Abstract
Background—
Although cardiac magnetic resonance (CMR) and positron emission tomography (PET) detect different pathological attributes of cardiac sarcoidosis (CS), the complementary value of these tests has not been evaluated. Our objective was to determine the value of combining CMR and PET in assessing the likelihood of CS and guiding patient management.
Methods and Results—
In this retrospective study, we included 107 consecutive patients referred for evaluation of CS by both CMR and PET. Two experienced readers blinded to all clinical data reviewed CMR and PET images and categorized the likelihood of CS as no (<10%), possible (10%–50%), probable (50%–90%), or highly probable(>90%) based on predefined criteria. Patient management after imaging was assessed for all patients and across categories of increasing CS likelihood. A final clinical diagnosis for each patient was assigned based on a subsequent review of all available imaging, clinical, and pathological data. Among 107 patients (age, 55±11 years; left ventricular ejection fraction, 43±16%), 91 (85%) had late gadolinium enhancement, whereas 82 (76%) had abnormal F18-fluorodeoxyglucose uptake on PET, suggesting active inflammation. Among the 91 patients with positive late gadolinium enhancement, 60 (66%) had abnormal F18-fluorodeoxyglucose uptake. When PET data were added to CMR, 48 (45%) patients were reclassified as having a higher or lower likelihood of CS, most of them (80%) being correctly reclassified when compared with the final diagnosis. Changes in immunosuppressive therapies were significantly more likely among patients with highly probable CS.
Conclusions—
Among patients with suspected CS, combining CMR and PET provides complementary value for estimating the likelihood of CS and guiding patient management.
Keywords: diagnosis, heart failure, inflammation, sarcoidosis
Cardiac magnetic resonance (CMR) and positron emission tomographic (PET) imaging have both emerged as useful methods to detect cardiac sarcoidosis (CS) and identify individuals who have a higher risk of future adverse events.1–4 Each of these techniques has unique advantages and disadvantages and is designed to evaluate different aspects of the pathobiology of this disease. Although CMR is useful for evaluating fibrosis, PET is best suited for visualizing and quantifying active inflammation. Several small studies have compared CMR and PET but did not have sufficient number of patients to assess the complementary value of these techniques.5–7
Therefore, we sought to compare the complementary value of CMR and PET to diagnose and manage CS. Specifically, our objective was to determine the value of combining both tests for determining the likelihood of CS, as well as guiding patient management.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
We included 111 consecutive patients referred for evaluation of known or suspected CS by both CMR and PET at Brigham and Women’s Hospital (Boston, MA) between May 2006 and July 2014. Patients with more than a year between PET and CMR (n=1) were excluded, as were those with poor image quality (n=2) and prior heart transplant (n=1).
The final cohort comprised 107 patients. The median time between CMR and PET was 8 days (interquartile range [25th–75th percentile], 3–32 days). When considering the order of testing, the majority of patients underwent CMR first, whereas only 12 were evaluated by PET first. All decisions on immunosuppressive therapy were made after both tests were available. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institution guidelines.
Adjudication of the Imaging-Based Likelihood of CS
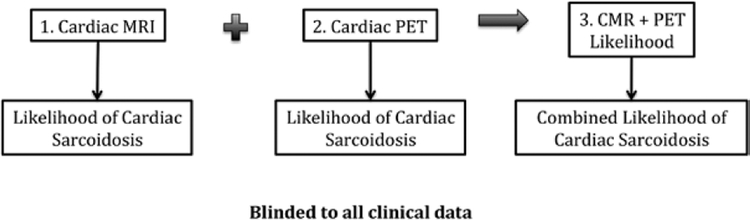
Two experienced cardiologists reviewed all CMR and PET images, blinded to all clinical data. For each patient, the likelihood of CS based on CMR images alone was first evaluated. Subsequently, the likelihood of CS based on PET imaging and on combining CMR and PET images was determined. This sequence of image analysis was specifically designed to simulate real-world practice where CMR is most often the initial test performed (Figure 1). Discrepancies in imaging interpretation were resolved by consensus reading.
Figure 1.
Image interpretation workflow. All images were interpreted blinded to clinical data. Cardiac magnetic resonance (CMR) images were interpreted first, followed by positron emission tomographic (PET) images, and then combined CMR+PET images. At each step, the likelihood of cardiac sarcoidosis was recorded. MRI indicates magnetic resonance imaging.
We used prior literature and expert consensus statements from the World Association of Sarcoidosis and Other Granulomatous Disorders8 and Heart Rhythm Society9 to categorize the likelihood of CS in each patient as follows:
No CS (likelihood, <10%): when there is no evidence of CS or an alternative diagnosis was established.
Possible CS (likelihood, 10%–50%): when imaging findings are not specific for CS.8 In such cases, CS could not be excluded, and an alternative diagnosis was more likely.
Probable CS (likelihood, 50%–90%): when imaging findings are suggestive but not definitive for CS.8
Highly probable (likelihood, >90%): when imaging findings are highly specific for CS.8
CMR Acquisition and Image Analysis
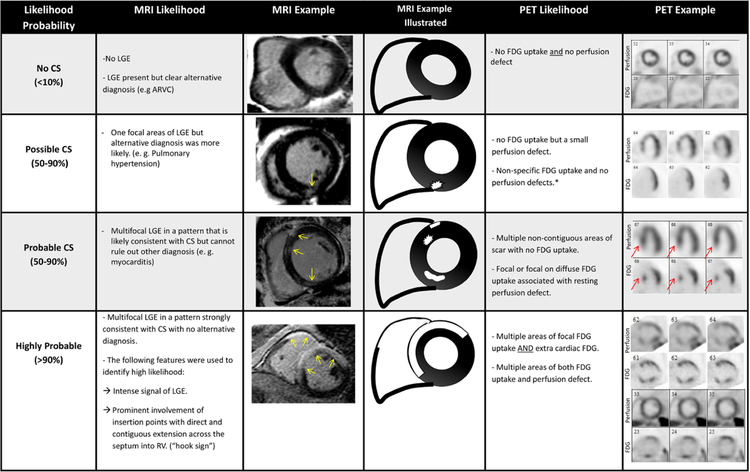
All CMR images were acquired on a 3.0-T system as detailed in the Data Supplement. The presence and pattern of late gadolinium enhancement (LGE) was classified as (1) subendocardial (2) midmyocardium, and (3) subepicardial. Based on previously described patterns,10–16 expert consensus,8 and our own clinical experience,17–19 the likelihood of sarcoidosis based on CMR was categorized according to the criteria listed in Figure 2.
