Abstract
Microvascular rarefaction is found in experimental uremia, but data from patients with chronic kidney disease (CKD) are limited. We therefore quantified absolute myocardial blood flow and coronary flow reserve (the ratio of peak to resting flow) from myocardial perfusion positron emission tomography scans at a single institution. Individuals were classified into standard CKD categories based on the estimated glomerular filtration rate. Associations of coronary flow reserve with CKD stage and cardiovascular mortality were analyzed in models adjusted for cardiovascular risk factors. The coronary flow reserve was significantly associated with CKD stage, declining in early CKD, but it did not differ significantly among individuals with stage 4, 5, and dialysis-dependent CKD. Flow reserve with preserved kidney function was 2.01, 2.06 in stage 1 CKD, 1.91 in stage 2, 1.68 in stage 3, 1.54 in stage 4, 1.66 in stage 5, and 1.55 in dialysis-dependent CKD. Coronary flow reserve was significantly associated with cardiovascular mortality in adjusted models (hazard ratio 0.76, 95% confidence interval: 0.63–0.92 per tertile of coronary flow reserve) without evidence of effect modification by CKD. Thus, coronary flow reserve is strongly associated with cardiovascular risk regardless of CKD severity and is low in early stage CKD without further decrement in stage 5 or dialysis-dependent CKD. This suggests that CKD physiology rather than the effects of dialysis is the primary driver of microvascular disease. Our findings highlight the potential contribution of microvascular dysfunction to cardiovascular risk in CKD and the need to define mechanisms linking low coronary flow reserve to mortality.
Keywords: cardiovascular disease, chronic kidney disease, end-stage renal disease
Cardiovascular disease (CVD) is highly prevalent in patients who have chronic kidney disease (CKD).1–3 Although the incidence of most CVD manifestations increases as glomerular filtration rate (GFR) declines, associations of CKD with sudden death from CV causes are particularly striking,1,2,4 and sudden death, not myocardial infarction, actually accounts for most deaths in patients in the most-advanced stages of CKD.
The exaggerated risk of sudden death compared with myocardial infarction in CKD suggests that non-atherosclerotic manifestations of CKD, including rarefaction of the myocardial microvasculature and/or microvascular dysfunction, may induce relative or absolute myocardial hypoxia in the absence of obstructive epicardial atherosclerosis or coronary thrombosis and may lower the threshold for arrhythmia propagation. Experimental studies have consistently demonstrated that ischemia-driven angiogenesis and myocardial capillary supply is reduced in uremic animals,5,6 and results from several small studies utilizing postmortem samples are consistent with a reduction in left ventricular capillary supply in moderate CKD and end-stage renal disease.7,8
Although these data suggest that the myocardial micro-vasculature may contribute to the increased risk of death from CV causes in CKD, characterizing the relationship between CKD and the myocardial microvasculature more broadly has been hampered by the impracticability of obtaining myocardial tissue from large numbers of individuals with and without CKD. However, microvascular function can be noninvasively assessed by measuring coronary flow reserve (CFR)—the ratio of peak to basal myocardial blood flow—using positron emission tomography (PET). We and others have shown that CFR is strongly and independently associated with death from CV causes, in a wide range of patient populations, and improves risk stratification, even after accounting for the presence of obstructive atherosclerosis.9–11 Similar associations between CV events and CFR are present in the setting of CKD, and CFR appears to be a strong predictor of CV risk in both moderate CKD and dialysis-dependent CKD.12,13 We have demonstrated that mildly reduced kidney function is associated with accelerated decline in CFR over time, in a small study of individuals without diabetes.14 Although these studies suggest that low CFR and microvascular dysfunction may play a role in the pathogenesis of uremic CVD, the broader association of kidney function with CFR remains poorly characterized.
Our objective in this study was to characterize myocardial microvascular function by analyzing the association between kidney function and CFR across the full spectrum of CKD. We hypothesized that CFR would be reduced in CKD and that low CFR would be associated with worse CV outcomes in CKD.
RESULTS
Baseline characteristics
In all, 3946 individuals underwent myocardial perfusion PET scanning during the study period and had sufficient information for calculation of estimated GFR (eGFR). A total of 198 (5.6%) individuals had preserved kidney function, 602 (17.2%) had stage 1 CKD, 1292 (37.0%) had stage 2 CKD, 901 (25.8%) had stage 3 CKD, 216 (6.2%) had stage 4 CKD, 112 (3.2%) had non–dialysis-dependent stage 5 CKD, and 175 (5.0%) had stage 5 CKD and were on dialysis (Table 1). Baseline characteristics differed by CKD stage. In general, increasing severity of CKD was associated with increasing comorbidity, including older age, hyper-tension, diabetes, prior myocardial infarction, heart failure, and prior revascularization (Table 1).
Table 1 |.
Baseline characteristics according to CKD stage
| Characteristic | Preserved kidney function (n = 198) |
Stage 1 CKD (n = 602) |
Stage 2 CKD (n = 1292) |
Stage 3 CKD (n = 901) |
Stage 4 CKD (n = 216) |
Stage 5 CKD (n = 112) |
Dialysis (n = 175) |
P value |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age, yr | 49.5 ± 9.8 | 58.5 ± 10.2 | 69.7 ± 14.0 | 77.7 ± 13.7 | 78.6 ± 15.8 | 69.7 ± 17.8 | 64.4 ± 19.8 | <0.001 |
