Abstract
Circadian rhythms of physiology are key to health and fitness, as dysregulation, by genetic mutations or environmental factors, increases disease risk and aggravates progression. Molecular and physiological studies have shed important light on an intrinsic clock that drives circadian rhythms and serves essential roles in metabolic homeostasis, organ physiology, and brain functions. One exciting new area in circadian research is pain, including headache and neuropathic pain for which new mechanistic insights have recently emerged. For example, cluster headache is an intermittent pain disorder with an exceedingly precise circadian timing, and preliminary evidence is emerging linking several circadian components (e.g., Clock and Nr1d1) with the disease. In this review, we first discuss the broad metabolic and physiological relevance of the circadian timing system. We then provide a detailed review of the circadian relevance in pain disease and physiology, including cluster headache, migraine, hypnic headache, and neuropathic pain. Finally, we describe potential therapeutic implications, including existing pain medicines and novel clock-modulating compounds. The physiological basis for the circadian rhythms in pain is an exciting new area of research with profound basic and translational impact.
Keywords: circadian rhythms, chronotherapy and clock-modulating compounds, cluster and hypnic headaches, metabolism, migraine, neuropathic pain
Introduction
An internal 24-hour timer, or circadian clock, is present in organisms ranging from bacteria to plants to animals1, 2. It coordinates essential functions, and in doing so conserves energy or promotes activity at the appropriate times3. Examples include body temperature (highest before midnight), blood pressure (highest at midday), pulmonary capacity (forced expiratory volume in one second or FEV1 highest in the afternoon), and sleep (tiredness is highest around midnight). Understanding these basic circadian functions has led to simple but important medical advances in chronotherapy, namely drug administration at specific circadian times. For example, blood pressure medications are given before the blood pressure peaks4, and asthma medications are given before the pulmonary function declines5, 6.
The internal circadian clock also affects metabolic homeostasis7, 8 and higher-order functions9, 10 such that there are peak times every day for gluconeogenesis, schoolwork, and athletic performance. Thus when the circadian system is disrupted, it increases the risk of a wide range of diseases. Animal and human studies have shown that debilitating mutations or disruptions of core circadian genes can have adverse effects on metabolic, physiologic and neurologic processes11, 12. For example, mutations in the human circadian gene Casein Kinase (Ck) 1delta lead not only to sleep cycle shifts, but also to an increased incidence of migraines13. Understanding these complex circadian functions, and their role in human disease, has the potential to lead to significant medical advances. In this article we review the role of circadian rhythms in physiology, and in particular highlight pain as an emerging field for circadian research.
Circadian clock and its broad relevance in physiology
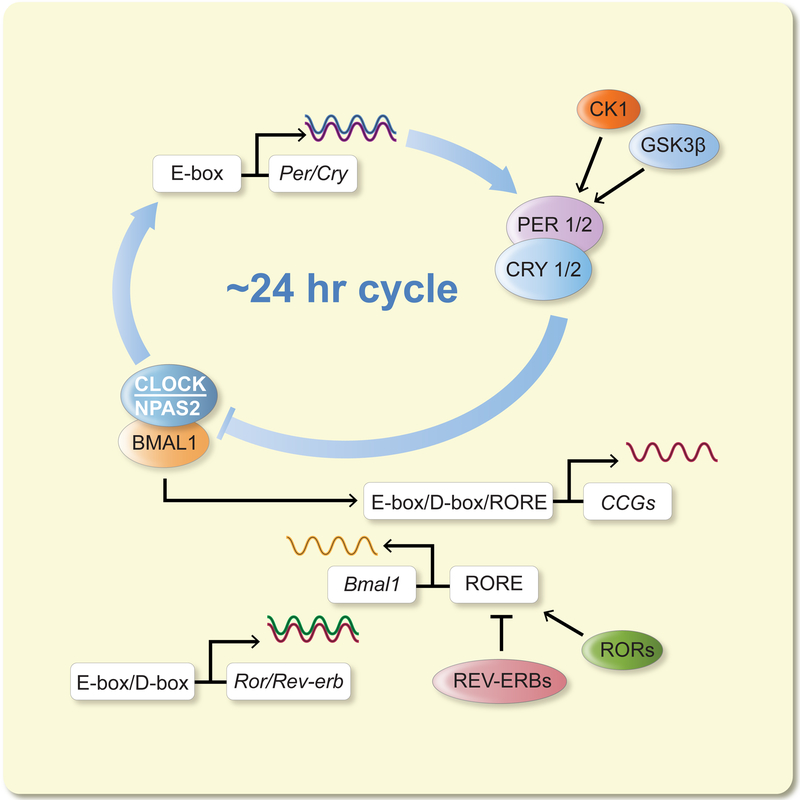
The circadian cycle is present at the single cell level: individual cells grown in vitro, such as fibroblasts, are capable of generating a circadian rhythm14, 15. Within the cell, the clock machinery is a series of intersecting transcription-translation feedback loops that activate and inhibit each other in a cycle lasting about 24 hours16 (Figure 1). These core circadian genes encode both positive (CLOCK: Circadian Locomotor Output Cycles Kaput; NPAS2: Neuronal PAS domain protein 2; BMAL1: Brain And Muscle ARNT-Like 1; RORα/β/γ: Retinoid acid-related Orphan Receptor a, b, c) and negative (PERIOD1/2/3, or PER1/2/3; CRYTOCHROME1/2, or CRY1/2; REV-ERBα/β: Reverse strand of erb) proteins. Additional factors have been identified, which either form auxiliary loops or regulate the core clock components16, 17. The core oscillator subsequently regulates tissue-specific expression of output genes via elaborate genetic and epigenetic mechanisms18.
Figure 1:
The cell-autonomous circadian oscillator present in brain and peripheral cells. In the oscillator, transcriptional-translational feedback loops are connected and reciprocally regulated, including the core loop (CLOCK/NPAS2, BMAL1 and PERs, CRYs) and secondary loop (BMAL1, REV-ERBs and RORs). The oscillators are fine-tuned by a wide array of regulatory factors (including the CK1 and GSK3β kinases) and input signals and in turn drive expression of output genes in a tissue-specific manner, thus controlling metabolic, physiological, behavioral and cognitive functions. Full name of genes encoding the clock components are as follows. Clock: Circadian Locomotor Output Cycles Kaput; Npas2: Neuronal PAS domain protein 2; Bmal1: Brain And Muscle ARNT-Like 1; Per: Period; Cry: Cryptochrome; Rev-erb: Reverse strand of erba; Ror: Retinoid acid-related Orphan Receptor; Ck1: casein kinase 1; Gsk3b: glycogen synthase kinase 3 beta. The genes encoding the nuclear receptors RORs and REV-ERBs are also known as Nr1f and Nr1d subfamilies, respectively. Modified from137 under Creative Commons Attribution license.
On a larger scale, the circadian rhythm is present throughout the body: organ explants isolated in vitro show a circadian rhythm regardless of tissue type, as brain, liver, lung, kidney, and other tissues have shown circadian rhythmicity19. Even though each cell has its own rhythm, cells of a single organ typically function together as a single circadian unit called a peripheral clock. These peripheral clocks run independently but are synchronized by a central pacemaker – the suprachiasmatic nucleus (SCN)20. The SCN was shown as the central clock in elegant experiments where the SCN was either lesioned (abolishing the 24 hour cycle) or where the SCN in a wild-type hamster was replaced with a mutant SCN with a 20 hour cycle (and the wild-type hamster began to display a 20 hour cycle)21–23. Located in the anterior hypothalamus, the SCN is a remarkably robust self-sustaining oscillator24. Both the SCN and peripheral clocks can be calibrated, or entrained, by various stimuli called zeitgebers (time givers)25. Light is the predominant zeitgeber and is relayed to the SCN via the retinohypothalamic tract. Light enters this tract not by rods or cones but by a third group of photosensitive cells, the photosensitive retinal ganglion cells, which contain melanopsin and use glutamate and pituitary adenylate cyclase-activating peptide (PACAP)26, 27. Food can also entrain body clocks: when light is kept constant over 24 hours, and thus is eliminated as a zeitgeber, rats exposed to food once a day will entrain to that food stimulus28. Other zeitgebers for the SCN and peripheral clocks include temperature, exercise, and circadian hormones (i.e., steroids and melatonin)29–31. The SCN communicates with peripheral clocks through circadian hormones, and communicates with other brain areas through neuronal connections. SCN neurons project to various locations in the thalamus, hypothalamus, and basal forebrain25. These include areas such as the periventricular nucleus of the hypothalamus and the nucleus of the vagus32, which are critical areas of the autonomic nervous system. It may also connect to the lateral hypothalamus, which contains orexin and is important in sleep regulation.