Figure 2.
Definitions and illustrations of criteria used to categorize cardiac sarcoidosis (CS) likelihood based on cardiac magnetic resonance and positron emission tomographic (PET) findings. *Nonspecific F18-fluorodeoxyglucose (FDG) uptake includes the following: (1) focal FDG, which demonstrated homogenous signal intensity and only involved the lateral wall; (2) diffuse uptake of FDG by left ventricle; and (3) small area of FDG uptake that has signal intensity, which is only minimally increased when compared with background/blood pool. LGE indicates late gadolinium enhancement; and MRI, magnetic resonance imaging.
Multifocal LGE was used to define a probable likelihood of CS (50%–90%) based on magnetic resonance imaging when there were at least 2 noncontiguous areas that had LGE in a noninfarct pattern (subepicardial or midmyocardial). Multifocal LGE could be classified as probable (50%–90%) or highly probable (>90%). When other diagnosis (eg, myocarditis) could not be ruled out, the likelihood was determined as probable. If no other alternative diagnosis was found, the presence of several features was used to designate highly probable CS: (1) involvement of the basal anteroseptum and inferoseptum, which demonstrated contiguous extension into the right ventricle (hook sign; Figure 2); (2) large amount of intense LGE uptake in multiple segments (Figures 2 and 3).
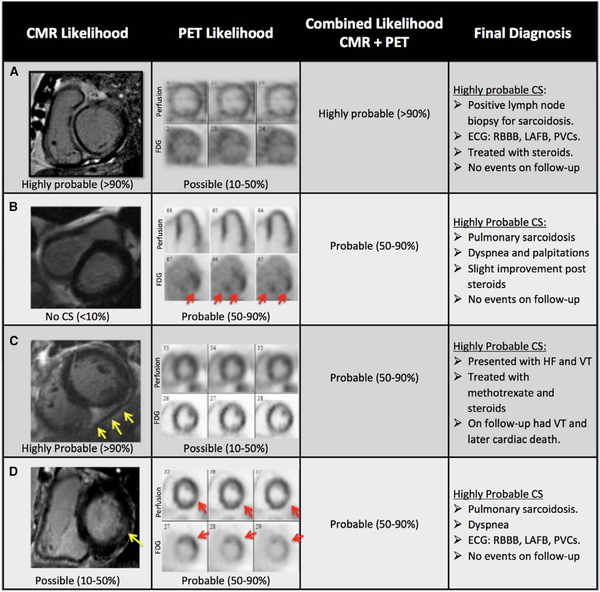
Figure 3.
Examples of complementary value of integrating cardiac magnetic resonance (CMR) and positron emission tomography (PET) results. A, Sixty-one-year-old man presented with systolic heart failure of unknown etiology. After CMR and PET, a lymph node biopsy showed noncaseating granuloma compatible with sarcoidosis. CMR showed late gadolinium enhancement (LGE) in the basal and midanteroseptal and inferoseptal segments that extends to the right ventricle (RV; hook sign) and was, therefore, categorized as highly probable cardiac sarcoidosis (CS; >90%). PET showed focal on diffuse F18-fluorodeoxyglucose (FDG) uptake with no perfusion defect and was classified as possible CS (10%–50%) but had reduced image quality. The combined diagnosis based on CMR and PET was highly probable (>90%) because our methods specify placing greater emphasis on whichever test is more definitive. The final diagnosis was highly probable CS (>90%) because the patient had biopsy-proven sarcoidosis, nonsustained ventricular tachycardia on follow-up, and CMR findings for CS. B, Fifty-four-year-old man with biopsy-proven lung sarcoidosis presented with palpitations and presyncope. CMR did not show perfusion defect, wall motion abnormalities, or LGE and was, therefore, categorized as no CS (<10%). On the contrary, PET showed severe FDG uptake of the basal inferolateral and inferoseptal segments with no perfusion defects and was classified as probable CS (50%–90%). The combined diagnosis based on CMR and PET was probable CS (50%–90%) reflecting that the PET results were more definitive, especially because the FDG uptake involved multiple areas (ie, not just the lateral wall) and occurred in the setting of complete suppression of FDG from all other areas. Because of having biopsy-confirmed extra-CS, cardiac involvement by FDG imaging, and clinical signs and symptoms, which were consistent with CS, the patient was categorized as having highly probable (>90%) CS. C, Fifty-five-year-old man with pulmonary sarcoidosis was referred for an evaluation of CS because of heart failure and ventricular tachycardia. CMR showed subepicardial LGE in the midinferior and inferoseptal segments that extended into the RV. CMR was interpreted as highly probable CS because of intense signal of LGE and prominent involvement of the RV insertion points with direct and contiguous extension across the septum into the RV. PET showed diffuse FDG uptake with no perfusion defects and was classified as possible CS (10%–50%). The combined diagnosis based on CMR and PET was probable CS (50%–90%) reflecting that the highly probable CMR results were more influential than the nonspecific FDG PET results but that the combined diagnosis now provided a lower likelihood of CS than magnetic resonance imaging alone because of the PET findings. The final diagnosis was highly probable (≥90%) considering the evidence of extra-CS and cardiac involvement demonstrated on CMR. D, Seventy-five-year-old woman with pulmonary sarcoidosis referred for an evaluation of CS because of dyspnea and abnormal electrocardiogram. On CMR, there was midwall LGE in the basal inferolateral segment, which was classified as possible CS (10%–50%) because there was only 1 affected segment. PET showed a mild perfusion defect in the basal inferolateral segment and FDG uptake involving the basal anterior and anterolateral segments and was subsequently classified as probable CS (50%–90%). The combined diagnosis based on CMR and PET was probable CS (50%–90%) reflecting that the PET results were more definitive than the nonspecific CMR finding of midwall LGE in 1 segment. The final diagnosis was highly probable CS (≥90%) based on pulmonary sarcoidosis with abnormalities on both CMR and PET. HF indicates heart failure; LAFB, left anterior fascicular block; PVC, premature ventricular contractions; and RBBB, right bundle branch block.