| Male | 67 (33.8) | 275 (45.7) | 651 (50.4) | 473 (52.5) | 123 (56.9) | 63 (56.3) | 96 (57.5) | <0.001 |
| Race | <0.001 | |||||||
| Black | 71 (35.9) | 121 (20.1) | 184 (14.2) | 117 (13.0) | 35 (16.2) | 27 (24.1) | 72 (43.4) | |
| White | 38 (19.2) | 374 (62.1) | 961 (74.4) | 709 (78.7) | 169 (78.2) | 68 (60.7) | 69 (41.6) | |
| Other | 89 (44.9) | 107 (17.8) | 147 (11.4) | 75 (8.3) | 12 (5.6) | 17(15.2) | 25 (15.1) | |
| Medical history | ||||||||
| Hypertension | 136 (68.7) | 444 (73.8) | 1050 (81.3) | 818 (90.8) | 200 (92.6) | 105 (93.8) | 155 (88.6) | <0.001 |
| Diabetes | 57 (28.8) | 165 (27.4) | 354 (27.4) | 331 (36.7) | 105 (48.6) | 51 (45.5) | 88 (50.3) | <0.001 |
| Myocardial infarction | 31 (15.7) | 140 (23.3) | 351 (27.2) | 313 (34.7) | 90 (41.7) | 28 (25.0) | 50 (28.6) | <0.001 |
| Heart failure | 5 (2.5) | 16 (2.7) | 69 (5.3) | 83 (9.2) | 34 (15.7) | 8 (7.1) | 15 (8.6) | <0.001 |
| PVD | 5 (2.5) | 18 (3.0) | 72 (5.6) | 87 (9.7) | 22 (10.2) | 4 (3.6) | 25 (14.3) | <0.001 |
| COPD | 27 (13.6) | 57 (9.5) | 141 (10.9) | 109 (12.1) | 26 (12.0) | 8 (7.1) | 14 (8.0) | 0.27 |
| Hyperlipidemia | 90 (45.5) | 393 (65.3) | 891 (69.0) | 682 (75.7) | 149 (69.0) | 78 (69.6) | 101 (57.7) | 0.003 |
| Prior CABG | 4 (2.0) | 44 (7.3) | 177 (13.7) | 178 (19.8) | 50 (23.1) | 15 (13.4) | 19 (10.9) | <0.001 |
| Prior PCI | 17 (8.6) | 127 (21.1) | 292 (22.6) | 244 (27.1) | 50 (23.1) | 14 (12.5) | 33 (18.9) | <0.001 |
| Physical examination | ||||||||
| Rest HR, bpm | 73.8 ± 14.1 | 71.3 ± 13.1 | 69.9 ± 12.9 | 70.3 ± 12.9 | 71.1 ± 12.4 | 73.2 ± 13.3 | 79.4 ± 60.5 | <0.001 |
| Rest SBP, mm Hg | 141.1 ± 26.3 | 142.2 ± 24.2 | 145.4 ± 25.3 | 145.9 ± 26.8 | 147.1 ± 28.2 | 157.1 ± 26.2 | 151.4 ± 31.2 | <0.001 |
| Rest DBP, mm Hg | 74.4 ± 13.4 | 73.4 ± 12.1 | 72.8 ± 12.4 | 71.4 ± 13.4 | 71.9 ± 14.2 | 77.0 ± 15.1 | 74.8 ± 14.7 | <0.001 |
| BMI, kg/m2 | 33.1 ± 10.0 | 31.6 ± 8.46 | 30.2 ± 7.5 | 29.8 ± 7.3 | 29.1 ± 7.1 | 29.1 ± 6.7 | 27.7 ± 6.5 | <0.001 |
| Medications | ||||||||
| ACE or ARB | 60 (30.3) | 212 (35.2) | 540 (41.8) | 388 (43.1) | 54 (25.0) | 39 (34.8) | 62 (35.4) | <0.001 |
| Aspirin | 95 (48.0) | 358 (59.5) | 854 (66.1) | 610 (67.7) | 148 (68.5) | 55 (49.1) | 95 (54.3) | <0.001 |
| Beta blockers | 89 (44.9) | 333 (55.3) | 809 (62.6) | 658 (73.1) | 163 (75.5) | 81 (72.3) | 127 (72.6) | <0.001 |
| Calcium channel blocker | 26 (13.1) | 100 (16.6) | 291 (22.5) | 258 (28.6) | 76 (35.2) | 50 (44.6) | 68 (38.9) | <0.001 |
| Lipid-lowering | 80 (40.4) | 337 (56.0) | 826 (63.9) | 658 (73.0) | 157 (72.7) | 72 (64.3) | 110 (62.9) | <0.001 |
| Digoxin | 2 (1.0) | 13 (2.2) | 47 (3.6) | 61 (6.8) | 7 (3.2) | 1 (0.9) | 1 (0.6) | <0.001 |
| Insulin | 24 (12.1) | 78 (13.0) | 178 (13.8) | 175 (19.4) | 63 (29.2) | 35 (31.3) | 55 (31.4) | <0.001 |
| Oral hypoglycemics | 29 (14.6) | 74 (12.3) | 142 (11.0) | 98 (10.9) | 15 (6.9) | 5 (4.5) | 6 (3.4) | 0.001 |
| Nitrates | 18 (9.1) | 67 (11.1) | 160 (12.4) | 163 (18.1) | 51 (23.6) | 18 (16.1) | 18 (10.3) | <0.001 |
Baseline characteristics of the study population. Values are n (%) or mean ± SD. Categorical P values using analysis of variance for continuous variables, and χ2 tests for categorical values.
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; bpm, beats per minute; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; HR, heart rate; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SBP, systolic blood pressure.
Aside from serum creatinine and calcium, which were universally available, other laboratory parameters were often missing (Table 2). Among individuals with available values, those with more severe CKD had lower low-density lipoprotein cholesterol and hemoglobin concentrations and higher parathyroid hormone and phosphorous concentrations.
Table 2 |.
Laboratory values according to CKD stage
| Laboratory value | Preserved kidney function (n = 198) |
Stage 1 CKD (n = 602) |
Stage 2 CKD (n = 1292) |
Stage 3 CKD (n = 901) |
Stage 4 CKD (n = 216) |
Stage 5 CKD (n = 122) |
Dialysis (n = 175) |
P valuea |
|---|---|---|---|---|---|---|---|---|
| Creatinine, mg/dl | 0.67 ± 0.14 | 0.77 ± 0.13 | 0.94 ± 0.16 | 1.35 ± 0.29 | 2.44 ± 0.61 | 5.83 ± 2.46 | 6.96 ± 2.98 | <0.001 |
| N | 198 | 602 | 1292 | 901 | 216 | 112 | 175 | |
| eGFR,b ml/min/ | 119.13 ± 7.83 | 98.38 ± 5.43 | 75.29 ± 8.59 | 46.84 ± 8.22 | 23.49 ± 4.40 | 9.82 ± 3.12 | 0.50 ± 0.00 | <0.001 |
| 1.73m2 | ||||||||
| N | 198 | 602 | 1292 | 901 | 216 | 112 | 175 | |
| Calcium, mg/dl | 9.01 ± 0.47 | 9.05 ± 0.52 | 9.11 ± 0.49 | 9.10 ± 0.51 | 9.03 ± 0.53 | 9.00 ± 0.71 | 9.15 ± 0.80 | 0.01 |
| N | 194 | 588 | 1269 | 895 | 216 | 112 | 174 | |
| HbA1C, % | 7.19 ± 1.95 | 7.03 ± 1.56 | 6.73 ± 1.51 | 6.96 ± 1.54 | 7.16 ± 1.45 | 6.74 ± 1.40 | 6.59 ± 1.47 | 0.01 |
| N | 72 | 255 | 571 | 438 | 114 | 48 | 79 | |
| Hemoglobin, g/dl | 12.41 ± 1.81 | 12.64 ± 1.67 | 12.65 ± 1.71 | 11.87 ± 1.70 | 10.83 ± 1.66 | 10.55 ± 1.43 | 11.05 ± 1.70 | <0.001 |
| N | 189 | 572 | 1232 | 874 | 214 | 111 | 171 | |
| LDL cholesterol, mg/dl | 102.13 ± 32.19 | 115.36 ± 47.45 | 93.10 ± 33.66 | 87.52 ± 39.08 | 88.75 ± 52.20 | 95.76 ± 47.74 | 83.93 ± 38.08 | 0.01 |
| N | 19 | 46 | 93 | 65 | 22 | 45 | 26 | |
| Phosphorous, md/dl | 3.25 ± 0.65 | 3.15 ± 0.62 | 3.23 ± 0.60 | 3.35 ± 0.69 | 3.86 ± 0.86 | 4.71 ± 1.07 | 4.76 ± 1.43 | <0.001 |
| N | 67 | 236 | 485 | 436 | 152 | 103 | 147 | |
| PTH, pg/ml | 67.42 ± 34.57 | 57.91 ± 49.48 | 57.90 ± 26.18 | 106.95 ± 97.93 | 172.22 ± 181.45 | 280.03 ± 241.94 | 410.96 ± 452.50 | <0.001 |
| N | 3 | 9 | 28 | 52 | 60 | 91 | 138 |
CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HbA1C, hemoglobin A1C; LDL, low-density lipoprotein; PTH, parathyroid hormone.