Commonly used biomarkers for the circadian system are the hormones melatonin and cortisol. Melatonin is a biological marker of darkness, rising at dusk and declining before dawn, and a light pulse will acutely reduce the production of melatonin. Melatonin is produced and released primarily by the pineal gland, which is directly controlled by the SCN via sympathetic neurons. Melatonin interacts with most if not all cell types and may be one way that the SCN synchronizes all of the peripheral clocks30, 33. Melatonin may be used as a measure of the yearly cycle: the duration of melatonin release is a marker of day length, and day length varies from the summer to the winter solstice. Corticosteroids, in contrast, are a biological marker of day and display a pattern opposite to melatonin. Like melatonin, corticosteroids synchronize the peripheral clocks and are often used for this purpose experimentally34: in vitro, individual cells each display a slightly different circadian cycle, and dexamethasone is used to synchronize the cycles of an entire cell population. Thus either by direct neuronal connections from the SCN or by hormonal communication, the entire circadian system can be synchronized.
The majority of all genes are cyclically expressed in at least one organ in mammals35, 36. Affirming the physiological importance of the circadian clock, the subset of genes expressed in each tissue often encompasses regulatory genes for various physiologies associated with organ function37. For example, in a transcriptional cascade required for myocardial repolarization, CLOCK/BMAL1 activates Klf15 gene expression; subsequently, KLF15 proteins activate expression of the gene encoding the cardiac ion channel component KvCHIP238. Furthermore, PER2 proteins were found to be stabilized by adenosine signaling in response to myocardial ischemia, and play an important role to reprogram hypoxic and metabolic pathways39. These circadian changes in the cardiovascular system may be relevant for human medicine. In a recent study, patients were randomized to morning or afternoon surgery for aortic valve replacement. Improved surgical outcomes were found in the afternoon group and were linked to changes in the expression of the core circadian component REV-ERBα40.
Finally, circadian patterns are not the only type of cyclical pattern in humans, and hormones and transcriptional-translational feedback loops are not the only mechanism for generating them. Ultradian rhythms refer to cycles that are shorter than 24 hours: an example is heart rate, which cycles 70–100 times per minute in a resting human and is controlled by the nervous system. In contrast, infradian rhythms have a cycle that is longer than 24 hours. Infradian cycles can be hormonal such as the monthly menstrual cycle, or behavioral such as the weekly pattern of sleep that changes from the workweek to the weekend.
Circadian rhythms in pain
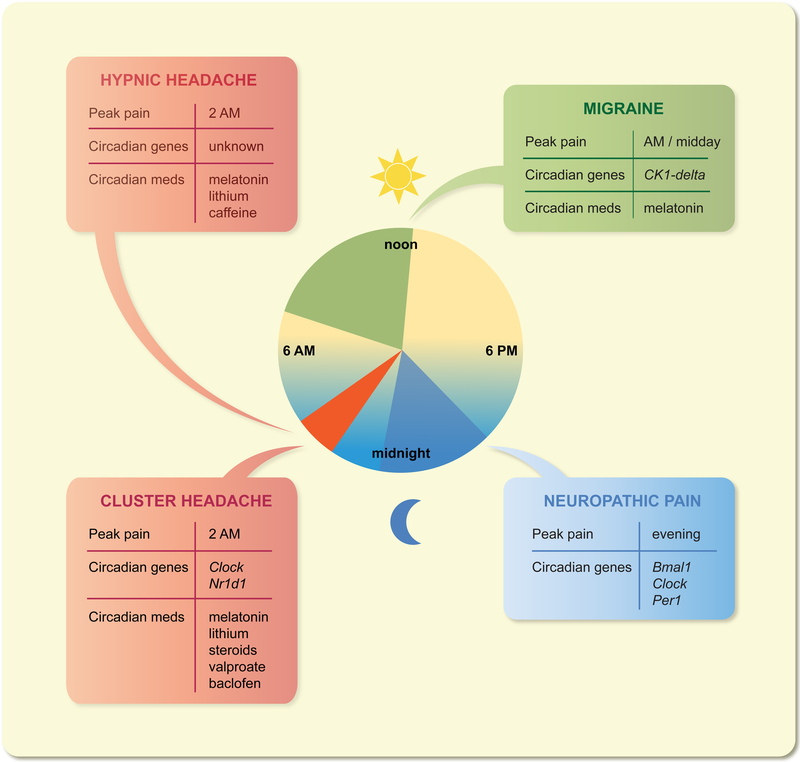
The most common reason to seek medical care is pain, and patients with a variety of pain disorders have reported distinct daily rhythms of pain intensity41–43. A well-known pain disorder with circadian timing is rheumatoid arthritis: morning stiffness and morning pain have been described for over a half-century, with morning stiffness proposed as a diagnostic criterion in the 1950’s44. Recent studies have linked arthritic morning pain to the daily cycle of inflammatory cytokines45. Moreover, there appears to be a role for chronotherapy of immunosuppressants in treating rheumatoid arthritis45. The circadian biology of other pain disorders is also emerging. Below, we will focus on headaches (including cluster headache, migraine, and hypnic headache) and neuropathic pain (chronic pain after nerve injury) for which new mechanistic information has emerged (Figure 2).
Figure 2:
Circadian rhythms in headaches and neuropathic pain. Headache disorders, including cluster headache, migraine and hypnic headache, and neuropathic pain display circadian rhythmicity of attack occurrence. Several clock components have also been implicated in these diseases in either rodent models or in small human studies. Note that Nr1d1 encodes REV-ERBα. Several pain medicines, such as lithium, valproic acid and melatonin and corticoids, are known to reset the circadian clock.
Cluster headache
Cluster headache affects 1 in 1000 people and starts between the ages of 20–40, with a 3:1 ratio of male to female46–48. It is often described as one of the most painful human experiences and has been nicknamed “suicide headaches,” as over 50% of patients have contemplated suicide49, 50. Cluster headache is characterized by unilateral attacks of exquisite pain lasting 15–180 minutes, occurring between once every other day and eight times per day, and is associated with restlessness (patient pace during a headache) and autonomic changes of the face (like a bloodshot or watering eye)51. The vast majority (90%) of cluster headache patients will have headaches every day for weeks-to-months and then will have no headaches for several months, as if the headaches are clustering together. This cyclical pattern is termed episodic cluster headache; the remaining 10% of patients have chronic cluster headache with no headache-free period lasting longer than three months. The exact mechanisms of cluster headache are not known, but current research from imaging and animal studies suggests that three systems are involved. First, the trigeminovascular pain system receives inputs from the meninges and large cranial blood vessels and transmits information to the trigeminal nucleus and the dorsal horn of the adjacent three cervical levels (C1–3). Second, the autonomic system connects from the superior salivatory nucleus to the sphenopalatine ganglion and is thought to be responsible for the cranial autonomic features of the disease. Third, imaging data suggests hypothalamic activation during a cluster headache attack52. In this imaging study, nine cluster headache patients and eight controls were exposed to nitroglycerin, which induced a cluster headache attack in the cluster headache subjects but not in the control subjects. Positron emission tomography (PET) was performed at baseline, during nitroglycerin infusion, during the headache, and after the headache. The ipsilateral hypothalamus showed increased regional cerebral blood flow only during the headache period and only in the cluster headache group. The hypothalamus contains several areas with potential roles in cluster headache, including the central clock (the SCN) and an area anterior and medial to the fornix that has been implicated in defensive rage and might explain the restlessness and self-aggression seen in the disease53, 54.