Myocardial Perfusion and Metabolic Imaging
All patients underwent evaluation of regional myocardial perfusion and metabolic imaging using either PET (n=84) or an integrated hybrid protocol that includes single photon emission computer tomography myocardial perfusion imaging and F18-fluorodeoxyglucose (FDG) PET (n=23). For simplicity, we will refer to PET as an operational term that is inclusive of both perfusion with PET or single photon emission computer tomography and metabolic imaging throughout the article. Full details on the imaging protocol are available in the Data Supplement.
FDG images were categorized as normal, abnormal, or nonspecific. In abnormal cases, FDG uptake was analyzed for each of the 17 myocardial segments and categorized as no uptake, mild, or moderate/severe.20 Based on previously described patterns,1,21–23 the likelihood of CS based on PET/computed tomographic imaging pattern was categorized according to the classification shown in Figure 2.
Integrated CMR and PET Image Analysis
After the CMR and PET images were both reviewed, the combined likelihood of CS based on all imaging results, but blinded to all clinical information, was determined. In cases where the likelihood based on CMR and the likelihood based on PET imaging differed, factors such as image quality, potential alternative diagnosis, and certainty of diagnosis established by each individual modality were all used to determine the combined likelihood. For example, if CMR findings suggested probable CS (50%–90%) but PET imaging suggested possible CS (10%–50%) in the setting of incomplete FDG suppression, then the combined likelihood was categorized as probable CS. In such cases, the CMR results were more influential in determining the final likelihood of CS. When either CMR or PET was limited in quality, the alternative examination was also more influential in determining the combined likelihood of CS. In other cases, when either CMR or PET imaging offered an alternative diagnosis, the combined interpretation reflected the test that offered the lower likelihood of CS because the clear presence of an alternative diagnosis would make CS unlikely. Finally, if both exams were determined by readers to offer a similar level of certainty, then the combined likelihood was categorized in between the likelihood offered by the 2 individual exams.
Integration of Imaging and Clinical Information in the Final Adjudication of the Likelihood of CS
Because of the lack of a reliable gold standard for CS (which is a challenge related to all studies in the field of CS), we used the Final Likelihood of CS as the reference against which to examine the performance of CMR, PET, and the combination of both. The final likelihood of CS was determined based on a comprehensive review of all available medical records, clinical data, pathology data, imaging findings, and follow-up data. In addition, the Japanese Ministry of Health, Labour, and Welfare criteria and Heart Rhythm Society expert consensus statement criteria on the diagnosis and management of arrhythmias associated with CS criteria were applied to each patient.9 The final likelihood of CS was determined according to the aforementioned categories and classified as no sarcoidosis, possible CS, probable CS, and highly probable CS.
After reviewing both CMR and PET results, clinical features that increased the likelihood of CS included improvement of FDG after immunosuppressive therapy, or the presence of clinical sequelae of CS (eg, ventricular tachycardia and heart block) in individuals that had known extra-CS and imaging features consistent with cardiac involvement. Conversely, features that decreased the likelihood of CS included a known alternative diagnosis, which in some cases relied on integrating family history or genetic testing. In addition, when present, other explanation for myocardial inflammation, such as myocarditis or underlying inflammatory rheumatologic disease, decreased the likelihood of CS on the final diagnosis. For example, if a subject categorized by imaging as having probable (50%–90%) CS was found to have a clear alternative diagnosis by pathology or genetic testing, then the final diagnosis was categorized as no CS (<10% likelihood).
On the contrary, the final diagnosis was categorized as highly probable (>90%) when there was biopsy-proven sarcoidosis, cardiac imaging findings that were conclusive for cardiac involvement by both CMR and PET, and clinical signs and symptoms that were consistent with having CS.
Clinical Follow-Up and Event Adjudication
All patients were evaluated for downstream (ie, post-imaging) patient management and for incident adverse events, including death from any cause and documented sustained ventricular tachycardia. Change in immunosuppressive therapy was defined as initiation of steroids therapy or when a nonsteroid immunosuppressive medication was added to a patient who was already on steroid therapy.
Statistical Analysis
All continuous variables were tested for normality. Continuous data are presented as mean±SD, whereas categorical variables are presented as percentages. Statistical significance was assessed using Fisher exact or Cochran–Armitage trend test for ordinal and dichotomous variables. A 2-sided P value of <0.05 was deemed significant. To assess the incremental diagnostic value of adding PET imaging to CMR, we evaluated the number of patients who were reclassified as having a higher or lower likelihood of CS when both CMR and PET imaging were evaluated together. Annualized event rates are expressed as the number of patients having events (ventricular tachycardia or death) as a proportion of the number of patients at risk divided by the number of patient-years follow-up. The event rates were compared using the log-rank test.
Results
Baseline Characteristics
The final study cohort consisted of 107 individuals, of whom most (n=104; 97%) had no history of CS. At baseline, 37 patients (34%) had biopsy-proven (n=32) or clinically diagnosed (n=5) extra-CS. Based on the Japanese Ministry of Health, Labour, and Welfare criteria, 33 patients (31%) had CS, whereas when using the Heart Rhythm Society criteria, 33 (31%) had probable or definite CS.
Table 1 summarizes the baseline characteristics of the study population. The mean left ventricular ejection fraction was 43±16%, and right ventricular ejection fraction was 40±13%. There were 25 patients (23%) with severely reduced left ventricular ejection fraction (≤30%). Nearly one third of the cohort (n=38; 35%) presented with ventricular tachycardia, of whom 29 did not have previous diagnosis of sarcoidosis.
Table 1.
