
Multiple-partial F-test used to test whether exposure from different time points relates in the same manner to nonverbal IQ scores.
Diethyl alkyl phosphates is the sum of DEDTP, DETP, and DEP.
Dimethyl alkyl phosphates is the sum of DMDTP, DMTP, and DMP.
Figure 3.
Restricted cubic spline of adjusted child nonverbal IQ scores and (untransformed) total DAP concentration. The solid line represents the estimated mean value of nonverbal IQ scores at each total DAP metabolite concentration, and the shaded area indicates the corresponding 95% confidence band for these estimates. Adjusted for age of the mother, sex of child, ethnicity categories (Dutch, other-Western, and non-Western), education (low, intermediate, and high), income (low, middle, and high), marital status, maternal alcohol consumption (no alcohol consumption during pregnancy, alcohol consumption until pregnancy was known, occasional alcohol consumption during pregnancy, and frequent alcohol consumption during pregnancy), maternal nonverbal IQ, BMI categories (, 18.5–25, 25–30, ), height of the mother, parity categories (0, 1, ), IT-Home quartiles, and smoking categories (no smoking during pregnancy, smoked until pregnancy was known, smoked during pregnancy). Note: BMI, body mass index; DAP, dialkylphosphates; IT-HOME, Infant/Toddler Home Observation for Measurement of the Environment inventory.
The results among the subset of mother–child pairs with child data on genotype showed that there was no strong statistical support for heterogeneity in the nonverbal child IQ–DAP association by genotype, particularly considering the number of comparisons made (see Tables S4–S11). When the genetic analysis was restricted to Dutch national origin participants, the findings were again unremarkable (see Tables S12–S15).
Sensitivity Analyses
As noted above, the sensitivity analyses examined the effects of a) the substitution method (see Table S16), b) adjustment of creatinine concentration as a separate covariate (see Table S17), c) the removal of extreme exposure and outcome values (see Table S18), d) adjustment for prenatal fruit and vegetable consumption (see Table S19), e) analyzing the data using a multiple informants model (see Table S20), f) examining effect measure modification by maternal educational attainment (see Table S21), g) examining effect measure modification by child sex (see Table S22), h) fitting a “complete case” only model (see Table S23–S24), and i) excluding DEDTP (i.e., including only those metabolites with at least 80% of values ) (see Table S25). Finally, Mosaics (see Table S26) and Categories (see Table S27) were modeled as the outcome of interest in place of nonverbal IQ. The sensitivity analyses supported the results shown in Table 2 and the absence of important differences when examining effect measure modification. However, with adjustment for fruit and vegetable intake, the associations tended to be more inverse.
Discussion
In this analysis of data on nonverbal IQ in children in relation to prenatal DAP concentrations in a diverse, urban population in Europe, evidence of an adverse association was weak overall, although there was some suggestion of an inverse relation between nonverbal IQ and late pregnancy urinary DAP concentration. Among the three groups of DAP metabolites analyzed, diethyl metabolites showed the weakest associations with child nonverbal IQ. Where inverse associations were suggested, the adjusted results were generally more strongly inverse than the crude results, consistent with negative confounding.