Categorical P value using analysis of variance. Tests for trends were performed using Cuzick nonparametric test for trend.
For eGFR, GFR was estimated as 0.5 ml/min per 1.72 m2 for individuals on chronic dialysis.
Myocardial perfusion
As shown in Table 3, compared with those who had preserved kidney function, resting myocardial blood flow (MBF) was significantly lower in individuals who had stage 3 CKD, and higher among those with stage 5 CKD and those on dialysis. In contrast, coronary vascular resistance (P = 0.07) did not differ across CKD stages. Both peak MBF (P < 0.001) and CFR (P < 0.001) declined at higher CKD stages. Although peak MBF and CFR values were lower among those who had stage 3 or higher CKD, compared with individuals with preserved kidney function, CFR in individuals on dialysis did not differ significantly from CFR in those who had stage 3 CKD. Similarly, corrected CFR reached its nadir in stage 4 CKD, at 1.54 ± 0.77, without evidence of a further decline in stage 5 CKD (1.66 ± 0.50) or dialysis-dependent CKD (1.55 ± 0.54). Differences in CFR were similarly apparent in the subset of individuals who did not reveal regional perfusion defects on PET imaging, with values that were 21%–28% lower with stages 4 and 5, and those who had dialysis-dependent CKD, compared with individuals with preserved kidney function. Although CFR was significantly lower among patients with stage 5 CKD who were not on dialysis than among those with stage 3 CKD, values were not significantly lower for those on dialysis or those with stage 4 CKD compared with those who had stage 3 CKD (Table 4).
Table 3 |.
Myocardial perfusion PET results, according to CKD stage
| Imaging parameter | Preserved kidney function (n = 198) |
Stage 1 CKD (n = 602) |
Stage 2 CKD (n = 1292) |
Stage 3 CKD (n = 901) |
Stage 4 CKD (n = 216) |
Stage 5 CKD (n = 112) |
Dialysis (n = 175) |
P valuea |
|---|---|---|---|---|---|---|---|---|
| Peak myocardial blood flow, ml/min/g | 2.27 ± 1.07b | 2.17 ± 0.99b,c | 1.97 ± 0.91b,c | 1.69 ± 0.77c | 1.50 ± 0.72c | 1.70 ± 0.73c | 1.82 ± 0.82c | <0.001 |
| Resting blood flow, ml/min/g | 1.17 ± 0.53b | 1.09 ± 0.44 | 1.07 ± 0.47 | 1.03 ± 0.40c | 1.05 ± 0.38 | 1.17 ± 0.45b | 1.20 ± 0.47b | <0.001 |
| CFR | 2.01 ± 0.67b | 2.06 ± 0.74b | 1.91 ± 0.72b | 1.68 ± 0.59c | 1.47 ± 0.62b,c | 1.47 ± 0.42b,c | 1.55 ± 0.54c | <0.001 |
| Corrected CFR | 2.10 ± 0.91b | 2.06 ± 0.85b | 1.92 ± 0.85b | 1.71 ± 0.69c | 1.54 ± 0.77c | 1.66 ± 0.50c | 1.89 ± 2.13 | <0.001 |
| CVR, mm Hg/ml/min/g | 99.91 ± 52.11 | 102.35 ± 43.65 | 105.06 ± 44.44 | 105.67 ± 42.31 | 108.78 ± 98.49 | 99.29 ± 36.08 | 95.59 ± 44.89 | 0.07 |
| Agatston score, median | 0.0 (0.0, 6.0) | 1.0 (0.0, 125.0) | 58.0, (0.0, 389.0) | 249.5 (20.0, 984.0) | 274.0 (35.0, 1134.0) | 165.0 (0.0, 707.0) | 96.0 (0.0, 999.0) | <0.001 |
| Ejection fraction, % | 59.98 ± 10.46b | 58.93 ± 12.58b | 56.01 ± 13.91b,c | 52.63 ± 15.70c | 47.35 ± 14.74b,c | 50.82 ± 12.62c | 50.35 ± 14.27c | <0.001 |
| LV mass, g | 137.77 ± 26.89 | 136.70 ± 33.61 | 137.64 ± 37.74 | 142.63 ± 41.48 | 159.85 ± 44.97b,c | 161.28 ± 44.10b,c | 158.37 ± 39.50b,c | <0.001 |
| Ischemic percentage, % | 2.20 ± 5.88b | 3.65 ± 7.18b | 4.07 ± 7.24b,c | 5.43 ± 8.42c | 5.59 ± 8.13c | 4.70 ± 6.85 | 4.35 ± 8.22 | <0.001 |
| Summed difference score | 1.50 ± 4.00b | 2.48 ± 4.88b | 2.77 ± 4.92b,c | 3.69 ± 5.73c | 3.80 ± 5.53c | 3.20 ± 4.66 | 2.96 ± 5.59 | <0.001 |
| Summed rest score | 1.14 ± 3.46b | 1.69 ± 4.59b | 2.82 ± 6.36b,c | 3.67 ± 7.05c | 5.25 ± 8.03b,c | 2.40 ± 6.39 | 2.30 ± 5.05 | <0.001 |
| Summed stress score | 2.64 ± 6.06b | 4.17 ± 7.42b | 5.59 ± 8.69b,c | 7.37 ± 9.68c | 9.05 ± 10.39c | 5.60 ± 8.58 | 5.26 ± 8.51 | <0.001 |
CFR, coronary flow reserve; CKD, chronic kidney disease; CVR, coronary vascular resistance; LV, left ventricular; min, minute; PET, positron emission tomography.
Categorical P value using analysis of variance. Tests for trends were performed using Cuzick nonparametric test for trend.
P < 0.05 versus stage 3 CKD.
P < 0.05 versus preserved kidney function.
Table 4 |.