Baseline Characteristics of Study Population
| Variables | All Patients (n=107) |
|---|---|
| Demographics | |
| Age, y (mean, SD) | 55±11 |
| Women | 34 (31%) |
| Black | 14 (13%) |
| History of heart failure | 22 (20%) |
| Medical history | |
| Biopsy-proven extra-CS | 32 (30%) |
| CS | 3 (2%) |
| Ventricular tachycardia (before first study) | 38 (35%) |
| Obstructive coronary artery disease | 3 (2%) |
| Sign and symptoms* | |
| Syncope | 18 (16%) |
| Heart failure | 32 (30%) |
| Palpitations | 43 (40%) |
| Dyspnea | 42 (39%) |
| Electrocardiographic characteristics | |
| Advanced heart block (second degree type II or third degree) | 9 (8%) |
| Left bundle branch block | 10 (9%) |
| Right bundle branch block | 23 (21%) |
| Medications | |
| Steroid treatment (baseline) | 20 (19%) |
| Other immunosuppressive agent (baseline) | 4 (4%) |
| Steroid treatment (on follow-up) | 35 (32%) |
| Steroid treatment stopped (on follow-up) | 2 (1%) |
| Other immunosuppressive agent (on follow-up) | 13 (12%) |
| Cardiac MRI | |
| Left ventricular ejection fraction, % (mean, SD) | 43±16 |
| Right ventricular ejection fraction, % (mean, SD) | 40±13 |
| End diastolic volume, indexed to BSA, mL/m2 | 97±37 |
| LGE (any) | 91 (85%) |
| FDG PET | |
| Resting perfusion defect | 59 (55%) |
| Normal or nonspecific FDG uptake | 39 (37%) |
| Abnormal FDG uptake | 68 (63%) |
| Criteria | |
| Positive JMHW (2006) | 33 (31%) |
| Probable or positive Heart Rhythm Society expert consensus statement (2014) | 33 (31%) |
BSA indicates body surface area; CS, cardiac sarcoidosis; FDG, F18-fluorodeoxyglucose; JMHW, Japanese Ministry of Health, Labour, and Welfare; LGE, late gadolinium enhancement; MRI, magnetic resonance imaging; and PET, positron emission tomography.
Because patients may have had >1 sign or symptom, the total number of signs/symptoms is >100%.
Final Diagnosis of CS
When using all available information, 45 patients (42%) were classified as having a high probability (ie, >90%) of CS as the final diagnosis, whereas 21 (19%) patients were categorized as not having sarcoidosis, and of them, 18 (85%) ultimately had a clear alternative diagnosis.
CMR Findings
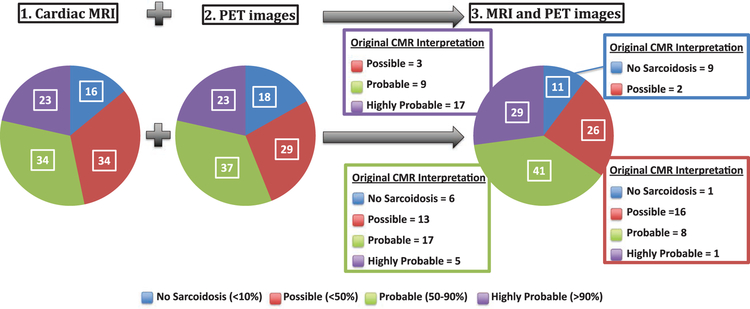
Overall, 91 patients (85%) had LGE. Among these 91 patients with positive LGE, only 54 (59%) had perfusion defects on PET. When evaluating the likelihood of CS based on CMR, 57 patients (53%) had highly probable or probable CS, whereas 16 (15%) had no evidence of CS (Figure 4, left circle). There was 1 patient who had LGE but was categorized as not having CS because the CMR demonstrated a clear alternative diagnosis of arrythmogenic right ventricular cardiomyopathy.
Figure 4.
Distribution of likelihood of cardiac sarcoidosis (CS) based on cardiac magnetic resonance (CMR), positron emission tomo-graphic (PET) images, and the combination of both tests. The likelihood of CS for the entire study population is illustrated in each pie chart. The combined likelihood of CS is based on simultaneous evaluation of both CMR and PET study results blinded to all clinical data. For each category, the blowout box depicts the distribution that was originally assigned to those patients based on the CMR interpretation alone. MRI indicates magnetic resonance imaging.
Among patients who had LGE, the median number of involved segments was 4 (25th–75th percentile, 2–6). The most common LGE pattern (n=90; 84%) was midwall and subepicardial, although 31 (29%) patients also had subendocardial LGE involving at least 1 segment. Only 2 of these 31 patients were found to have obstructive coronary artery disease on follow-up. Among the 91 patients with LGE, 21 (23%) had right ventricular involvement.
PET Findings
Abnormal FDG uptake was present in 68 patients (63%), whereas a rest perfusion defect was observed in 59 patients (55%). Thirty-nine (36%) patients were categorized as having normal FDG. Fourteen of these patients had diffuse (nonspecific) FDG uptake, whereas the remainder (25 patients) had no FDG uptake.
Among the 68 patients with abnormal FDG uptake, the median number of involved segments was 3 (25th–75th percentile, 2–5), and 47 (69%) also had a rest perfusion defect. On the contrary, among the 39 individuals with negative or nonspecific FDG, 12 (31%) had a rest perfusion defect.
Twenty-three patients (21%) were categorized as having highly probable (ie, >90%) CS by PET. In contrast, 18 patients (16%) had no evidence of active CS. Figure 4 shows the distribution of CS likelihood by PET.
Combined CMR and PET Findings
Table 2 shows the distribution of the PET and CMR results. Among 16 patients with negative LGE, 8 (50%) had FDG uptake, and half of them (n=4) had probable or highly probable CS (Table 2). Among the 91 patients with positive LGE, 60 (66%) had abnormal FDG uptake. However, even among these 60 patients that had abnormal LGE and FDG uptake, not all of them had probable or highly probable CS, reflecting the fact that the pattern and extent of imaging abnormalities (Figure 2), rather than just a binary interpretation of each imaging technique, were important in identifying the final likelihood of CS.
Table 2.