These three systems – the trigeminovascular pain system, the autonomic system, and the hypothalamus – appear to be strongly interconnected in cluster headache patients. Deep brain stimulation of the hypothalamus is a proposed treatment for cluster headache based on preliminary studies55, 56, and cluster headache patients with implanted hypothalamic deep brain stimulators show higher cold pain thresholds57. Pain thresholds in general, such as the nociceptive flexion reflex in the leg, show circadian variation in episodic cluster headache patients compared to controls or chronic cluster headache patients58. Modulation of the autonomic system via the sphenopalatine ganglion can induce or relieve cluster headache pain depending on the stimulation setting, and stimulation of the superior salivatory nucleus in rodents activates neurons in the trigeminal nucleus59. One clear link between the circadian system and the mechanisms of cluster headache is that the central clock (the SCN) resides in the hypothalamus.
One hallmark feature of cluster headache is its prominent circadian and circannual patterns60. Patients generally have headaches at precisely the same time every day, and have headache cycles at precisely same time every year or every other year. For example, a typical patient may say that he has an attack at 2 AM every day April, then is headache-free until the following April. Another may say she has headaches at 10 PM and midnight every night in autumn, though it will sometimes skip a year. A large study of 1134 patients showed that 82% of cluster headache patients have headaches at the same time every day49. The most common time of day for an attack was 2 AM in three large studies49, 61, 62, and the most common times of year appear to be spring and autumn. Possibly the most strikingly circadian example reported is a Danish woman who underwent a sleep study over 2 nights and had 9 headaches, each almost exactly 90 minutes apart with one exception where she awoke but did not have a headache63. Several circadian zeitgebers can trigger an extra cluster headache attack, including exercise and temperature49, 64. Alcohol is the most common cluster headache trigger49, 62, and ethanol is known to disrupt multiple aspects of the circadian cycle65. Likewise, an overwhelming number of cluster headache patients smoke66–68, and nicotine has been reported to entrain the circadian clock69. Interestingly, these triggers work only during a headache cycle, and do not trigger a headache in the headache-free cycle, suggesting a circannual pattern of neural sensitivity.
The circadian properties of cluster headache extend beyond behavior, as cluster headache patients demonstrate alterations in key components of the circadian system. Several studies have noted changes in melatonin rhythms in cluster headache patients, including an overall decrease in melatonin levels, abolished daily and nightly rhythms of melatonin, and desynchrony between melatonin and cortisol70–72. In episodic cluster headache patients, these melatonin changes appear to be more pronounced during a headache cycle than during a headache-free cycle71, 73. Cortisol levels are also dysregulated in cluster headache70, 73. Furthermore, there are abnormal levels of vasoactive intestinal peptide (VIP) and arginine vasopressin (AVP) in cluster headache74, 75. VIP and AVP are peptides found in a large proportion of SCN neurons: VIP-positive neurons are primarily found in the core area of the SCN, and AVP-positive neurons are found in the shell area20. Preliminary studies also suggest cluster headache-associated changes in other molecules indirectly modulated by the SCN, including PACAP, orexin, orexin receptors, testosterone, prolactin, and growth hormone76–82.
Although earlier attempts did not identify a molecular association of clock genes with cluster headache83–85, more recent studies elucidated changes in circadian transcription-translation feedback loops. In an association study of large Swedish cohorts, a single nucleotide polymorphism (SNP: rs12649507) in the Clock gene was found to be significantly associated with cluster headache86. Although Clock mRNA expression was similar in primary fibroblasts between healthy controls and cluster headache patients, the disease-associated SNP above was found to increase mRNA expression. In another study of 8 cluster headache patients who responded to lithium as a treatment and were currently or recently in a headache cycle, lymphoblasts were immortalized with Epstein-Barr virus and subjected to microarray analysis. Compared to 10 healthy controls, cluster headache patients showed markedly decreased expression of the core circadian gene Nr1d1 (encoding REV-ERBα, a circadian transcription repressor)87. Interestingly, the most significantly altered gene in cluster headache in this study was RBM3 (encoding RNA binding motif protein 3). RBM3 is a cold-induced RNA binding protein that has previously been shown to regulate alternative polyadenylation and thereby circadian gene expression level and amplitude88. These findings provide initial evidence for alterations in the transcription-translation feedback loops, highlighting potential mechanistic functions of the core clock and regulatory genes Clock, Nr1d1 and RBM3. In summary, cluster headaches appear to be integrated with the circadian system at multiple levels including behavior, neuroanatomy, molecular biomarkers, and transcriptomics.
Migraine
Migraine is a very common headache disorder, as 17.6% of females and 5.7% of males have at least one migraine per year89. It is also the 6th most disabling disease worldwide90. Migraine is characterized by throbbing, moderate-or-severe unilateral pain, lasting 4–72 hours and associated with features such as nausea, vomiting, and sensitivity to light, noise, and motion51. The pain is thought to be related to the same trigeminovascular system discussed above. Similar to cluster headache, migraine is accompanied by an early activation of the hypothalamus: the pain is preceded by 1–2 days of a “premonitory phase” of irritability, yawning, and other features that is thought to originate in the hypothalamus, though the exact location in the hypothalamus is unclear91. Other mechanisms of migraine include the trigeminovascular system (similar to cluster headache) and the pain neuromatrix. In particular, the hypothalamus and the dorsal rostral pons have traditionally been thought of as migraine generators, though more recent evidence suggests a more complicated mechanism92. Thus like cluster headache, migraine patients show early activation of the hypothalamus, which contains the central biological clock.
Migraines are rhythmic at several levels. On a circadian time scale, migraines peak in the morning and midday93, 94. In a recent study, migraine subjects who were “morning larks” (i.e. go to sleep early and wake up early) or “night owls” (i.e. go to sleep late and wake up late) were more likely to have migraines in the morning or in the afternoon/evening, respectively95. Overall there were more migraines in the mornings because migraine patients had a higher likelihood of being morning larks than controls. Other rhythmic features include a weekly cycle (less common on Sundays)96, a monthly cycle tied to menses and estrogen levels93, 97, 98, and even a yearly cycle where migraines are more common in the spring and fall93, 99. Behaviorally, migraines can be triggered by stress, menses, alcohol, weather changes, and skipping meals, as well as possibly bright lights and sleep disturbances100, 101. Many of these triggers are related to circadian zeitgebers, including the stress hormone cortisol, the neuropeptide PACAP involved in light entrainment, and meal timing102, 103. Indeed, eating at a specific period of time (late at night) was associated with reduction in the odds of a headache104. At the molecular level, multiple changes have been noted in melatonin. First, patients have lower levels of melatonin on days with a headache than days when they are headache-free105. Second, when triggered by a light pulse, melatonin suppression is more pronounced in migraine patients than in controls106. Third, in patients with chronic migraine, which is defined as 15 or more days of headache per month for at least 3 months, a variety of nighttime changes in melatonin have been found, including decreased nocturnal melatonin, decreased melatonin in REM sleep, and a delayed nocturnal melatonin peak71, 107.
Genetically, there have been several individual gene mutations linked to migraine. Two families with migraines have been identified with distinct missense mutations in the circadian gene Ck1delta; the affected subjects also display advanced sleep phases13. The latter finding is consistent with previous studies where Ck1delta mutations can lead to familial advanced sleep phase syndrome108. The best known genetic causes of migraine are three forms of familial hemiplegic migraine, which are linked to three separate ion channel mutations109. Interestingly, a mouse model of familial hemiplegic migraine type I has been created, and these mice display signs of an overactive circadian system such as increased wheel-running activity and shifts of in vivo SCN activity110. Migraine displays circadian behavioral and anatomical features, and a direct genetic connection has been identified in a subset of patients.