Myocardial perfusion PET results according to CKD stage, among those without a perfusion defect
| Imaging parameter | Preserved kidney function (n = 146) |
Stage 1 CKD (n = 385) |
Stage 2 CKD (n = 736) |
Stage 3 CKD (n = 388) |
Stage 4 CKD (n = 84) |
Stage 5 CKD (n = 56) |
Dialysis (n = 97) | P valuea |
|---|---|---|---|---|---|---|---|---|
| Peak myocardial blood flow, ml/min/g |
2.33 ± 1.09b | 2.41 ± 1.00b | 2.25 ± 0.92b | 2.01 ± 0.77c | 1.86 ± 0.81c | 1.93 ± 0.75c | 2.02 ± 0.82 | <0.001 |
| Resting blood flow, ml/min/g |
1.17 ± 0.52 | 1.16 ± 0.46 | 1.14 ± 0.47 | 1.12 ± 0.42 | 1.16 ± 0.40 | 1.27 ± 0.50 | 1.23 ± 0.49 | 0.19 |
| CFR | 2.06 ± 0.64 | 2.18 ± 0.77b | 2.07 ± 0.75b | 1.88 ± 0.62 | 1.69 ± 0.76c | 1.56 ± 0.38b,c | 1.71 ± 0.58c | <0.001 |
| Corrected CFR | 2.16 ± 0.89 | 2.22 ± 0.91b | 2.12 ± 0.90 | 1.95 ± 0.69 | 1.84 ± 0.93 | 1.77 ± 0.47 | 2.24 ± 2.79 | <0.001 |
| CVR, mm Hg/ml/min/g | 101.40 ± 55.67 | 97.27 ± 40.93 | 100.06 ± 42.96 | 100.05 ± 41.67 | 109.19 ± 149.21 | 93.70 ± 34.65 | 97.95 ± 51.95 | 0.61 |
| Agatston score | 0.0 (0.0,0.0) | 0.0 (0.0, 80.0) | 31.0 (0.0, 206.0) | 146.0 (4.0, 538.0) | 220.0 (2.0, 615.0) | 71.0 (0.0, 305.0) | 53.0 (0.0, 873.0) | <0.001 |
| Ejection fraction, % | 61.21 ± 9.97 | 61.85 ± 10.56b | 60.66 ± 11.18 | 59.17 ± 12.79 | 55.87 ± 10.79c | 55.09 ± 9.69c | 54.95 ± 11.66b,c | <0.001 |
| LV mass, g | 136.01 ± 27.31 | 129.84 ± 28.46c | 126.58 ± 30.90 | 128.35 ± 34.64 | 138.78 ± 33.59 | 156.12 ± 38.18b,c | 146.85 ± 34.30b | <0.001 |
| Ischemic percentage, % | 0.08 ± 0.57 | 0.12 ± 0.55 | 0.13 ± 0.60 | 0.12 ± 0.71 | 0.18 ± 0.66 | 0.08 ± 0.33 | 0.24 ± 0.79 | 0.57 |
| Summed difference score | 0.06 ± 0.39 | 0.08 ± 0.37 | 0.09 ± 0.40 | 0.08 ± 0.48 | 0.08 ± 0.39 | 0.05 ± 0.23 | 0.16 ± 0.53 | 0.57 |
| Summed rest score | 0.15 ± 1.20 | 0.11 ± 0.77 | 0.07 ± 0.47 | 0.08 ± .37 | 0.06 ± 0.28 | 0.07 ± 0.32 | 0.03 ± 0.23 | 0.72 |
| Summed stress score | 0.21 ± 1.54 | 0.19 ± 0.88 | 0.17 ± 0.68 | 0.16 ± 0.58 | 0.14 ± 0.47 | 0.13 ± 0.43 | 0.20 ± 0.67 | 0.98 |
CFR, coronary flow reserve; CKD, chronic kidney disease; CVR, coronary vascular resistance; LV, left ventricular; min, minute; PET, positron emission tomography.
Categorical P value using analysis of variance. Tests for trends were performed using Cuzick nonparametric test for trend.
P < 0.05 versus stage 3 CKD.
P < 0.05 versus preserved kidney function.
In adjusted models, CKD stages 3 and higher were each associated with a lower CFR. Among those without a regional perfusion defect (Table 5), a worsening CKD stage remained associated with lower CFR (P < 0.001) after adjustment for demographics, other CV conditions, blood pressure, CV medications, and ejection fraction. However, after adjustment, the association of stage 3 CKD (β 0.02, 95% CI: −0.05, 0.09) with CFR was no longer statistically significant, whereas stage 4 (β −0.11, 95% CI: −0.21, −0.01), stage 5 (β −0.20, 95% CI: −0.31, −0.10), and dialysis-dependent CKD (β −0.12, 95% CI: −0.21, −0.03) were associated with a lower CFR value than preserved kidney function or stage 1, 2, or 3 CKD (P < 0.05 for all pairwise comparisons). Results were qualitatively similar in models adjusting only for the subset of baseline variables (from the prespecified list) with univariate P < 0.10 (data not shown).
Table 5 |.
Crude and adjusted association of CFR with renal function in individuals without a perfusion defect at baseline
| CKD stage | Crude β | SE | 95% Cl | P value | Adjusted β | SE | 95% Cl | P value |
|---|---|---|---|---|---|---|---|---|
| Preserved kidney function | Ref | Ref | Ref | Refa,b | Ref | Ref | Ref | Ref |
| CKD stage | ||||||||
| 1 | 0.05 | 0.03 | −0.13, 0.11 | 0.12b | 0.08 | 0.03 | 0.01, 0.14 | 0.02b |
| 2 | −0.00 | 0.03 | −0.06, 0.06 | 0.91a | 0.07 | 0.03 | 0.004, 0.13 | 0.04b |
| 3 | −0.09 | 0.03 | −0.16, −0.03 | 0.003 | 0.02 | 0.04 | −0.05, 0.09 | 0.57a |
| 4 | −0.22 | 0.05 | −0.31, −0.13 | <0.001c | −0.11 | 0.05 | −0.21, −0.01 | 0.03c |
| 5 | −0.26 | 0.05 | −0.36, −0.16 | <0.001c | −0.20 | 0.05 | −0.31, −0.10 | <0.001c |
| Chronic dialysis | −0.19 | 0.05 | −0.27, −0.10 | <0.001c | −0.12 | 0.04 | −0.21, −0.03 | 0.01c |
Crude and adjusted associations of natural log–transformed CFR with CKD stage. For crude model, P < 0.001 for global association of CKD stage with CFR. Models are adjusted for age, gender, race, hypertension, diabetes, history of myocardial infarction, history of heart failure, chronic lung disease, hyperlipidemia, surgical or percutaneous revascularization, peripheral vascular disease, resting systolic blood pressure, obesity, left ventricular ejection fraction and use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blocker, cholesterol-lowering medications, and nitrates. P < 0.001 for overall adjusted association of CKD stage and CFR. CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; Ref, referent.
Categories with a shared footnote letter are not significantly different from each other at P = 0.05 level. R2 = 0.10; adjusted R2 = 0.09.
Results were similar in the overall study population in models that included all subjects but adjusted for the presence of a regional perfusion defect (Supplementary Table S1). To better understand the impact of diabetes, hypertension, and heart failure on our findings, we analyzed results according to the presence or absence of each. Among individuals without a perfusion defect, no significant interactions were found between CKD stage and these conditions. Results were qualitatively similar in each of the subgroups (Supplementary Table S2).