The results of our study share some consistencies with other published data. For example, in a pooled analysis of data on developmental indices at 2 y of age and DAPs measured in one or two prenatal urine specimens, there was a negative association at lower, as opposed to higher, concentrations of DAPs in three of the four pooled studies (with the fourth study showing linearity throughout the range of exposure), a finding similar to the pattern observed in our data (Figure 3) (Engel et al. 2016). A larger negative association at lower concentrations was also present in Bouchard’s study with IQ measured at 7 y of age (Bouchard et al. 2011). In that analysis of 7-y-old children from the CHAMACOS cohort, each 10-fold difference in total DAP concentration—in the second half of pregnancy—was associated with a 3.5-point lower Weschler Intelligence Scale for Children (WISC)-IV Full-Scale IQ score and a 3.1-point lower Verbal Comprehension score (Bouchard et al. 2011). Results from the present study are similar: Each 10-fold difference in total DAP concentration measured at was associated with a 3.9-point lower nonverbal IQ score. Results of our restricted cubic spline models illustrate that associations between DAPs and nonverbal IQ may be stronger at lower levels of exposure. There is evidence from animal and human studies that exposure–disease associations may not be linear (Calabrese and Baldwin 2001). For instance, studies of lead exposure and child IQ have observed nonlinearity in the lead–IQ association across different persons, places, and times (Bellinger and Needleman 2003; Canfield et al. 2003; Jusko et al. 2008; Lanphear et al. 2005). For organophosphate pesticides, low-dose developmental toxicity may occur through noncholinergic mechanisms (Flaskos 2014; Terry 2012), which may have nonlinear dose–effect modes of action.
The timing of the DAP exposure assessment may explain differences across studies. Bouchard et al. (2011) examined child IQ at 7 y of age in relation to urinary DAPs from either the first or second half of pregnancy; the DAP-IQ coefficient was negative for both periods, but more so for the DAPs in the second half of pregnancy. Our results also showed a larger negative association later in pregnancy. Other factors may also explain variation in the results across studies (Engel et al. 2016). For example, variation across studies in the degree of confounding by fruit and vegetable intake, or the degree to which urine DAP metabolites concentrations reflect exposure to the active pesticide rather than degradation products, especially in regions where a larger proportion of measured DAP concentrations is due to agricultural pesticide exposure (Gunier et al. 2017) may explain differences across studies. Other potential reasons for variation may be in the types of pesticides used on food consumed in different countries or in the socioeconomic status (SES) of the studied populations. The authors of two recent studies of organophosphate pesticide metabolites and IQ speculated that a reason for the lack of association in higher SES populations was due to the protective effects of higher SES (Cartier et al. 2016; Donauer et al. 2016). In the present data, adjusted results were more inverse than the crude results, consistent with the possibility of residual confounding by SES or other lifestyle factors. Additionally, the instruments used to measure IQ differed across studies. The present study utilized a global, nonverbal measure of intelligence. Other studies utilized more complete IQ batteries such as the WISC that may be more sensitive to cognitive deficits because the instrument measures both verbal and nonverbal domains of intellectual function. For instance, in the CHAMACOS cohort, total DAP concentrations were associated with lower scores on the Verbal Comprehension Index, but scores on the Perceptual Reasoning and Processing Speed indices of the WISC-IV were less strongly associated with DAP concentrations, if at all (Eskenazi et al. 2014).
In the present study, we did not observe any evidence of effect measure modification by the PON1 gene allele. When we restricted the total sample to only those of Dutch national origin, results also did not show evidence of interaction by PON1 genotype status. In other studies of effect measure modification by genotype, the results on cognitive measures and DAPs have been inconsistent (Engel et al. 2011; Eskenazi et al. 2014). For example, Eskenazi et al. (2014) observed that the association between DAPs and Mental Development Index scores was the strongest in children with PON1-108T allele, but this and other interactions between DAPs and PON1 polymorphisms or enzymes were not statistically significant. On the other hand, Engel et al. (2011) observed a statistically significant interaction (), showing a stronger inverse association between -DAP exposure and perceptual reasoning in children with the PON192QQ allele. Results based on mother and child genotypes tended to be similar within a given study (Engel et al. 2011; Eskenazi et al. 2014).