CMR and PET Result Distribution
| PET Examination Results | |||||
|---|---|---|---|---|---|
| Normal | Rest Perfusion Defect | FDG Uptake | FDG Uptake and Perfusion Defect | Total | |
| CMR examination results | |||||
| Negative LGE | 8 | 0 | 3 | 5 | 16 |
| Positive LGE | 19 | 12 | 18 | 42 | 91 |
| No CS | 0 | 0 | 1 | 0 | 1 |
| Possible CS | 6 | 5 | 10 | 12 | 33 |
| Probable CS | 9 | 4 | 5 | 16 | 34 |
| Highly probable CS | 4 | 3 | 2 | 14 | 23 |
| Total | 27 | 12 | 21 | 47 | 107 |
CMR indicates cardiac magnetic resonance; CS, cardiac sarcoidosis; FDG, F18-fluorodeoxyglucose; LGE, late gadolinium enhancement; and PET, positron emission tomography.
Comparison of CMR With PET Findings on Per-Segment Analysis
The concordance between LGE and perfusion on a per-segment level was modest to high with 82% of the segments being concordant (1500 of 1819). The concordance between LGE and FDG was 76% (1384 of 1819).
Reclassification of CS Likelihood
Although the overall distribution of CS likelihood remained similar after adding PET data to CMR, there was significant reclassification. Overall, 48 (45%) patients were reclassified as having a higher or lower likelihood of CS (Figure 4; Table 3), and most of these reclassified cases occurred in patients who were initially classified as having possible or probable CS based on CMR. Specifically, 17 of the 34 patients with probable CS on CMR were reclassified to a higher (n=9) or lower (n=8) likelihood group after adding the PET information. Similarly, 16 of the 34 patients with possible CS on CMR were reclassified to a higher likelihood group (Table 3).
Table 3.
Final Diagnosis and Events in Individuals Reclassified by Combining PET With CMR
| CMR Likelihood→Likelihood After Adding PET | Final Diagnosis | Events (Death or VT) | Death |
|---|---|---|---|
| Reclassification to a higher likelihood (n=32) | |||
| Probable→highly probable (n=9) | Highly probable=7 | 4 | 1 |
| Probable=1 | 0 | 0 | |
| Possible=1 | 0 | 0 | |
| Possible→highly probable (n=3) | Highly probable=3 | 0 | 0 |
| Possible→probable (n=13) | Highly probable=9 | 1 | 1 |
| Probable=0 | 0 | 0 | |
| Possible=4 | 1 | 0 | |
| No sarcoidosis→probable (n=6) | Highly probable=1 | 0 | 0 |
| Probable=3 | 0 | 0 | |
| Possible=1 | 0 | 0 | |
| No sarcoidosis=1 | 0 | 0 | |
| No sarcoidosis→possible (n=1) | Probable=1 | 0 | 0 |
| Reclassification to a lower likelihood (n=16) | |||
| Highly probable→probable (n=5) | Highly probable=3 | 2 | 1 |
| Probable=1 | 0 | 0 | |
| Possible=1 | 0 | 0 | |
| Highly probable→possible (n=1) | Highly probable=1 | 0 | 0 |
| Probable→possible (n=8) | Highly probable=1 | 0 | 0 |
| Possible=5 | 2 | 0 | |
| No sarcoidosis=2 | 0 | 0 | |
| Possible→no sarcoidosis (n=2) | No sarcoidosis=2 | 1 | 0 |
CMR indicates cardiac magnetic resonance; PET, positron emission tomography; and VT, ventricular tachycardia.
Notably, combining both CMR and PET findings resulted in 12 new patients being reclassified as highly probable (>90%) likelihood of CS. Among these 12 patients, 10 had a final diagnosis of highly probable CS, and 5 experienced adverse events on follow-up.
Table 3 shows the final diagnosis and absolute numbers of events of all patients that were reclassified by adding PET imaging after CMR. Among the 32 patients who were reclassified to a higher likelihood of CS, 25 (78%) had a higher likelihood of CS on the final diagnosis than was initially categorized based on CMR alone. Among the 16 patients who were reclassified to a lower likelihood of CS, 11 (68%) had a lower likelihood of CS on the final diagnosis than was initially categorized based on CMR alone.
Yield of Endomyocardial Biopsy Versus Imaging
Overall, 38 patients (35%) underwent endomyocardial biopsy (EMBx). In 3 of them, the EMBx was positive, 11 had nonspecific fibrosis, and the remaining 24 were negative.
When evaluating the imaging findings of the 38 patients who underwent EMBx, 34 had positive LGE, and 21 (55%) had abnormal FDG uptake. Overall, 21 of these 38 patients were ultimately diagnosed as having highly probable CS on follow-up, and all 21 had both abnormal LGE and abnormal FDG findings.
Clinical Follow-Up
Downstream Patient Management
During follow-up, an implantable cardiac defibrillator was implanted in 65 (60%) patients. Likewise, there was a significant increase in the use of immunosuppressive therapies, as the use of steroids increased from 19% to 32%, whereas the use of other immunosuppressive therapies increased from 4% to 12% (Table 1).
When examining these changes in patient management by the combined likelihood of CS based on integrating both CMR and PET, there was no difference in implantable cardiac defibrillator or pacemaker implantation between groups. However, there was a significantly higher rate of changes in immunosuppressive therapy in patients reclassified as having a higher likelihood of CS (Table 4).
Table 4.
Changes in Downstream Patient Management Stratified by Likelihood of CS Based on Combined Cardiac Magnetic Resonance and Positron Emission Tomographic Imaging
| Combined Likelihood Images | Δ Immunosuppressive Therapy* | Pacemaker Implantation | ICD Implantation | Any Change† |
|---|---|---|---|---|
| No sarcoid (<10%), n=11 | 0 (0%) | 2 (18%) | 9 (81%) | 9 (81%) |
| Possible CS (10%–50%), n=26 | 2 (7%) | 3 (11%) | 13 (50%) | 18 (69%) |
| Probable CS (50%–90%), n=41 | 6 (14%) | 5 (12%) | 21 (51%) | 25 (61%) |
| Highly probable CS (≥90%), n=29 | 12 (41%) | 4 (14%) | 22 (75%) | 23 (79%) |
| Total, n=107 | 20 (18%) | 15 (13%) | 65 (60%) | 75 (70%) |
Δ=Change in immunosuppressive therapy was defined as initiation of steroid therapy or addition of a nonsteroid immunosuppressive drug. CS indicates cardiac sarcoidosis; and ICD, implantable cardiac defibrillator.
There was a significant difference in change in immunosuppressive therapy across categories of increasing CS likelihood (P<0.01).