To understand the relationship between flow reserve and parameters of anemia and mineral and bone metabolism, we analyzed association among individuals without perfusion defects at baseline. Serum calcium (r = 0.13, P < 0.001), serum phosphorous (r = −0.14, P < 0.001), and hemoglobin (r = 0.33, P < 0.001) were all correlated with CFR in univariate analyses, whereas parathyroid hormone was not (r = −0.07, P = 0.29). In the subset of individuals for whom values were available for all parameters (n = 176), neither CKD, stage, parathyroid hormone level, or calcium level was significantly associated with CFR, in models adjusting simultaneously for CKD stage, demographics, CV risk factors, ejection fraction, and calcium, phosphorous, parathyroid hormone, and hemoglobin levels. In contrast, serum phosphorous (β = −0.06, 95% CI: −0.10, −0.02) and hemoglobin (β = 0.08, 95% CI: 0.05, 0.11) levels were associated with CFR after full adjustment (Supplementary Table S3).
Cardiovascular-related mortality
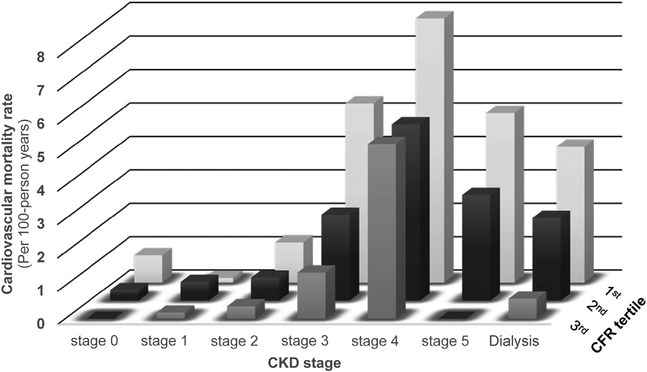
A total of 313 deaths from CV causes occurred during the follow-up period. In crude models, the risk of mortality decreased by 65% for each tertile increase in CFR (hazard ratio [HR] 0.45, 95% CI: 0.39, 0.53). A worse CKD stage (P < 0.001) was associated with higher risks of mortality from CV causes in univariate models than was preserved kidney function, with significant increases in risk across stage 3 or higher chronic dialysis (Supplementary Table S4). In fully adjusted models that included both CFR and CKD stage, CFR remained associated with mortality risk from CV causes (HRper tertile 0.76, 95% CI: 0.63, 0.92, P = 0.004). Similarly, CKD stage overall (global P < 0.001), stage 4 (HR 3.54, 95% CI: 1.05, 12.00, P = 0.04), stage 5 (HR 4.38, 95% CI: 1.24, 15.48, P = 0.02) and dialysis-dependent CKD (HR 3.60, 95% CI: 1.03, 12.55, P = 0.04) remained significantly associated with mortality from CV causes. Adjustment for CFR attenuated risks associated with stage 4, stage 5, and dialysis-dependent CKD by approximately 10%, suggesting that low CFR partly underlies the increase in mortality from CV causes in CKD. No evidence was found of effect modification between CFR and CKD stage (Pinteraction = 0.64), such that within each CKD category, risk of mortality from CV causes increased with lower CFR (Figure 1).
Figure 1 |. Mortality rate from CV causes per 100 patient years (unadjusted) according to CKD stage and tertile of CFR.
CFR, coronary flow reserve; CKD, chronic kidney disease; CV, cardiovascular.
DISCUSSION
We analyzed the association of CKD with coronary micro-vascular function in a large population of patients undergoing stress testing and found that impaired CFR was associated with early CKD and remained associated with kidney function after adjustment for relevant confounders. We found that CFR was 23% percent lower with dialysis-dependent CKD than with preserved kidney function. However, the decrease in CFR became detectable in early CKD and reached a nadir in stage 4 CKD, without additional decrements in stage 5 CKD. We found that CFR was strongly associated with mortality from CV causes with a 17% decrease in relative risk of mortality from CV causes per quintile increase in CFR, after adjustment for CKD.
Ischemia-driven angiogenesis and myocardial capillary supply are clearly reduced in experimental models of uremia.5,6 However, relatively few human studies have investigated microvascular function in CKD. Several autopsy studies have demonstrated a reduction in myocardial capillary supply in humans with CKD or end-stage renal disease,7,8 but the cohorts were small, and the representativeness of individuals undergoing postmortem examination for the overall CKD population is unclear.
Non-invasive imaging modalities offer an alternative means of investigating the myocardial microvasculature that circumvent logistical and ethical hurdles to obtaining myocardial tissue from humans. Several studies have used invasive or non-invasive imaging modalities to assess the microvasculature. Mohandas et al.15 recently demonstrated that eGFR and CFR were highly correlated in older women who did not have obstructive coronary disease. In a study by Chade et al.16 of 605 individuals who did not have obstructive coronary disease, flow-wire–measured left anterior descending artery fractional flow reserve was lower in individuals with stage 3 or higher CKD. The crude odds ratio for low CFR was 2.03 in individuals with stage 3 or higher CKD, compared with an eGFR of >60 ml/min per 1.73 m, but the difference was largely attenuated after adjustment for age and gender. Koivuviita et al.17 studied 31 individuals who did not have CV disease, and they did not find a significant association between CFR and CKD, with myocardial PET imaging. In contrast, a transthoracic echocardiography study of a cohort of 10 dialysis patients and 14 control participants with normal coronaries and preserved eGFR found a marked decrement in peak flow in the dialysis patients, but the small cohort size precluded multivariable adjustment.18 Finally, in a previous study of 435 clinical trial participants without diabetes who underwent protocol-driven PET scanning, we did not detect an adjusted association between CKD and baseline CFR, but we did find that baseline creatinine clearance was associated with a greater rate of decline in CFR during follow-up.14
To our knowledge, the current study is the largest cohort in which associations between kidney function and CFR have been investigated. Compared with prior studies, the large sample size and inclusion of sufficient numbers of patients at each stage of CKD increased power, so we were able to investigate changes in CFR across CKD stages and assess the roles of CKD-specific laboratory abnormalities. Additionally, the combination of the large sample size and the detailed characterization of our study subjects allowed for us to adjust for relevant CV and demographic risk factors, as well as CV medications.
Our findings that impaired CFR is associated with early CKD are consistent with those of Tok et al.,18 as well as with our previous report14 demonstrating an association between lower baseline creatinine clearance value and a greater rate of decline in CFR. Several prior studies did not detect an independent association of CKD and baseline CFR.14,17 The differing findings could reflect differences in technique or study population, but they more likely reflect the greater power of the current study.