The concentration of DAPs in urine only captures short-term exposure, which varies substantially from day to day, depending on diet. Although DAPs were measured at three time points, the average exposure may not have been accurately captured (Perrier et al. 2016). Furthermore, the proportion of the DAPs measured that reflected exposure to the active pesticide rather than to inactive degradation products was unknown. The rate of degradation of organophosphate pesticides under specific field conditions is hard to predict (Fenner et al. 2013). As noted above, the SES of the Generation R population in the present study was higher than for the Generation R cohort as a whole, which may be a reflection of our exclusion criterion of having three urine specimens during pregnancy. Our results therefore may not be generalizable to the Generation R population; however, as noted above, we saw no evidence of effect measure modification by education, suggesting that potential selection bias is unlikely to have materially affected our results. Among the strengths of our study were the relatively large size, the measurement of maternal DAPs at three time points during pregnancy, and a well-standardized and validated instrument for measuring outcome. Furthermore, as documented elsewhere, the median total DAPs among the Generation R Study mothers was more than 2-fold higher compared with background-exposed pregnant women in the United States living in nonagricultural communities, which suggests a greater range of exposure and statistical power with which to evaluate exposure–disease associations (Ye et al. 2008).
Conclusions
Organophosphate pesticide exposure is ubiquitous, and experimental data and evidence from accidental poisoning in humans indicate that it is neurotoxic. The present study, utilizing a well-characterized pregnancy cohort in Rotterdam, Netherlands, examined maternal organophosphate exposure in 708 pregnant women in relation to child nonverbal IQ at 6 y of age. OP pesticide exposure was characterized by six measured dialkylphosphate metabolite concentrations in pregnancy urine specimens at three time points, and child nonverbal IQ was assessed at 6 y of age, after school entry, when IQ tests tend to have greater predictive validity for aspects of learning such as school achievement (Sattler 2008). Our results suggest that typical, background OP exposures during pregnancy are not consistently associated with lower child IQ at 6 y of age in this population; however, there was some evidence that late pregnancy may be a susceptible period for adverse effects on cognition.
Supplementary Material
Acknowledgments
The authors thank F. Pierik for laying the groundwork for the National Institute of Environmental Health Sciences–Netherlands Organisation for Applied Scientific Research (NIEHS-TNO)-Erasmus University collaboration and R. Hauser for alerting M.P.L. to the possibility of this project. The Generation R Study is conducted by the Erasmus Medical Center, Rotterdam in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University, Rotterdam, the Municipal Health Service, Rotterdam Homecare Foundation, and Stichting Trombosedienst & Artsenlaboratorium Rijnmond. The authors are thankful for the contribution of participating parents and their children, general practitioners, hospitals, midwives, and pharmacies. The authors also thank A. Neumann for providing detailed information concerning genotyping and N. Russo for assistance with manuscript preparation.
This research received support from National Institutes of Health (NIH) grants K12 ES019852, P30 ES001247, and the intramural research program of the NIEHS/NIH. M.G. is funded by a Miguel Servet fellowship (MS13/00054) awarded by the Spanish Institute of Health Carlos III (Ministry of Economy and Competitiveness). ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP3024).
While this manuscript was being prepared, M.P.L. was working part-time at Ramboll Environ, with support from the American Chemistry Council. The work on this manuscript was done solely with NIEHS support (M.P.L. as a government contractor). Each author certifies that their freedom to design, conduct, interpret, and publish research was not compromised by any sponsor.