Hypnic headache
Hypnic headache is a very rare headache disorder, with only a few hundred reported cases in the literature. The symptoms usually begin after the age of 50. Also termed “alarm clock headaches,” these headaches occur only during sleep, last 15–240 minutes, and have no autonomic features or restlessness51. These headaches typically occur between 1–3 am, and in one study over half of patients had headaches between 2–3 am111, which coincides with the peak of cluster headache attacks. Like cluster headache and migraine, alcohol may be a trigger in hypnic headache112. The pathophysiology of hypnic headaches is poorly understood, but it has been postulated that the disease is either a circadian or a REM sleep disorder112. Several studies have suggested that the disorder originates in REM sleep113, 114, though there is evidence that it occurs in other sleep stages as well115, 116. Melatonin levels do not appear to differ in hypnic headache patients compared to healthy controls117. However, medications that alter the circadian cycle have been effective in hypnic headache, such as lithium, melatonin, and caffeine51.
Other headache syndromes
Cluster headache, migraine, and hypnic headache are not the only headache / facial pain syndromes that have connections to the circadian system. Sinusitis has a peak in several symptoms (sneezing, congestion, runny nose) at 6 AM, and choronotherapy of histamine 1 receptor blockers has been proposed118. Patients with temporomandibular disorder show evidence of increased daytime cortisol, altered nocturnal heart rate variability, and possibly daily variation in pain119, 120. Limited circadian data does exist for other disorders such as tension-type headache (altered melatonin levels)71 and burning mouth syndrome (pain worsening in the evening)121, 122.
Chronotherapy for headache disorders
Various behavioral, dietary and environmental strategies have clear circadian effects. Bright light in the morning, meal-timing and melatonin are commonly employed in clinical trials to reset circadian rhythms123. Melatonin and melatonin receptor agonists have also been tested in jetlag, sleep and other neurological disorders33, 124. Melatonin and a number of compounds have also been applied to pain125. For cluster headaches, there are currently nine preventive medications available in the United States that are recommended by either American or European headache guidelines126, 127 (Figure 2), including melatonin and corticosteroids. Several other cluster headache preventive medications also have circadian effects. Lithium, also a commonly used mood stabilizer, inhibits GSK-3β and alters clock protein abundance and oscillation128, 129. The antiepileptic valproate results in phase-shifts of PER2 reporter rhythms, and the GABAB agonist baclofen can reset the SCN in vitro130, 131. Verapamil, a calcium channel blocker commonly used in migraine and cluster headache, can alter circadian rhythm of blood pressure, and is often applied as a chronotherapy132. Other pain medications, including NSAIDs and opioids, can also be applied in chronotherapeutic regimens133. Melatonin and valproate, and perhaps steroids to a certain degree, have also shown efficacies in migraine prevention, whereas lithium and baclofen do not appear to be effective134. In summary, the circadian system appears to play a role in headache at the behavioral, molecular, and treatment levels, and is an enticing area for future study.
Significant efforts have also been dedicated to development of novel clock-modulating small molecules135. In several recent studies, such compounds, either naturally occurring or synthetic, have shown promising efficacies in rodent models for various diseases including metabolic disease, mood disorder, and aging135, 136. For emerging clock-related diseases such as pain, establishment of model systems will allow testing of novel therapeutics. As mentioned above, the critical next step is to identify the key circadian characteristic or factors that may be causal or serve as biomarkers for the disease. For example, while attenuation of circadian oscillation (amplitude reduction) is characteristic of many chronic diseases and aging137, it will be interesting to investigate whether circadian gene oscillation is in fact enhanced in cluster headache. Studies described above have implicated several circadian genes in pain, including Clock and Nr1d1 for cluster headache and Ck1delta for migraine (Figure 2). Endogenous and synthetic ligands for REV-ERBα have been extensively studied138, and several ligands have in fact shown efficacies to modulate brain functions in mouse models136, 139. CK1δ plays an important role in PER protein turnover and multiple screening studies have revealed a wide variety of compounds targeting CK1δ (or its close homolog CK1ε) to alter circadian periodicity140–143. Small molecules targeting other components of the circadian feedback loops have also been described144, 145. Collectively, this small-molecule toolset can be applied to human cells or mouse disease models, with the ultimate goal to evaluate their potential efficacies to mitigate circadian-related symptoms.
Neuropathic pain
Neuropathic pain is pain caused by a lesion or disease of the somatosensory system and is generally a burning, electric, or shooting pain. Neuropathic pain can occur both in the central and the peripheral nervous system: central neuropathic pain includes thalamic strokes and spinal cord injuries, while peripheral neuropathic pain includes painful diabetic peripheral neuropathy, chemotherapy-induced peripheral neuropathy, post-herpetic neuralgia, and trigeminal neuralgia146. Mechanistically, the gate theory of pain was first proposed in the 1960s and considers pain a balance between inhibitory and excitatory inputs as well as between painful and nonpainful sensory inputs147; chronic pain, then, would suggest an imbalance. One of the hallmarks of neuropathic pain is a shift in pain sensitivity, including allodynia and hyperalgesia148. Allodynia is pain from a non-painful stimulus, such as pain when putting on socks in painful diabetic peripheral neuropathy. Hyperalgesia is increased pain from a painful stimulus, such as exquisite pain with a light pinch. When a shift in pain interferes with basic activities like putting on socks, significant disability can occur.
Daily patterns in nerve pain have been well-described. Electrical stimulation of the sural nerve, for example, is most painful in the late evening and early morning149. Multiple studies have reported that pain from diabetic peripheral neuropathy and post-herpetic neuralgia worsen throughout the day and are worst at night150–152. An animal model of neuropathic pain has mirrored these findings. For nocturnal rodents, the hypothesis would be that neuropathic pain in rats would be worse during the day. Indeed, when the sciatic nerve is surgically ligated, rats and mice have increased pain sensitivity during the day153, 154. Pain rhythmicity in other experimental models155, 156 remains to be investigated.
There are multiple proposed mechanisms for the circadian variation of neuropathic pain. Daily variations of mu opioid receptors, β-endorphin, and naloxone response have been reported in rodents149, 157, 158. Circadian fluctuations have also been noted in calcium channels involved in pain, as well as in inflammatory modulators159–161. In the aforementioned studies of sciatic nerve injury, adenovirus-mediated alteration in the NR2B-CREB-CRTC1 signaling pathway was shown to improve pain behavior153, whereas glucocorticoid-induced ATP release from spinal astrocytes aggravates daily hypersensitivity154. Changes in the circadian expression of melatonin receptors in the hypothalamus were observed in rodents after peripheral nerve injuries, suggesting a possible role of the central clock162. Pioneering studies have further provided molecular evidence that core circadian genes may play an important role in pain. For example, CLOCK:BMAL1 was shown to drive circadian transcription of the Tac1 gene which encodes the pain signaling molecule substance P in the dorsal root ganglia, suggesting a direct molecular mechanism linking the circadian oscillator and neuropathic pain163. Furthermore, partial sciatic nerve ligation in mice was found to suppress circadian oscillation of Per genes, and knock-down of Per1 in the spinal cord by intrathecal injection of siRNA induced mechanical hypersensitivity164. In summary, neuropathic pain in general, and diabetic peripheral neuropathy and post-herpetic neuralgia specifically, show circadian alterations concordant with oscillations in receptors, ion channels, and small molecules that modulate nociception.
Future directions and conclusions
Circadian rhythms are clearly important for physiological health. In addition to well-established roles in sleep, mood, metabolism and cardiovascular functions, the role of circadian regulation in other organ systems, systemic processes, and gut microbiota is increasingly appreciated3. The key challenge is to delineate causal events where circadian changes at the molecular level (e.g., transcription-translation feedback loops) can be propagated to physiological and pathophysiological adaptation. Given the circadian rhythmicity in pain, it is imperative to establish experimental systems, including cultured cells (e.g., human fibroblasts) or rodent models, to delineate key circadian components or focal events that can be targeted for therapy. For example, it will be interesting to investigate a potential functional mechanism whereby the Clock gene influences circadian and physiological functions in cluster headache patients. On the one hand, the cluster headache-associated Clock SNP (rs12649507) appears to correlate with increased Clock mRNA expression in a limited set of samples86. On the other hand, whereas genetic ablation of Clock in mice leads to diabetes11, recent evidence from two large studies shows a decreased prevalence of diabetes in cluster headache patients66, 67. Future studies should investigate whether enhanced Clock gene expression may contribute to the precise circadian timing while diminishing diabetes risk in cluster headache.