Our results extend these findings by analyzing the change in CFR across the full spectrum of CKD. Unexpectedly, CFR was not lower in dialysis patients than in individuals with stage 4 or predialysis stage 5 CKD. Our findings suggest that microvascular damage is at least partly driven by physiologic changes that occur with small decrements in GFR and become apparent after a medium duration of exposure to moderate loss of kidney function. The findings further suggest that myocardial stunning, and repetitive insults during dialysis, are unlikely to fully account for microvascular rare-faction in late-stage CKD. Additional studies designed to investigate longitudinal changes in CFR, and to delineate the factors involved, would be highly informative.
We also observed associations of serum phosphorous and hemoglobin with CFR, as well as a decrease in the strength of association between CKD stage and CFR after adjustment for these factors. In the context of previous studies demonstrating associations between anemia19,20 or serum phosphorus21 and mortality from CV causes in CKD, our findings suggest a potential mechanistic role for disordered erythropoiesis and bone and mineral metabolism in the microvascular changes that occur in CKD. Alternatively, changes in vascular function and microvascular supply may mediate the observed associations between anemia or hyperphosphatemia and mortality from CV causes. Confirmation of our findings and additional exploration in other data sets are needed.
Finally, we analyzed associations of CFR with mortality from CV causes and found that CFR was inversely associated with the risk of death. These results are consistent with prior studies in which we and others found lower CFR to be predictive of mortality from CV causes and to improve risk stratification in stage 3–5 CKD12,22 and dialysis-dependent CKD.13 This study builds upon earlier work by demonstrating that CFR is similarly associated with CV risk, regardless of the underlying kidney function. The modest attenuation of CKD-associated risks after adjustment for CFR, as found in the current study, is also consistent with the concept that microvascular disease is an important contributor to CV risk in the setting of CKD.
How a decrease in CFR contributes to risk of death from CV causes merits further investigation, but several explanations are appealing. First, endothelial dysfunction and capillary loss could impair systolic and diastolic function, causing heart failure, and may be important mechanisms underlying the “reni-cardiac” variants—type 3 (acute) and type 4 (chronic) of cardio-renal syndrome.23 Given that CFR may reflect myocardial capillary rarefaction that impairs myocardial ischemia tolerance and increases myocardial necrosis in experimental models of uremia,24 reduced CFR may increase the likelihood of myocardial necrosis and the likelihood of clinically manifested myocardial infarction in the setting of CKD. Alternatively, reduced CFR may induce basal tissue hypoxia and anaerobic metabolism that impairs cellular function, disrupting conduction and lowering the threshold for arrhythmia propagation, particularly when CV demand is increased due to stressors such as ultrafiltration, hypertension, and dialysis. Further studies to determine whether CFR is associated specifically with the risk of death owing to myocardial infarction or arrhythmia in CKD would be highly informative.
An understanding of why CFR declines in CKD is of clear interest. CKD was not associated with coronary vascular resistance, which may indicate that changes in CFR are more likely to be related to a loss of small vessels than to a change in vascular function. Such a loss could occur through mechanisms present in uremia that directly induce either endothelial cell dropout or the failure of compensatory angiogenesis in response to ischemia, such as the destabilization of hypoxia-inducible factor25 and the excessive inhibition of nitric oxide synthase by asymmetric dimethyl arginine.26 Alternatively, increases in asymmetric dimethyl arginine and the concentration of additional inhibitors of angiogenesis as kidney function declines may simultaneously induce myocardial fibrosis and capillary rarefaction via a process of endothelial to mesenchymal transformation—the transformation of endothelial cells into fibroblasts.7,27
The detected differences in serum phosphorous, as well as an independent association of serum phosphorous with CFR, suggest the possibility of mechanistic links between disordered bone and mineral metabolism in CKD and microvascular dysfunction. Our findings are thus consistent with the association found between microvascular supply and both serum phosphorous28 and serum calcium and parathyroid hormone29 in 2 previous investigations that used nailfold microscopy to measure capillary density. These results are also consistent with an experimental study in which use of a calcimimetic agent ameliorated capillary rarefaction.30 Although our study was not designed to assess physiologic mechanisms, our findings suggest that clinical and experimental investigations of connections between mineral and bone metabolism and microvascular function in CKD are warranted.
Our study should be interpreted within the context of study design. This was a single-center investigation of patients referred for PET scanning. We adjusted for multiple clinical risk factors and our findings were consistent in subgroup analyses, but we cannot rule out residual confounding or the possibility that mortality in early CKD impacts the observed distribution of CFR in late CKD. An estimate of GFR was used rather than a calculation. However, misclassification of eGFR is unlikely to explain our findings, as any misclassification should have been nondifferential and therefore would have biased our findings toward the null. Additionally, our study was retrospective, and CFR is a surrogate measure for microvascular supply and function, the biologic entity of interest. Without myocardial tissue, we cannot assess the contribution of changes in cardiac structure, endothelial function, or vasodilatory capacity of the coronary vessels to the changes in CFR that we observed. However, previous studies have confirmed that CFR is highly correlated with myocardial microvascular supply31–34; myocardial blood flow measurements by PET, as assessed in this study, show excellent correlation with tissue perfusion measured by radio-labeled microspheres in animals,35 and with radiolabeled water in humans.36 In the absence of epicardial coronary artery obstruction, reduced myocardial blood flow and CFR measured by PET reflect microvascular dysfunction caused by arteriolar and capillary remodeling, as well as endothelial and smooth muscle cell dysfunction.37,38Associations were strong and readily apparent in those without perfusion detectable at baseline, as well as in analyses of the overall cohort with models adjusting for the presence of perfusion defects. Thus, the changes we detected are unlikely to be solely attributable to an increasing burden of coronary atherosclerosis or changes in ventricular structure and function as eGFR declines. In addition, we did not have information on urinary protein or albumin excretion and thus are unable to assess association of this important component of kidney function with myocardial blood flow. Finally, bone and mineral metabolism test results were available in a minority of subjects and were more likely to be tested in subjects with CKD. Our finding of an association between phosphorous and CFR, although intriguing, should be interpreted cautiously and considered to be “hypothesis-forming.”
In conclusion, we studied coronary microvascular function across the spectrum of CKD and found the following: CKD stage was associated with CFR in multivariable adjusted analyses; CFR was predictive of the risk of death from CV causes, regardless of CKD stage; and changes in CFR are detectable in stages 1–4 CKD without further changes in more-severe CKD. These data suggest that changes in the myocardial microvasculature may partly underlie the excess CV risk in CKD and that additional studies are warranted, to better define the underlying pathophysiology and potential interventions.
METHODS
Subjects and clinical data
Consecutive patients undergoing rest/vasodilator stress myocardial perfusion PET scanning at the Brigham & Women’s Hospital (Boston, MA), from January 1, 2006 through December 30, 2014, for whom age, race, gender, and serum creatinine data were available were included. When multiple scans were conducted, only the first was analyzed. Patient demographics, medical history, and medications were ascertained at the time of PET scanning, by review of medical records and patient interview. Laboratory data from within 90 days before or after the index scan were extracted from the electronic medical record, and average values were calculated. The study was approved by the hospital institutional review board.