All other authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. . 2015. A global reference for human genetic variation. Nature 526(7571):68–74, PMID: 26432245, 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarelli A, Pfeiffer R, Consonni D, Pesatori AC, Bonzini M, Patterson DG Jr, et al. . 2005. Handling of dioxin measurement data in the presence of non-detectable values: overview of available methods and their application in the Seveso chloracne study. Chemosphere 60(7):898–906, PMID: 15992596, 10.1016/j.chemosphere.2005.01.055. [DOI] [PubMed] [Google Scholar]
- Bellinger DC, Needleman HL. 2003. Intellectual impairment and blood lead levels. N Engl J Med 349(5):500–502, PMID: 12890850, 10.1056/NEJM200307313490515. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. . 2011. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect 119(8):1189–1195, PMID: 21507776, 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Driskell WJ, Whitehead RD Jr, Needham LL, Barr DB. 2002. Quantitation of dialkyl phosphate metabolites of organophosphate pesticides in human urine using GC-MS-MS with isotopic internal standards. J Anal Toxicol 26(5):245–252, PMID: 12166810, 10.1093/jat/26.5.245. [DOI] [PubMed] [Google Scholar]
- Burke RD, Todd SW, Lumsden E, Mullins RJ, Mamczarz J, Fawcett WP, et al. . 2017. Developmental neurotoxicity of the organophosphorus insecticide chlorpyrifos: from clinical findings to preclinical models and potential mechanisms. J Neurochem 142 (suppl 2):162–177, PMID: 28791702, 10.1111/jnc.14077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AR. 1975. The Jaffé reaction. Part II. A kinetic study of the Janovsky complexes formed from creatinine (2-imino-1-methylimazolidin-4-one) and acetone. J Chem Soc Perkin Trans 2 0:853–857, 10.1039/P29750000853. [DOI] [Google Scholar]
- Calabrese EJ, Baldwin LA. 2001. The frequency of U-shaped dose responses in the toxicological literature. Toxicol Sci 62(2):330–338, PMID: 11452146, 10.1093/toxsci/62.2.330. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. 1984. Home Observation for Measurement of the Environment. Littlerock, AR:University of Arkansas at Littlerock. [Google Scholar]
- Canfield RL, Henderson CR Jr, Cory-Slechta DA, Cox C, Jusko TA, Lanphear BP. 2003. Intellectual impairment in children with blood lead concentrations below 10 μg per deciliter. N Engl J Med 348(16):1517–1526, PMID: 12700371, 10.1056/NEJMoa022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier C, Warembourg C, Le Maner-Idrissi G, Lacroix A, Rouget F, Monfort C, et al. . 2016. Organophosphate insecticide metabolites in prenatal and childhood urine samples and intelligence scores at 6 years of age: results from the mother–child PELAGIE cohort (France). Environ Health Perspect 124(5):674–680, PMID: 26394442, 10.1289/ehp.1409472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2009. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA:CDC. [Google Scholar]
- Chiesi F, Ciancaleoni M, Galli S, Primi C. 2012. Using the Advanced Progressive Matrices (Set I) to assess fluid ability in a short time frame: an item response theory–based analysis. Psychol Assess 24(4):892–900, PMID: 22449036, 10.1037/a0027830. [DOI] [PubMed] [Google Scholar]
- Donauer S, Altaye M, Xu Y, Sucharew H, Succop P, Calafat AM, et al. . 2016. An observational study to evaluate associations between low-level gestational exposure to organophosphate pesticides and cognition during early childhood. Am J Epidemiol 184(5):410–418, PMID: 27539379, 10.1093/aje/kwv447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, et al. . 2008. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol 38 (suppl 2):1–125, PMID: 18726789, 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. . 2007. Prenatal organophosphate metabolite and organochlorine levels and performance on the Brazelton Neonatal Behavioral Assessment Scale in a multiethnic pregnancy cohort. Am J Epidemiol 165(12):1397–1404, PMID: 17406008, 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Bradman A, Wolff MS, Rauh VA, Harley KG, Yang JH, et al. . 