It will be interesting to integrate headache and circadian models in the laboratory for a better understanding of the interactions between pain and circadian rhythms. Multiple models are currently utilized to further understand the pathways and molecules involved in headache disorders. To study the trigeminovascular pain system that may underlie many headache disorders, a commonly used model involves experimental irritation of the dura in rodents via chemical or electrical stimulation165. For cluster headache in specific, a rodent model with a lesion of the superior salivatory nucleus has yielded important insights into possible autonomic effects of medications such as oxygen gas59. In migraine, two mouse strains have been created as discussed in the migraine section above: the Ck1delta human mutation and the SCN1A mutation in patients with familial hemiplegic migraine type I108, 110. Other animal models have been investigated for temporomandibular disorder such as connective tissue genetic mutations and partial temporomandibular joint discecetomy166. Among circadian knockout mouse models, Per1 and Per2 KO mice have been reported to display altered pain behavior. Specifically, stress-induced antinociception in Per1 KO mice was partially-reversed (mechanical sensitivity) or over-reversed to hyperalgesia (thermal sensitivity) compared with WT mice167. In a morphine-induced tolerance paradigm, Per2 KO mice developed less tolerance and showed attenuated withdrawal compared to WT168.
In conclusion, circadian rhythms permeate many aspects of physiology and pathophysiology. We describe here an emerging link between circadian timing and pain. With a detailed knowledge of circadian mechanisms available, studies of circadian regulation of pain should yield important new insights that can be translated to actionable regimens to alleviate or manage pain symptoms.
Acknowledgments
The authors would like to acknowledge funding from the American Headache Society, National Headache Foundation, and Will Erwin Headache Research Foundation to M.J.B., the Robert A. Welch Foundation (AU-1731) and National Institute on Aging (R01AG045828) to Z.C., and the Robert A. Welch Foundation (AU-1971–20180324) and National Institute of General Medical Sciences (R01GM114424) to S.-H.Y. The authors declare no conflict of interest.
Footnotes
Conflict of interest: we declare no conflict of interest.
References
- 1.Bell-Pedersen D, Cassone VM, Earnest DJ et al. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nature reviews. Genetics 2005;6:544–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 2010;72:517–49. [DOI] [PubMed] [Google Scholar]
- 3.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science 2016;354:994–999. [DOI] [PubMed] [Google Scholar]
- 4.Hermida RC, Ayala DE, Smolensky MH et al. Chronotherapy improves blood pressure control and reduces vascular risk in CKD. Nat Rev Nephrol 2013;9:358–68. [DOI] [PubMed] [Google Scholar]
- 5.Burioka N, Fukuoka Y, Koyanagi S et al. Asthma: Chronopharmacotherapy and the molecular clock. Adv Drug Deliv Rev 2010;62:946–55. [DOI] [PubMed] [Google Scholar]
- 6.Sundar IK, Sellix MT, Rahman I. Redox regulation of circadian molecular clock in chronic airway diseases. Free Radic Biol Med 2018;119:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green CB, Takahashi JS, Bass J. The meter of metabolism. Cell 2008;134:728–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panda S Circadian physiology of metabolism. Science 2016;354:1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harfmann BD, Schroder EA, Esser KA. Circadian rhythms, the molecular clock, and skeletal muscle. J Biol Rhythms 2015;30:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colwell CS. Linking neural activity and molecular oscillations in the SCN. Nat Rev Neurosci 2011;12:553–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marcheva B, Ramsey KM, Buhr ED et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature 2010;466:627–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones CR, Huang AL, Ptacek LJ, Fu YH. Genetic basis of human circadian rhythm disorders. Experimental neurology 2013;243:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brennan KC, Bates EA, Shapiro RE et al. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med 2013;5:183ra56, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell 1998;93:929–37. [DOI] [PubMed] [Google Scholar]
- 15.Welsh DK, Yoo SH, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol 2004;14:2289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017;18:164–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoo SH, Kojima S, Shimomura K et al. Period2 3’-UTR and microRNA-24 regulate circadian rhythms by repressing PERIOD2 protein accumulation. Proc Natl Acad Sci U S A 2017;114:E8855–E8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Millius A, Ueda HR. Systems Biology-Derived Discoveries of Intrinsic Clocks. Front Neurol 2017;8:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo SH, Yamazaki S, Lowrey PL et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 2004;101:5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzog ED, Hermanstyne T, Smyllie NJ, Hastings MH. Regulating the Suprachiasmatic Nucleus (SCN) Circadian Clockwork: Interplay between Cell-Autonomous and Circuit-Level Mechanisms. Cold Spring Harbor perspectives in biology 2017;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990;247:975–8. [DOI] [PubMed] [Google Scholar]
- 22.Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 1972;42:201–6. [DOI] [PubMed] [Google Scholar]
- 23.Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A 1972;69:1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu AC, Welsh DK, Ko CH et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell 2007;129:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Albrecht U Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 2012;74:246–60. [DOI] [PubMed] [Google Scholar]
- 26.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci 2014;15:443–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes S, Jagannath A, Hankins MW, Foster RG, Peirson SN. Photic regulation of clock systems. Methods Enzymol 2015;552:125–43. [DOI] [PubMed] [Google Scholar]
- 28.Edmonds SC. Food and light as entrainers of circadian running activity in the rat. Physiol Behav 1977;18:915–9. [DOI] [PubMed] [Google Scholar]
- 29.Marchant EG, Mistlberger RE. Entrainment and phase shifting of circadian rhythms in mice by forced treadmill running. Physiol Behav 1996;60:657–63. [DOI] [PubMed] [Google Scholar]
- 30.Redman JR. Circadian entrainment and phase shifting in mammals with melatonin. J Biol Rhythms 1997;12:581–7. [DOI] [PubMed] [Google Scholar]
- 31.Horseman ND, Ehret CF. Glucocorticosteroid injection is a circadian zeitgeber in the laboratory rat. Am J Physiol 1982;243:R373–8. [DOI] [PubMed] [Google Scholar]
- 32.Buijs RM, la Fleur SE, Wortel J et al. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. J Comp Neurol 2003;464:36–48. [DOI] [PubMed] [Google Scholar]
- 33.Pevet P, Challet E. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 2011;105:170–82. [DOI] [PubMed] [Google Scholar]
- 34.Balsalobre A, Marcacci L, Schibler U. Multiple signaling pathways elicit circadian gene expression in cultured Rat-1 fibroblasts. Curr Biol 2000;10:1291–4. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proceedings of the National Academy of Sciences of the United States of America 2014;111:16219–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mure LS, Le HD, Benegiamo G et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 2018;359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang X, Downes M, Yu RT et al. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006;126:801–10. [DOI] [PubMed] [Google Scholar]