Any change was considered when ICD or a pacemaker was implanted or when there was a change in immunosuppressive therapy.
Adverse Events
During a median follow-up of 1.7 years (25th–75th percentile, 0.76–3.43), there were 27 events (7 deaths and 20 ventricular tachycardias). Of note, all 7 deaths were attributed to cardiovascular causes.
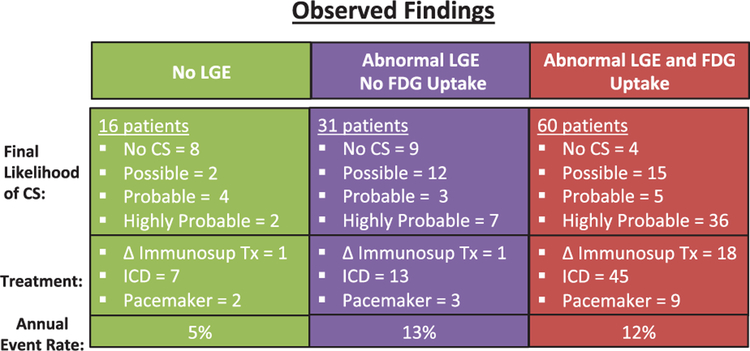
There were no significant differences in event rates when stratified by the various classifications used to determine likelihood of CS. Although patients with no sarcoidosis had the lowest numeric event rates, this difference was not statistically significant owing to the small size of the subgroups that had no disease. Furthermore, among patients with LGE, the presence or absence of FDG uptake was not associated with any differences in adverse events (Figure 5; P=0.85).
Figure 5.
Observed findings for cardiac sarcoidosis (CS) likelihood. This figure shows the final likelihood of CS, the observed change (Δ) in patient management with respect to immunosuppressive therapy, or implantation of ICD/pacemaker, as well as the annual event rate for each group. FDG indicates F18-fluorodeoxyglucose; ICD, implantable cardiac defibrillator; and LGE, late gadolinium enhancement.
Discussion
Our study showed that in selected patients with suspected CS, the combination of CMR and PET findings provides complementary value for the diagnosis and management of CS. We found that when PET information was added to CMR, ≈45% of patients were reclassified to having a higher or lower likelihood of CS, with 11% being reclassified to having highly probable (eg, >90%) likelihood. Furthermore, we found that 2 of every 3 patients with abnormal LGE uptake had myocardial inflammation by FDG PET. In such patients, having both LGE and FDG uptake yielded an even higher likelihood of CS and identified candidates for immunosuppressive therapies.
To our knowledge, this is the largest study to evaluate the complementary value of CMR and FDG PET in CS. Although several small prior studies have compared the findings of CMR to FDG PET,5–7,24,25 these have been limited by small sample sizes and older CS classification schemes.5,6 Importantly, these prior studies offered a head-to-head comparison rather than evaluating the complementary clinical value that results when both tests are used together.
The complementary value of CMR and PET imaging likely reflects the fact that each of these tests evaluates different aspects of the pathobiology of CS, which are relevant in clinical decision making. In other words, if the 2 tests offered similar data, the value of combining them would be substantially lower. Consistent with our results, the discordance between CMR and PET has also been described in other studies comparing these 2 techniques.
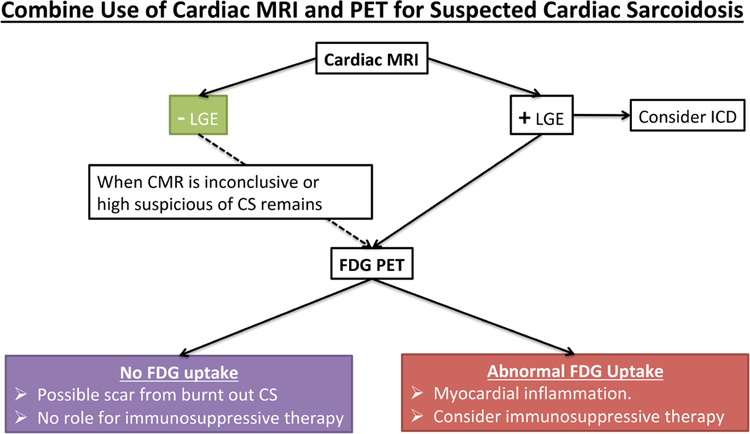
Nevertheless, not all patients being evaluated for CS require both CMR and PET imaging. Based on our findings, individuals who are most likely to benefit from PET after CMR include the following groups: (1) equivocal or negative magnetic resonance imaging findings in the setting of high clinical suspicion; (2) magnetic resonance imaging findings with highly probable CS—in such cases, FDG PET could serve to identify inflammation/potential role for immunosuppressive therapies. Conversely, there may be situations where CMR may be helpful after an inconclusive PET examination. For example, there may be diffuse FDG uptake involving the myocardium—a finding which could be because of incomplete suppression of FDG26 from presumably normal myocardium rather than diffuse inflammation.
Although both CMR and PET have been shown to have an important diagnostic and prognostic role in evaluating patients with known or suspected CS,17,18 there remain limitations to each technique and thus an important need to determine how to overcome these limitations by combining data from both exams. Our results suggest that combining technique enables clinicians to better determine the likelihood of CS and decide on the role of immunosuppressive therapies. Although the absence of LGE on CMR can rule out disease in most patients and identify a favorable prognosis, FDG PET provides a better assessment of the presence and extent of myocardial inflammation. Indeed, we found that among patients with LGE, approximately one third had no FDG uptake, and thus would not benefit from any immunosuppressive therapies. Although we observed that detecting inflammation by FDG PET was associated with higher use of immunosuppressive therapies, there is no randomized trial data to support the benefit of such treatments. Nevertheless, there are several small retrospective studies that suggest that immunosuppressive therapy may be associated with improved outcomes and a higher ejection fraction.2,27
Our study did not include T2 mapping by CMR, and it is possible that in the future, T2 mapping techniques may improve the ability of CMR to detect active inflammation,28 although to date, FDG imaging seems superior for such purposes. On the other hand, PET imaging can be limited when FDG cannot be adequately suppressed by the myocardium, and in the future, better tracers may offer more specific methods to detect myocardial inflammation.