2016. Prenatal organophosphorus pesticide exposure and child neurodevelopment at 24 months: an analysis of four birth cohorts. Environ Health Perspect 124(6):822–830, PMID: 26418669, 10.1289/ehp.1409474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. . 2011. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect 119(8):1182–1188, PMID: 21507778, 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Bradman A, Castorina R. 1999. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 107 (suppl 3):409–419, PMID: 10346990, 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Kogut K, Huen K, Harley KG, Bouchard M, Bradman A, et al. . 2014. Organophosphate pesticide exposure, PON1, and neurodevelopment in school-age children from the CHAMACOS study. Environ Res 134:149–157, PMID: 25171140, 10.1016/j.envres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. . 2007. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect 115(5):792–798, PMID: 17520070, 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner K, Canonica S, Wackett LP, Elsner M. 2013. Evaluating pesticide degradation in the environment: blind spots and emerging opportunities. Science 341(6147):752–758, PMID: 23950532, 10.1126/science.1236281. [DOI] [PubMed] [Google Scholar]
- Flaskos J. 2014. The neuronal cytoskeleton as a potential target in the developmental neurotoxicity of organophosphorothionate insecticides. Basic Clin Pharmacol Toxicol 115(2):201–208, PMID: 24476507, 10.1111/bcpt.12204. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, El Marroun H, Peeters RP, Jaddoe VW, Hofman A, Verhulst FC, et al. . 2014. Downstream effects of maternal hypothyroxinemia in early pregnancy: nonverbal IQ and brain morphology in school-age children. J Clin Endocrinol Metab 99(7):2383–2390, PMID: 24684462, 10.1210/jc.2013-4281. [DOI] [PubMed] [Google Scholar]
- Gunier RB, Bradman A, Harley KG, Kogut K, Eskenazi B. 2017. Prenatal residential proximity to agricultural pesticide use and IQ in 7-year-old children. Environ Health Perspect 125(5):057002, PMID: 28557711, 10.1289/EHP504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell FE. 2018. rms: regression modeling strategies. R package version 5.1-2. https://CRAN.R-project.org/package=rms [accessed 25 May 2018].
- Health Canada. 2010. Report on Human Biomonitoring of Environmental Chemicals in Canada: Results of the Canadian Health Measures Survey Cycle 1 (2007–2009). Ottawa, Ontario, Canada:Government of Canada. [Google Scholar]
- Huen K, Harley K, Bradman A, Eskenazi B, Holland N. 2010. Longitudinal changes in PON1 enzymatic activities in Mexican–American mothers and children with different genotypes and haplotypes. Toxicol Appl Pharmacol 244(2):181–189, PMID: 20045427, 10.1016/j.taap.2009.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko TA, Henderson CR, Lanphear BP, Cory-Slechta DA, Parsons PJ, Canfield RL. 2008. Blood lead concentrations < 10 μg/dL and child intelligence at 6 years of age. Environ Health Perspect 116(2):243–248, PMID: 18288325, 10.1289/ehp.10424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH, van Ijzendoorn MH, et al. . 2016. The Generation R Study: design and cohort update 2017. Eur J Epidemiol 31(12):1243–1264, PMID: 28070760, 10.1007/s10654-016-0224-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof CJ, Kooijman MN, van Duijn CM, Franco OH, de Jongste JC, Klaver CC, et al. . 2014. The Generation R Study: Biobank update 2015. Eur J Epidemiol 29(12):911–927, PMID: 25527369, 10.1007/s10654-014-9980-6. [DOI] [PubMed] [Google Scholar]
- Lanphear BP, Hornung R, Khoury J, Yolton K, Baghurst P, Bellinger DC, et al. . 2005. Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis. Environ Health Perspect 113(7):894–899, PMID: 16002379, 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. 2010. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol 34(8):816–834, PMID: 21058334, 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llop S, Murcia M, Iñiguez C, Roca M, González L, Yusà V, et al. . 2017. Distributions and determinants of urinary biomarkers of organophosphate pesticide exposure in a prospective Spanish birth cohort study. Environ Health 16(1):46, PMID: 28514952, 10.1186/s12940-017-0255-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- London L, Beseler C, Bouchard MF, Bellinger DC, Colosio C, Grandjean P, et al. . 