Positron emission tomography imaging
The PETscans were conducted using Discovery RX or STE Lightspeed 64 systems (GE Healthcare, Milwaukee, WI) and either 82Rubidium or 13N-ammonia as the flow tracer at rest and stress as previously described.39 Vasodilator stress was achieved by infusion of dipyridamole, adenosine, or regadenoson, using standard protocols. The extent and severity of myocardial scar and ischemia were semi-quantitatively, visually analyzed40; left ventricular ejection fraction was calculated from gated myocardial rest and stress perfusion images, using commercial algorithms (4DM, INVIA, Ann Arbor, MI). Absolute myocardial blood flow (MBF) was calculated using hybrid factor analysis and a 2-compartment tracer kinetic model.
Coronary flow reserve was defined as the ratio between hyper-emic and resting MBF. An index of coronary vascular resistance was calculated by dividing the mean aortic blood pressure by MBF. Unless otherwise noted, MBF, CFR, and coronary vascular resistance are presented as averages of all the left ventricular values. To normalize for the impact of cardiac workload on MBF, we calculated the corrected CFR, in which resting myocardial blood flow was normalized by dividing by the rate–pressure product, an index of cardiac workload, and multiplying by 10,000.
Kidney function
The eGFR was calculated from the averaged values of the baseline serum creatinine levels using the CKD–Epidemiology Collaboration creatinine equation.41 Kidney function was categorized according to standard guidelines42 (eGFR in ml/min per 1.73 m2): preserved renal function, ≥110; stage 1, 90–109; stage 2, 60–89; stage 3, 30–59; stage 4, 15–30; stage 5, <15; and dialysis-dependent CKD.
Follow-up
Subjects were followed from the date of the index PET scan through August 31, 2015. Vital status was ascertained using the Social Security Death Index, the National Death Index, and the Partners Healthcare Research Patient Data Registry, which includes records from Massachusetts General Hospital (Boston, MA), Newton Wellesley Hospital (Newton, MA), and Brigham & Women’s Hospital (Boston, MA). In cases for which clinical records were available, death was adjudicated as being due to CV causes or non-CV causes by 2 independent cardiologists as described previously.9
Statistical analysis
Data are presented as mean ± SD or median (interquartile ratio), according to the baseline distribution. Differences in baseline characteristics and myocardial blood flow parameters across categories of CKD were assessed by analysis of variance or χ2 tests, and detection of differences among groups by Cuzick nonparametric trend tests to assess trends across categories of CKD. Post hoc analyses of variance, to assess for significant differences among groups, were used with the Tukey correction for multiple comparisons. Multivariable linear regression was used to adjust for potential confounding of the association of CFR with CKD. Models were prespecified and included factors with potential to confound associations between CKD and CFR on the basis of clinical intuition and published evidence of association with death, CV outcomes, and CFR.43–46 The following factors were included: age, gender, race, hypertension, diabetes, history of myocardial infarction, heart failure, chronic lung disease, hyperlipidemia, prior revascularization, peripheral vascular disease, resting systolic blood pressure, obesity, ejection fraction, and use of medications, including angiotensin receptor blockers and converting enzyme inhibitors, calcium channel blockers, cholesterol-lowering medications, and nitrates.
Given that our primary interest was in CFR as an index of microvascular function, our primary analyses examined this association in those who did not have perfusion defects at baseline, because the presence of a perfusion defect can indicate an infarct or high-grade coronary stenosis, which can affect blood flow independent of microvascular function. Sensitivity to the model selection strategy was checked in models that incorporated only those factors from the prespecified list that had univariate associations at the P < 0.10 level of significance. In addition, secondary analyses assessed the overall population while further adjusting for the presence or absence of perfusion deficits.
Associations of hemoglobin calcium, phosphorous, and parathyroid hormone with CFR, among those without a perfusion defect, were analyzed by adjusting for these parameters in the subset with complete data for indices of bone and mineral metabolism. Given the smaller size of the cohort with available laboratory test values, we adjusted only for baseline demographics, blood pressure, diabetes, hypertension, blood pressure, ejection fraction, and presence of a perfusion defect. Missing values were not imputed, because these parameters were missing both in a nonrandom fashion according to CKD stage and in a high proportion of subjects.
Univariate and multivariable Cox proportional hazards regression was used to assess the association of CFR with mortality from CV causes, after controlling for the effects of CV risk factors. Model fit was tested using graphical techniques, inspection of residual distribution, and tests of model specification, and/or tests of proportional hazards assumptions. A P value of <0.05 was considered significant. All statistical analyses were performed with Stata version 13.1 (Stata Corp, College Station, TX).
Supplementary Material
Table S1. Crude and adjusted association of CFR (coronary flow reserve) with renal function in the overall population. CKD, chronic kidney disease; Ref, referent.
Table S2. Adjusted association of CFR (coronary flow reserve) with renal function in individuals without flow defects according to the presence of diabetes, hypertension, and heart failure. CI, confidence interval; CKD, chronic kidney disease; Ref, referent.
Table S3. Crude and adjusted association of parameters of bone and mineral metabolism with CFR in individuals without a baseline flow defect. CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; Ref, referent.
Table S4. Crude and adjusted hazard ratios (HRs) of kidney disease stage and coronary flow reserve (CFR) with death from CV causes. CI, confidence interval; CKD, chronic kidney disease.
Footnotes
DISCLOSURE
NRS has equity in General Electric. SD has equity in General Electric and research support from Astellas Global Pharma. RB has received research grant support from Gilead Sciences. MFDC has received research grant support from Gilead Sciences. All the other authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at www.kidney-international.org.