A common question faced by clinicians is whether to use CMR or PET for the initial evaluation of patients with suspected CS. Based on prior recommendations18,26,29 and our study results (Figure 6), CMR is a reasonable first test in such cases. This recommendation is partly based on the fact that absence of LGE on CMR is associated with an excellent prognosis. Indeed, in a recent meta analysis of 7 studies, there were no cases of ventricular tachycardia among patients with suspected CS and no LGE, with an annual rate of cardiovascular death of only 0.6%.4,30,31 However, although rare, it remains possible to have CS, which has active inflammation by FDG PET but no LGE by CMR. In our study, 8 of 107 individuals had abnormal FDG uptake in the absence of LGE, although only 2 of them were ultimately categorized as having a high probability of CS on the final diagnosis. Similarly, Soussan et al24 evaluated 35 patients with suspected CS by CMR and PET and found 3 individuals with positive FDG who had negative LGE, noting that all of them were negative by the Japanese Ministry of Health, Labour, and Welfare criteria. Ohira et al7 evaluated 30 patients with suspected CS and found that 4 of 30 patients had abnormal FDG but negative CMR. In this study, the Japanese Ministry of Health, Labour, and Welfare 2006 criteria was used as the reference standard, and thus it remains unclear what proportion of individuals with isolated FDG uptake truly have CS versus having an alternative explanation for the observed FDG uptake.
Figure 6.
Algorithm for cardiac sarcoidosis (CS) diagnosis. A suggested algorithm for incorporating cardiac magnetic resonance imaging (MRI) and positron emission tomography (PET) for evaluating individuals with suspected CS. CMR indicates cardiac magnetic resonance; FDG, F18-fluorodeoxyglucose; and LGE, late gadolinium enhancement.
It is important to highlight that all of our CMR and PET interpretations were performed blinded to all clinical data. Interestingly, most cases read as highly probable had histo-logical evidence of sarcoidosis. Conversely, most cases interpreted as no CS had a clear alternative diagnosis once all clinical information was revealed.
Study Limitations
This study, as with all studies of the diagnostic accuracy for CS, is limited by the lack of an appropriate reference standard. Several prior studies have shown the limitation of various clinical criteria and of EMBx.19,32 Reflecting the low sensitivity and yield of EMBx, in our study, among 35 patients who had a negative EMBx, 32 had evidence of CS by CMR; of them, 21 also had abnormal FDG uptake by PET (Data Supplement). To overcome these limitations, our study created a comprehensive final diagnosis, which was used as the reference standard and which was based on all available medical records, imaging findings, pathology data, and follow-up data. By incorporating all available information available by longitudinal follow-up, our reference provides the optimum available method to determine the ultimate likelihood of CS. A similar method for establishing the reference standard has also been used by studies in other fields evaluating conditions in which a true gold standard may not be available. Although our final diagnosis (ie, reference standard) was influenced by imaging data, given the importance of imaging in the diagnosis of CS, it was not possible to have a reference standard that did not include CMR and PET findings. Finally, the fact that there were significant changes in in immunosuppressive therapy based on the combined CMR and PET imaging interpretation (which was performed blinded to all clinical data) reinforces the notion that this is clinically meaningful classification system.
Because the diagnosis of CS is often uncertain, particularly because there are other entities that can cause myocar-dial inflammation, we used 4 ordinal categories to estimate the likelihood of CS instead of using presence/absence of disease. Such a classification provides different degrees of certainty on the likelihood of CS, as is also often required in clinical practice when the diagnosis is unclear, and has also been used in prior statements.8 In such cases, when the diagnosis is unclear, the term inflammatory cardiomyopathy may be used when myocardial inflammation is detected. Although less specific, this term may be helpful when other inflammatory conditions could be present.
Our predefined criteria (Figure 2) were developed a priori and were based on available publications and prior experience on interpreting CMR and PET. Although these categories have not been validated previously, they were applied consistently to all imaging data in an objective manner, blinded to all clinical data. If further validated by others, these criteria could possibly serve as a useful clinical or research tool in the future. Although our results were based on the most robust imaging techniques available during the time of the study, future advances in imaging (eg, improved detection of inflammation by CMR via T2 mapping) could influence our findings.
Another important limitation of our study is referral bias, because the study only included individuals who were clinically referred to both CMR and PET. Although some patients were ordered to have both tests a priori, in many cases, the second test (often PET) could have been performed because of discrepant or inconclusive results from the first test (often magnetic resonance imaging). As a result, there was likely a higher than expected proportion of patients who had positive or inconclusive findings than would be expected. Nevertheless, these are precisely the type of patients who would be most likely to benefit from having both CMR and PET testing (ie, ones that have inconclusive or positive findings), and thus our findings are highly relevant for the subgroup of individuals for whom both tests are being considered.
Conclusions
Among selected patients with suspected CS, combining CMR and PET imaging provides important value for estimating the likelihood of CS and guiding patient management. Our results show that integrating data from both of these techniques can enhance the diagnostic certainty, particularly after inconclusive CMR or PET imaging results. Because 2 of every 3 patients with abnormal LGE uptake have myocardial inflammation, combining both tests is also helpful in identifying the need for immunosuppressive therapies.26,33
Supplementary Material
CLINICAL PERSPECTIVE.
The diagnosis and management of patients with suspected cardiac sarcoidosis present a significant challenge to general cardiologists, heart failure specialists, and electrophysiologists. In part, these challenges are related to the absence of a single reliable method to detect cardiac involvement, and accordingly, clinicians often must combine imaging and clinical data to estimate the likelihood of cardiac involvement. Several recent articles have suggested that advanced imaging techniques such as cardiac magnetic resonance (CMR) and F18-fluorodeoxyglucose positron emission tomography (PET) each provide useful diagnostic and prognostic information. However, no prior studies have examined how data from both of these tests can be combined. We evaluated 107 patients with suspected cardiac sarcoidosis who underwent both CMR and PET as part of their clinical care. We used prior studies and expert consensus statements to categorize the likelihood of cardiac sarcoidosis as absent, possible, probable, or highly probable. We found that when PET information was added to CMR, ≈45% of patients were reclassified to having a higher or lower likelihood of cardiac sarcoidosis. We also found that the findings provided by these tests had a significant impact on the use of immunosuppressive therapies. Specifically, only ≈66% of patients with abnormal late gadolinium enhancement uptake had myocardial inflammation by F18-fluorodeoxyglucose PET. Our study supports the selective use of combining CMR and F18-fluorodeoxyglucose PET in cases where any 1 test provides equivocal results, or after CMR when F18-fluorodeoxyglucose PET could serve to identify inflammation, and thus a potential role for immunosuppressive therapies.