2012. Neurobehavioral and neurodevelopmental effects of pesticide exposures. Neurotoxicology 33(4):887–896, PMID: 22269431, 10.1016/j.neuro.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Gomez C, Felix JF, Estrada K, Peters MJ, Herrera L, Kruithof CJ, et al. . 2015. Challenges in conducting genome-wide association studies in highly admixed multi-ethnic populations: the Generation R Study. Eur J Epidemiol 30(4):317–330, PMID: 25762173, 10.1007/s10654-015-9998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KM, Upson K, Cook NR, Weinberg CR. 2016. Environmental chemicals in urine and blood: improving methods for creatinine and lipid adjustment. Environ Health Perspect 124(2):220–227, PMID: 26219104, 10.1289/ehp.1509693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Malley M. 1997. Clinical evaluation of pesticide exposure and poisonings. Lancet 349(9059):1161–1166, PMID: 9113024, 10.1016/S0140-6736(96)07222-4. [DOI] [PubMed] [Google Scholar]
- Perrier F, Giorgis-Allemand L, Slama R, Philippat C. 2016. Within-subject pooling of biological samples to reduce exposure misclassification in biomarker-based studies. Epidemiology 27(3):378–388, PMID: 27035688, 10.1097/EDE.0000000000000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieler J. 2003. Raven’s Advanced Progressive Matrices. Mödling, Austria:Schufried. [Google Scholar]
- Rijlaarsdam J, Stevens GW, van der Ende J, Arends LR, Hofman A, Jaddoe VW, et al. . 2012. A brief observational instrument for the assessment of infant home environment: development and psychometric testing. Int J Methods Psychiatr Res 21(3):195–204, PMID: 22836590, 10.1002/mpr.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez BN, Hu H, Litman HJ, Téllez-Rojo MM. 2011. Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants. Environ Health Perspect 119(3):409–415, PMID: 21362588, 10.1289/ehp.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler JM. 2008. Assessment of Children: Cognitive Foundations. 5th ed San Diego:Sattler. [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. 2005. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 113(7):853–857, PMID: 16002372, 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, et al. . 2016. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada—results from the MIREC study. Environ Int 94:133–140, PMID: 27243443, 10.1016/j.envint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- Spaan S, Pronk A, Koch HM, Jusko TA, Jaddoe VW, Shaw PA, et al. . 2015. Reliability of concentrations of organophosphate pesticide metabolites in serial urine specimens from pregnancy in the Generation R Study. J Expo Sci Environ Epidemiol 25(3):286–294, PMID: 25515376, 10.1038/jes.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenweg-de Graaff J, Roza SJ, Steegers EA, Hofman A, Verhulst FC, Jaddoe VW, et al. . 2012. Maternal folate status in early pregnancy and child emotional and behavioral problems: the Generation R Study. Am J Clin Nutr 95(6):1413–1421, PMID: 22572645, 10.3945/ajcn.111.030791. [DOI] [PubMed] [Google Scholar]
- Tellegen PJ, Winkel M, Wijnberg-Williams BJ, Laros JA. 1998. “Snijders-Oomen Nonverbal Intelligence Test. SON-R 2½-7 Manual and Research Report.” Lisse, Netherlands: Swets & Zeitlinger B.V. [Google Scholar]
- Terry AV., Jr. 2012. Functional consequences of repeated organophosphate exposure: potential non-cholinergic mechanisms. Pharmacol Ther 134(3):355–365, PMID: 22465060, 10.1016/j.pharmthera.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Buuren S, Groothuis-Oudshoorn K. 2011. mice: multivariate imputation by chained equations in R. J Stat Softw 45(3):1–67, 10.18637/jss.v045.i03. [DOI] [Google Scholar]
- van den Dries MA, Pronk A, Guxens M, Spaan S, Voortman T, Jaddoe VW, et al. . 2018. Determinants of organophosphate pesticide exposure in pregnant women: a population-based cohort study in the Netherlands. Int J Hyg Environ Health 221(3):489–501, PMID: 29499913, 10.1016/j.ijheh.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, et al. . 2008. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R Study. Environ Res 108(2):260–267, PMID: 18774129, 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.