- 38.Jeyaraj D, Haldar SM, Wan X et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012;483:96–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckle T, Hartmann K, Bonney S et al. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med 2012;18:774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Montaigne D, Marechal X, Modine T et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbalpha antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet 2017. [DOI] [PubMed] [Google Scholar]
- 41.Gilron I, Ghasemlou N. Chronobiology of chronic pain: focus on diurnal rhythmicity of neuropathic pain. Curr Opin Support Palliat Care 2014;8:429–36. [DOI] [PubMed] [Google Scholar]
- 42.Smolensky MH, Portaluppi F, Manfredini R et al. Diurnal and twenty-four hour patterning of human diseases: acute and chronic common and uncommon medical conditions. Sleep Med Rev 2015;21:12–22. [DOI] [PubMed] [Google Scholar]
- 43.Segal JP, Tresidder KA, Bhatt C, Gilron I, Ghasemlou N. Circadian control of pain and neuroinflammation. J Neurosci Res 2018;96:1002–1020. [DOI] [PubMed] [Google Scholar]
- 44.Scott JT. Morning stiffness in rheumatoid arthritis. Ann Rheum Dis 1960;19:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buttgereit F, Smolen JS, Coogan AN, Cajochen C. Clocking in: chronobiology in rheumatoid arthritis. Nat Rev Rheumatol 2015;11:349–56. [DOI] [PubMed] [Google Scholar]
- 46.Fischera M, Marziniak M, Gralow I, Evers S. The incidence and prevalence of cluster headache: a meta-analysis of population-based studies. Cephalalgia 2008;28:614–8. [DOI] [PubMed] [Google Scholar]
- 47.Manzoni GC, Taga A, Russo M, Torelli P. Age of onset of episodic and chronic cluster headache - a review of a large case series from a single headache centre. J Headache Pain 2016;17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broner SW, Cohen JM. Epidemiology of cluster headache. Curr Pain Headache Rep 2009;13:141–6. [DOI] [PubMed] [Google Scholar]
- 49.Rozen TD, Fishman RS. Cluster headache in the United States of America: demographics, clinical characteristics, triggers, suicidality, and personal burden. Headache 2012;52:99–113. [DOI] [PubMed] [Google Scholar]
- 50.Goadsby PJ, Cohen AS, Matharu MS. Trigeminal autonomic cephalalgias: diagnosis and treatment. Curr Neurol Neurosci Rep 2007;7:117–25. [DOI] [PubMed] [Google Scholar]
- 51.Society IH. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 52.May A, Bahra A, Buchel C, Frackowiak RS, Goadsby PJ. Hypothalamic activation in cluster headache attacks. Lancet 1998;352:275–8. [DOI] [PubMed] [Google Scholar]
- 53.Haller J The neurobiology of abnormal manifestations of aggression--a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res Bull 2013;93:97–109. [DOI] [PubMed] [Google Scholar]
- 54.Luerding R, Henkel K, Gaul C et al. Aggressiveness in different presentations of cluster headache: results from a controlled multicentric study. Cephalalgia 2012;32:528–36. [DOI] [PubMed] [Google Scholar]
- 55.Fontaine D, Lazorthes Y, Mertens P et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain 2010;11:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franzini A, Ferroli P, Leone M, Broggi G. Stimulation of the posterior hypothalamus for treatment of chronic intractable cluster headaches: first reported series. Neurosurgery 2003;52:1095–9; discussion 1099–101. [PubMed] [Google Scholar]
- 57.Jurgens TP, Leone M, Proietti-Cecchini A et al. Hypothalamic deep-brain stimulation modulates thermal sensitivity and pain thresholds in cluster headache. Pain 2009;146:84–90. [DOI] [PubMed] [Google Scholar]
- 58.Nappi G, Sandrini G, Alfonsi E et al. Impaired circadian rhythmicity of nociceptive reflex threshold in cluster headache. Headache 2002;42:125–31. [DOI] [PubMed] [Google Scholar]
- 59.Akerman S, Holland PR, Lasalandra MP, Goadsby PJ. Oxygen inhibits neuronal activation in the trigeminocervical complex after stimulation of trigeminal autonomic reflex, but not during direct dural activation of trigeminal afferents. Headache 2009;49:1131–43. [DOI] [PubMed] [Google Scholar]
- 60.Burish MJ, Chen Z, Yoo SH. Cluster Headache Is in Part a Disorder of the Circadian System. JAMA Neurol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barloese M, Lund N, Petersen A et al. Sleep and chronobiology in cluster headache. Cephalalgia 2015;35:969–78. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg A, Fourier C, Ran C et al. Cluster headache - clinical pattern and a new severity scale in a Swedish cohort. Cephalalgia 2017;333102417731773. [DOI] [PubMed] [Google Scholar]
- 63.Barloese MC, Jennum PJ, Lund NT, Jensen RH. Sleep in cluster headache - beyond a temporal rapid eye movement relationship? Eur J Neurol 2015;22:656–e40. [DOI] [PubMed] [Google Scholar]
- 64.Blau JN, Engel HO. A new cluster headache precipitant: increased body heat. Lancet 1999;354:1001–2. [DOI] [PubMed] [Google Scholar]
- 65.Rosenwasser AM. Chronobiology of ethanol: animal models. Alcohol 2015;49:311–9. [DOI] [PubMed] [Google Scholar]
- 66.Choong CK, Ford JH, Nyhuis AW et al. Clinical Characteristics and Treatment Patterns Among Patients Diagnosed With Cluster Headache in U.S. Healthcare Claims Data. Headache 2017;57:1359–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Joshi S, Rizzoli P, Loder E. The comorbidity burden of patients with cluster headache: a population-based study. J Headache Pain 2017;18:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Govare A, Leroux E. Licit and illicit drug use in cluster headache. Curr Pain Headache Rep 2014;18:413. [DOI] [PubMed] [Google Scholar]
- 69.Gillman AG, Leffel JK, Kosobud AE, Timberlake W. Behavioral characteristics and pharmacological manipulations of a nicotine-entrainable circadian oscillator. Chronobiol Int 2013;30:855–69. [DOI] [PubMed] [Google Scholar]
- 70.Chazot G, Claustrat B, Brun J et al. A chronobiological study of melatonin, cortisol growth hormone and prolactin secretion in cluster headache. Cephalalgia 1984;4:213–20. [DOI] [PubMed] [Google Scholar]
- 71.Bruera O, Sances G, Leston J et al. Plasma melatonin pattern in chronic and episodic headaches: evaluation during sleep and waking. Funct Neurol 2008;23:77–81. [PubMed] [Google Scholar]
- 72.Leone M, Lucini V, D’Amico D et al. Abnormal 24-hour urinary excretory pattern of 6-sulphatoxymelatonin in both phases of cluster headache. Cephalalgia 1998;18:664–7. [DOI] [PubMed] [Google Scholar]
- 73.Waldenlind E, Gustafsson SA, Ekbom K, Wetterberg L. Circadian secretion of cortisol and melatonin in cluster headache during active cluster periods and remission. J Neurol Neurosurg Psychiatry 1987;50:207–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain 1994;117 ( Pt 3):427–34. [DOI] [PubMed] [Google Scholar]
- 75.Franceschini R, Leandri M, Cataldi A et al. Raised plasma arginine vasopressin concentrations during cluster headache attacks. J Neurol Neurosurg Psychiatry 1995;59:381–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tuka B, Szabo N, Toth E et al. Release of PACAP-38 in episodic cluster headache patients - an exploratory study. J Headache Pain 2016;17:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barloese M, Jennum P, Lund N et al. Reduced CSF hypocretin-1 levels are associated with cluster headache. Cephalalgia 2015;35:869–76. [DOI] [PubMed] [Google Scholar]
- 78.Rainero I, Gallone S, Valfre W et al. A polymorphism of the hypocretin receptor 2 gene is associated with cluster headache. Neurology 2004;63:1286–8. [DOI] [PubMed] [Google Scholar]
- 79.Waldenlind E, Gustafsson SA. Prolactin in cluster headache: diurnal secretion, response to thyrotropin-releasing hormone, and relation to sex steroids and gonadotropins. Cephalalgia 1987;7:43–54. [DOI] [PubMed] [Google Scholar]
- 80.Lepper A, Frese A, Summ O, Nofer JR, Evers S. Hypothalamic dopaminergic stimulation in cluster headache. Cephalalgia 2013;33:1155–9. [DOI] [PubMed] [Google Scholar]