REFERENCES
- 1.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3): S112–S119. [DOI] [PubMed] [Google Scholar]
- 4.Pun PH, Smarz TR, Honeycutt EF, et al. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76:652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amann K, Wiest G, Zimmer G, et al. Reduced capillary density in the myocardium of uremic rats—a stereological study. Kidney Int. 1992;42: 1079–1085. [DOI] [PubMed] [Google Scholar]
- 6.Jacobi J, Porst M, Cordasic N, et al. Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int. 2006;69:2013–2021. [DOI] [PubMed] [Google Scholar]
- 7.Charytan DM, Padera R, Helfand AM, et al. Increased concentration of circulating angiogenesis and nitric oxide inhibitors induces endothelial to mesenchymal transition and myocardial fibrosis in patients with chronic kidney disease. Int J Cardiol. 2014;176:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–1022. [DOI] [PubMed] [Google Scholar]
- 9.Taqueti VR, Hachamovitch R, Murthy VL, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taqueti VR, Everett BM, Murthy VL, et al. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farhad H, Dunet V, Bachelard K, et al. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging. 2013;14:1203–1210. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VL, Naya M, Foster CR, et al. Coronary vascular dysfunction and prognosis in patients with chronic kidney disease. JACC Cardiovasc Imaging. 2012;5:1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah NR, Charytan DM, Murthy VL, et al. Prognostic value of coronary flow reserve in patients with dialysis-dependent ESRD. J Am Soc Nephrol. 2016;27:1823–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charytan DM, Shelbert HR, Di Carli MF. Coronary microvascular function in early chronic kidney disease. Circ Cardiovasc Imaging. 2010;3:663–671. [DOI] [PubMed] [Google Scholar]
- 15.Mohandas R, Segal MS, Huo T, et al. Renal function and coronary microvascular dysfunction in women with symptoms/signs of ischemia. PloS One. 2015;10:e0125374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chade AR, Brosh D, Higano ST, et al. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006;69:266–271. [DOI] [PubMed] [Google Scholar]
- 17.Koivuviita N, Tertti R, Jarvisalo M, et al. Increased basal myocardial perfusion in patients with chronic kidney disease without symptomatic coronary artery disease. Nephrol Dial Transplant. 2009;24:2773–2779. [DOI] [PubMed] [Google Scholar]
- 18.Tok D, Gullu H, Erdogan D, et al. Impaired coronary flow reserve in hemodialysis patients: a transthoracic Doppler echocardiographic study. Nephron Clin Pract. 2005;101:c200–c206. [DOI] [PubMed] [Google Scholar]
- 19.Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis. 1996;28:53–61. [DOI] [PubMed] [Google Scholar]
- 20.Levin A, Thompson CR, Ethier J, et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis. 1999;34:125–134. [DOI] [PubMed] [Google Scholar]
- 21.Kanbay M, Goldsmith D, Akcay A, Covic A. Phosphate—the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27:220–230. [DOI] [PubMed] [Google Scholar]
- 22.Nakanishi K, Fukuda S, Shimada K, et al. Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol. 2013;112:928–932. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Bottiglieri B, McCulough PA. The central role of endothelial dysfunction in cardiorenal syndrome. Cardiorenal Med. 2017;7:104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dikow R, Kihm LP, Zeier M, et al. Increased infarct size in uremic rats: reduced ischemia tolerance? J Am Soc Nephrol. 2004;15:1530–1536. [DOI] [PubMed] [Google Scholar]
- 25.Schellinger IN, Cordasic N, Panesar J, et al. Hypoxia inducible factor stabilization improves defective ischemia-induced angiogenesis in a rodent model of chronic kidney disease. Kidney Int. 2017;91:616–627. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto Y, Ueda S, Yamagishi S, et al. Dimethylarginine dimethylaminohydrolase prevents progression of renal dysfunction by inhibiting loss of peritubular capillaries and tubulointerstitial fibrosis in a rat model of chronic kidney disease. J Am Soc Nephrol. 2007;18: 1525–1533. [DOI] [PubMed] [Google Scholar]
- 27.Wu M, Tang RN, Liu H, et al. Cinacalcet ameliorates aortic calcification in uremic rats via suppression of endothelial-to-mesenchymal transition. Acta Pharmacol Sin. 2016;37:1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thang OH, Serne EH, Grooteman MP, et al. Capillary rarefaction in advanced chronic kidney disease is associated with high phosphorus and bicarbonate levels. Nephrol Dial Transplant. 2011;26:3529–3536. [DOI] [PubMed] [Google Scholar]
- 29.Edwards-Richards A, DeFreitas M, Katsoufis CP, et al. Capillary rarefaction: an early marker of microvascular disease in young hemodialysis patients. Clin Kidney J. 2014;7:569–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koleganova N, Piecha G, Ritz E, et al. Interstitial fibrosis and microvascular disease of the heart in uremia: amelioration by a calcimimetic. Lab Invest. 2009;89:520–530. [DOI] [PubMed] [Google Scholar]
- 31.Kaul S The role of capillaries in determining coronary blood flow reserve: implications for stress-induced reversible perfusion defects. J Nucl Cardiol. 2001;8:694–700. [DOI] [PubMed] [Google Scholar]
- 32.Jayaweera AR, Wei K, Coggins M, et al. Role of capillaries in determining CBF reserve: new insights using myocardial contrast echocardiography. Am J Physiol. 1999;277:H2363–H2372. [DOI] [PubMed] [Google Scholar]
- 33.Tsagalou EP, Anastasiou-Nana M, Agapitos E, et al. Depressed coronary flow reserve is associated with decreased myocardial capillary density in patients with heart failure due to idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2008;52:1391–1398. [DOI] [PubMed] [Google Scholar]
- 34.Schubert S, Abdul-Khaliq H, Wellnhofer E, et al. Coronary flow reserve measurement detects transplant coronary artery disease in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:514–521. [DOI] [PubMed] [Google Scholar]
- 35.Kuhle WG, Porenta G, Huang SC, et al. Quantification of regional myocardial blood flow using 13N-ammonia and reoriented dynamic positron emission tomographic imaging. Circulation. 1992;86: 1004–1017. [DOI] [PubMed] [Google Scholar]
- 36.Nitzsche EU, Choi Y, Czernin J, et al. Noninvasive quantification of myocardial blood flow in humans. A direct comparison of the [13N] ammonia and the [15O]water techniques. Circulation. 1996;93: 2000–2006. [DOI] [PubMed] [Google Scholar]
- 37.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–840. [DOI] [PubMed] [Google Scholar]
- 38.Buus NH, Bottcher M, Hermansen F, et al. Influence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemia. Circulation. 2001;104:2305–2310. [DOI] [PubMed] [Google Scholar]
- 39.Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124:2215–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerqueira MD, Weissman NJ, Dilsizian V, et al. , American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. [DOI] [PubMed] [Google Scholar]
- 41.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 43.Nakamori S, Onishi K, Ishida M, et al. Myocardial perfusion reserve is impaired in patients with chronic obstructive pulmonary disease: a comparison to current smokers. Eur Heart J Cardiovasc Imaging. 2014;15: 180–188. [DOI] [PubMed] [Google Scholar]
- 44.Niiranen TJ, Vasan RS. Epidemiology of cardiovascular disease: recent novel outlooks on risk factors and clinical approaches. Expert Rev Cardiovasc Ther. 2016;14:855–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Labazi H, Trask AJ. Coronary microvascular disease as an early culprit in the pathophysiology of diabetes and metabolic syndrome. Pharmacol Res. 2017;123:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racial Graham G. and ethnic differences in acute coronary syndrome and myocardial infarction within the United States: from demographics to outcomes. Clin Cardiol. 2016;39:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Crude and adjusted association of CFR (coronary flow reserve) with renal function in the overall population. CKD, chronic kidney disease; Ref, referent.
Table S2. Adjusted association of CFR (coronary flow reserve) with renal function in individuals without flow defects according to the presence of diabetes, hypertension, and heart failure. CI, confidence interval; CKD, chronic kidney disease; Ref, referent.
Table S3. Crude and adjusted association of parameters of bone and mineral metabolism with CFR in individuals without a baseline flow defect. CFR, coronary flow reserve; CI, confidence interval; CKD, chronic kidney disease; Ref, referent.
Table S4. Crude and adjusted hazard ratios (HRs) of kidney disease stage and coronary flow reserve (CFR) with death from CV causes. CI, confidence interval; CKD, chronic kidney disease.