- 81.Meyer EL, Marcus C, Waldenlind E. Nocturnal secretion of growth hormone, noradrenaline, cortisol and insulin in cluster headache remission. Cephalalgia 2007;27:912–9. [DOI] [PubMed] [Google Scholar]
- 82.Facchinetti F, Nappi G, Cicoli C et al. Reduced testosterone levels in cluster headache: a stress-related phenomenon? Cephalalgia 1986;6:29–34. [DOI] [PubMed] [Google Scholar]
- 83.Ofte HK, Tronvik E, Alstadhaug KB. Lack of association between cluster headache and PER3 clock gene polymorphism. J Headache Pain 2015;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baumber L, Sjostrand C, Leone M et al. A genome-wide scan and HCRTR2 candidate gene analysis in a European cluster headache cohort. Neurology 2006;66:1888–93. [DOI] [PubMed] [Google Scholar]
- 85.Bacchelli E, Cainazzo MM, Cameli C et al. A genome-wide analysis in cluster headache points to neprilysin and PACAP receptor gene variants. J Headache Pain 2016;17:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fourier C, Ran C, Zinnegger M et al. A genetic CLOCK variant associated with cluster headache causing increased mRNA levels. Cephalalgia 2017;333102417698709. [DOI] [PubMed] [Google Scholar]
- 87.Costa M, Squassina A, Piras IS et al. Preliminary Transcriptome Analysis in Lymphoblasts from Cluster Headache and Bipolar Disorder Patients Implicates Dysregulation of Circadian and Serotonergic Genes. J Mol Neurosci 2015;56:688–95. [DOI] [PubMed] [Google Scholar]
- 88.Yoo SH, Mohawk JA, Siepka SM et al. Competing E3 Ubiquitin Ligases Govern Circadian Periodicity by Degradation of CRY in Nucleus and Cytoplasm. Cell 2013;152:1091–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992;267:64–9. [PubMed] [Google Scholar]
- 90.Global Burden of Disease Study C. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goadsby PJ, Holland PR, Martins-Oliveira M et al. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulte LH, May A. Of generators, networks and migraine attacks. Curr Opin Neurol 2017;30:241–245. [DOI] [PubMed] [Google Scholar]
- 93.Fox AW, Davis RL. Migraine chronobiology. Headache 1998;38:436–41. [DOI] [PubMed] [Google Scholar]
- 94.Alstadhaug K, Salvesen R, Bekkelund S. 24-hour distribution of migraine attacks. Headache 2008;48:95–100. [DOI] [PubMed] [Google Scholar]
- 95.van Oosterhout W, van Someren E, Schoonman GG et al. Chronotypes and circadian timing in migraine. Cephalalgia 2017;333102417698953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alstadhaug KB, Salvesen R, Bekkelund S. Weekend migraine. Cephalalgia 2007;27:343–6. [DOI] [PubMed] [Google Scholar]
- 97.Chai NC, Peterlin BL, Calhoun AH. Migraine and estrogen. Curr Opin Neurol 2014;27:315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Borsook D, Erpelding N, Lebel A et al. Sex and the migraine brain. Neurobiol Dis 2014;68:200–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brewerton TD, George MS. A study of the seasonal variation of migraine. Headache 1990;30:511–3. [DOI] [PubMed] [Google Scholar]
- 100.Pavlovic JM, Buse DC, Sollars CM, Haut S, Lipton RB. Trigger factors and premonitory features of migraine attacks: summary of studies. Headache 2014;54:1670–9. [DOI] [PubMed] [Google Scholar]
- 101.Ong JC, Taylor HL, Park M et al. Can Circadian Dysregulation Exacerbate Migraines? Headache 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Peris F, Donoghue S, Torres F, Mian A, Wober C. Towards improved migraine management: Determining potential trigger factors in individual patients. Cephalalgia 2017;37:452–463. [DOI] [PubMed] [Google Scholar]
- 103.Amin FM, Hougaard A, Schytz HW et al. Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 2014;137:779–94. [DOI] [PubMed] [Google Scholar]
- 104.Turner DP, Smitherman TA, Penzien DB et al. Nighttime snacking, stress, and migraine activity. J Clin Neurosci 2014;21:638–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Masruha MR, de Souza Vieira DS, Minett TS et al. Low urinary 6-sulphatoxymelatonin concentrations in acute migraine. J Headache Pain 2008;9:221–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Claustrat B, Brun J, Chiquet C, Chazot G, Borson-Chazot F. Melatonin secretion is supersensitive to light in migraine. Cephalalgia 2004;24:128–33. [DOI] [PubMed] [Google Scholar]
- 107.Peres MF, Sanchez del Rio M, Seabra ML et al. Hypothalamic involvement in chronic migraine. J Neurol Neurosurg Psychiatry 2001;71:747–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xu Y, Padiath QS, Shapiro RE et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature 2005;434:640–4. [DOI] [PubMed] [Google Scholar]
- 109.Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol 2011;10:457–70. [DOI] [PubMed] [Google Scholar]
- 110.van Oosterhout F, Michel S, Deboer T et al. Enhanced circadian phase resetting in R192Q Cav2.1 calcium channel migraine mice. Ann Neurol 2008;64:315–24. [DOI] [PubMed] [Google Scholar]
- 111.Dodick DW, Mosek AC, Campbell JK. The hypnic (“alarm clock”) headache syndrome. Cephalalgia 1998;18:152–6. [DOI] [PubMed] [Google Scholar]
- 112.Evers S, Goadsby PJ. Hypnic headache: clinical features, pathophysiology, and treatment. Neurology 2003;60:905–9. [DOI] [PubMed] [Google Scholar]
- 113.Evers S, Rahmann A, Schwaag S, Ludemann P, Husstedt IW. Hypnic headache - the first German cases including polysomnography. Cephalalgia 2003;23:20–3. [DOI] [PubMed] [Google Scholar]
- 114.Capuano A, Vollono C, Rubino M et al. Hypnic headache: actigraphic and polysomnographic study of a case. Cephalalgia 2005;25:466–9. [DOI] [PubMed] [Google Scholar]
- 115.Dolso P, Merlino G, Fratticci L et al. Non-REM hypnic headache: a circadian disorder? A clinical and polysomnographic study. Cephalalgia 2007;27:83–6. [DOI] [PubMed] [Google Scholar]
- 116.Manni R, Sances G, Terzaghi M, Ghiotto N, Nappi G. Hypnic headache: PSG evidence of both REM- and NREM-related attacks. Neurology 2004;62:1411–3. [DOI] [PubMed] [Google Scholar]
- 117.Naegel S, Huhn JI, Gaul C et al. No Pattern Alteration in Single Nocturnal Melatonin Secretion in Patients With Hypnic Headache: A Case-Control Study. Headache 2017;57:648–653. [DOI] [PubMed] [Google Scholar]
- 118.Smolensky MH, Lemmer B, Reinberg AE. Chronobiology and chronotherapy of allergic rhinitis and bronchial asthma. Adv Drug Deliv Rev 2007;59:852–82. [DOI] [PubMed] [Google Scholar]
- 119.Eze-Nliam CM, Quartana PJ, Quain AM, Smith MT. Nocturnal heart rate variability is lower in temporomandibular disorder patients than in healthy, pain-free individuals. J Orofac Pain 2011;25:232–9. [PubMed] [Google Scholar]
- 120.Glaros AG, Williams K, Lausten L. Diurnal variation in pain reports in temporomandibular disorder patients and control subjects. J Orofac Pain 2008;22:115–21. [PubMed] [Google Scholar]
- 121.Lopez-Jornet P, Molino Pagan D, Andujar Mateos P, Rodriguez Agudo C, Pons-Fuster A. Circadian rhythms variation of pain in burning mouth syndrome. Geriatr Gerontol Int 2015;15:490–5. [DOI] [PubMed] [Google Scholar]
- 122.Forssell H, Teerijoki-Oksa T, Kotiranta U et al. Pain and pain behavior in burning mouth syndrome: a pain diary study. J Orofac Pain 2012;26:117–25. [PubMed] [Google Scholar]
- 123.Chen Z What’s next for chronobiology and drug discovery. Expert Opin Drug Discov 2017;12:1181–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nohara K, Yoo SH, Chen ZJ. Manipulating the circadian and sleep cycles to protect against metabolic disease. Frontiers in endocrinology 2015;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gelfand AA, Goadsby PJ. The Role of Melatonin in the Treatment of Primary Headache Disorders. Headache 2016;56:1257–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.May A, Leone M, Afra J et al. EFNS guidelines on the treatment of cluster headache and other trigeminal-autonomic cephalalgias. Eur J Neurol 2006;13:1066–77. [DOI] [PubMed] [Google Scholar]
- 127.Robbins MS, Starling AJ, Pringsheim TM, Becker WJ, Schwedt TJ. Treatment of Cluster Headache: The American Headache Society Evidence-Based Guidelines. Headache 2016;56:1093–106. [DOI] [PubMed] [Google Scholar]
- 128.Yin L, Wang J, Klein PS, Lazar MA. Nuclear receptor Rev-erbalpha is a critical lithium-sensitive component of the circadian clock. Science 2006;311:1002–5. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Lu WQ, Beesley S, Loudon AS, Meng QJ. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PloS one 2012;7:e33292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Biggs KR, Prosser RA. GABAB receptor stimulation phase-shifts the mammalian circadian clock in vitro. Brain Res 1998;807:250–4. [DOI] [PubMed] [Google Scholar]
- 131.Johansson AS, Brask J, Owe-Larsson B, Hetta J, Lundkvist GB. Valproic acid phase shifts the rhythmic expression of Period2::Luciferase. J Biol Rhythms 2011;26:541–51. [DOI] [PubMed] [Google Scholar]
- 132.Glasser SP. Circadian variations and chronotherapeutic implications for cardiovascular management: a focus on COER verapamil. Heart Dis 1999;1:226–32. [PubMed] [Google Scholar]
- 133.Bruguerolle B, Labrecque G. Rhythmic pattern in pain and their chronotherapy. Adv Drug Deliv Rev 2007;59:883–95. [DOI] [PubMed] [Google Scholar]
- 134.Silberstein SD, Holland S, Freitag F et al. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78:1337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Z, Yoo SH, Takahashi JS. Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu Rev Pharmacol Toxicol 2018;58:231–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Banerjee S, Wang Y, Solt LA et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat Commun 2014;5:5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gloston GF, Yoo SH, Chen ZJ. Clock-Enhancing Small Molecules and Potential Applications in Chronic Diseases and Aging. Front Neurol 2017;8:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kojetin DJ, Burris TP. REV-ERB and ROR nuclear receptors as drug targets. Nature reviews. Drug discovery 2014;13:197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chung S, Lee EJ, Yun S et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 2014;157:858–68. [DOI] [PubMed] [Google Scholar]
- 140.Hirota T, Lee JW, Lewis WG et al. High-throughput chemical screen identifies a novel potent modulator of cellular circadian rhythms and reveals CKIalpha as a clock regulatory kinase. PLoS Biol 2010;8:e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Isojima Y, Nakajima M, Ukai H et al. CKIepsilon/delta-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci U S A 2009;106:15744–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chen Z, Yoo SH, Park YS et al. Identification of diverse modulators of central and peripheral circadian clocks by high-throughput chemical screening. Proc Natl Acad Sci U S A 2012;109:101–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chen Z, Yoo SH, Takahashi JS. Small molecule modifiers of circadian clocks. Cellular and molecular life sciences : CMLS 2013;70:2985–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.He B, Chen Z. Molecular Targets for Small-Molecule Modulators of Circadian Clocks. Current drug metabolism 2016;17:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.He B, Nohara K, Park N et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell metabolism 2016;23:610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol 2010;9:807–19. [DOI] [PubMed] [Google Scholar]
- 147.Melzack R, Wall PD. Pain mechanisms: a new theory. Science 1965;150:971–9. [DOI] [PubMed] [Google Scholar]
- 148.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet 1999;353:1959–64. [DOI] [PubMed] [Google Scholar]
- 149.Labrecque G, Vanier MC. Biological rhythms in pain and in the effects of opioid analgesics. Pharmacol Ther 1995;68:129–47. [DOI] [PubMed] [Google Scholar]
- 150.Galer BS, Gianas A, Jensen MP. Painful diabetic polyneuropathy: epidemiology, pain description, and quality of life. Diabetes Res Clin Pract 2000;47:123–8. [DOI] [PubMed] [Google Scholar]
- 151.Odrcich M, Bailey JM, Cahill CM, Gilron I. Chronobiological characteristics of painful diabetic neuropathy and postherpetic neuralgia: diurnal pain variation and effects of analgesic therapy. Pain 2006;120:207–12. [DOI] [PubMed] [Google Scholar]
- 152.Gilron I, Bailey JM, Vandenkerkhof EG. Chronobiological characteristics of neuropathic pain: clinical predictors of diurnal pain rhythmicity. Clin J Pain 2013;29:755–9. [DOI] [PubMed] [Google Scholar]
- 153.Xia T, Cui Y, Qian Y et al. Regulation of the NR2B-CREB-CRTC1 Signaling Pathway Contributes to Circadian Pain in Murine Model of Chronic Constriction Injury. Anesth Analg 2016;122:542–52. [DOI] [PubMed] [Google Scholar]
- 154.Koyanagi S, Kusunose N, Taniguchi M et al. Glucocorticoid regulation of ATP release from spinal astrocytes underlies diurnal exacerbation of neuropathic mechanical allodynia. Nat Commun 2016;7:13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yowtak J, Wang J, Kim HY et al. Effect of antioxidant treatment on spinal GABA neurons in a neuropathic pain model in the mouse. Pain 2013;154:2469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim HK, Hwang SH, Abdi S. Tempol Ameliorates and Prevents Mechanical Hyperalgesia in a Rat Model of Chemotherapy-Induced Neuropathic Pain. Front Pharmacol 2016;7:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Takada T, Yamashita A, Date A et al. Changes in the circadian rhythm of mRNA expression for micro-opioid receptors in the periaqueductal gray under a neuropathic pain-like state. Synapse 2013;67:216–23. [DOI] [PubMed] [Google Scholar]
- 158.Frederickson RC, Burgis V, Edwards JD. Hyperalgesia induced by naloxone follows diurnal rhythm in responsivity to painful stimuli. Science 1977;198:756–8. [DOI] [PubMed] [Google Scholar]
- 159.Kusunose N, Koyanagi S, Hamamura K et al. Molecular basis for the dosing time-dependency of anti-allodynic effects of gabapentin in a mouse model of neuropathic pain. Mol Pain 2010;6:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nakao A Temporal regulation of cytokines by the circadian clock. J Immunol Res 2014;2014:614529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cermakian N, Lange T, Golombek D et al. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int 2013;30:870–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Odo M, Koh K, Takada T et al. Changes in circadian rhythm for mRNA expression of melatonin 1A and 1B receptors in the hypothalamus under a neuropathic pain-like state. Synapse 2014;68:153–8. [DOI] [PubMed] [Google Scholar]
- 163.Zhang J, Li H, Teng H et al. Regulation of peripheral clock to oscillation of substance P contributes to circadian inflammatory pain. Anesthesiology 2012;117:149–60. [DOI] [PubMed] [Google Scholar]
- 164.Morioka N, Saeki M, Sugimoto T et al. Downregulation of the spinal dorsal horn clock gene Per1 expression leads to mechanical hypersensitivity via c-jun N-terminal kinase and CCL2 production in mice. Mol Cell Neurosci 2016;72:72–83. [DOI] [PubMed] [Google Scholar]
- 165.Munro G, Jansen-Olesen I, Olesen J. Animal models of pain and migraine in drug discovery. Drug Discov Today 2017;22:1103–1111. [DOI] [PubMed] [Google Scholar]
- 166.Ghassemi Nejad S, Kobezda T, Tar I, Szekanecz Z. Development of temporomandibular joint arthritis: The use of animal models. Joint Bone Spine 2017;84:145–151. [DOI] [PubMed] [Google Scholar]
- 167.Zhang J, Wu Z, Zhou L et al. Deficiency of antinociception and excessive grooming induced by acute immobilization stress in Per1 mutant mice. PLoS One 2011;6:e16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Perreau-Lenz S, Sanchis-Segura C, Leonardi-Essmann F, Schneider M, Spanagel R. Development of morphine-induced tolerance and withdrawal: involvement of the clock gene mPer2. Eur Neuropsychopharmacol 2010;20:509–17. [DOI] [PubMed] [Google Scholar]