Acknowledgments
Sources of Funding
Drs Vita and Bravo were supported by the National Institutes of Health training grant T32-HL094301–05.
Footnotes
The Data Supplement is available at http://circimaging.ahajournals.org/lookup/suppl/doi:10.1161/CIRCIMAGING.117.007030/-/DC1.
Disclosures
None.
References
- 1.Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, Kazemian P, Kwong RY, Tokuda M, Skali H, Padera R, Hainer J, Stevenson WG, Dorbala S, Di Carli MF. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osborne MT, Hulten EA, Singh A, Waller AH, Bittencourt MS, Stewart GC, Hainer J, Murthy VL, Skali H, Dorbala S, Di Carli MF, Blankstein R. Reduction in18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol. 2014;21:166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 3.Greulich S, Deluigi CC, Gloekler S, Wahl A, Zürn C, Kramer U, Nothnagel D, Bültel H, Schumm J, Grün S, Ong P, Wagner A, Schneider S, Nassenstein K, Gawaz M, Sechtem U, Bruder O, Mahrholdt H. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging. 2013;6:501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 4.Hulten E, Agarwal V, Cahill M, Cole G, Vita T, Parrish S, Bittencourt MS, Murthy VL, Kwong R, Di Carli MF, Blankstein R. Presence of late gadolinium enhancement by cardiac magnetic resonance among patients with suspected cardiac sarcoidosis is associated with adverse cardiovascular prognosis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2016;9:e005001. doi: 10.1161/CIRCIMAGING.116.005001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orii M, Hirata K, Tanimoto T, Ota S, Shiono Y, Yamano T, Matsuo Y, Ino Y, Yamaguchi T, Kubo T, Tanaka A, Akasaka T. Comparison of cardiac MRI and 18F-FDG positron emission tomography manifestations and regional response to corticosteroid therapy in newly diagnosed cardiac sarcoidosis with complet heart block. Heart Rhythm. 2015;12:2477–2485. doi: 10.1016/j.hrthm.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Ohira H, Birnie DH, Pena E, Bernick J, Mc Ardle B, Leung E, Wells GA, Yoshinaga K, Tsujino I, Sato T, Manabe O, Oyama-Manabe N, Nishimura M, Tamaki N, Dick A, Dennie C, Klein R, Renaud J, deKemp RA, Ruddy TD, Chow BJ, Davies R, Hessian R, Liu P, Beanlands RS, Nery PB. Comparison of (18)F-fluorodeoxyglucose positron emission tomography (FDG PET) and cardiac magnetic resonance (CMR) in corticosteroid-naive patients with conduction system disease due to cardiac sarcoidosis. Eur J Nucl Med Mol Imaging. 2016;43:259–269. doi: 10.1007/s00259-015-3181-8. [DOI] [PubMed] [Google Scholar]
- 7.Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. doi: 10.1007/s00259-007-0650-8. [DOI] [PubMed] [Google Scholar]
- 8.Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, Sweiss N, Crouser E, Morgenthau AS, Lower EE, Azuma A, Ishihara M, Morimoto S, Tetsuo Yamaguchi T, Shijubo N, Grutters JC, Rosenbach M, Li HP, Rottoli P, Inoue Y, Prasse A, Baughman RP, Organ Assessment Instrument Investigators TW. The WASOG sarcoidosis organ assessment instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 9.Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Nagai T, Kohsaka S, Okuda S, Anzai T, Asano K, Fukuda K. Incidence and prognostic significance of myocardial late gadolinium enhancement in patients with sarcoidosis without cardiac manifestation. Chest. 2014;146:1064–1072. doi: 10.1378/chest.14-0139. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Cawley PJ, Heitner JF, Klem I, Parker MA, Jaroudi WA, Meine TJ, White JB, Elliott MD, Kim HW, Judd RM, Kim RJ. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120:1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe E, Kimura F, Nakajima T, Hiroe M, Kasai Y, Nagata M, Kawana M, Hagiwara N. Late gadolinium enhancement in cardiac sarcoidosis: characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging. 2013;28:60–66. doi: 10.1097/RTI.0b013e3182761830. [DOI] [PubMed] [Google Scholar]
- 13.Ichinose A, Otani H, Oikawa M, Takase K, Saito H, Shimokawa H, Takahashi S. MRI of cardiac sarcoidosis: basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR Am J Roentgenol. 2008;191:862–869. doi: 10.2214/AJR.07.3089. [DOI] [PubMed] [Google Scholar]
- 14.Cheong BY, Muthupillai R, Nemeth M, Lambert B, Dees D, Huber S, Castriotta R, Flamm SD. The utility of delayed-enhancement magnetic resonance imaging for identifying nonischemic myocardial fibrosis in asymptomatic patients with biopsy-proven systemic sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 2009;26:39–46. [PubMed] [Google Scholar]
- 15.Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29:89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 16.Crawford T, Mueller G, Sarsam S, Prasitdumrong H, Chaiyen N, Gu X, Schuller J, Kron J, Nour KA, Cheng A, Ji SY, Feinstein S, Gupta S, Ilg K, Sinno M, Abu-Hashish S, Al-Mallah M, Sauer WH, Ellenbogen K, Morady F, Bogun F. Magnetic resonance imaging for identifying patients with cardiac sarcoidosis and preserved or mildly reduced left ventricular function at risk of ventricular arrhythmias. Circ Arrhythm Electrophysiol. 2014;7:1109–1115. doi: 10.1161/CIRCEP.113.000156. [DOI] [PubMed] [Google Scholar]
- 17.Blankstein R, Waller AH. Evaluation of known or suspected cardiac sarcoidosis. Circ Cardiovasc Imaging. 2016;9:e000867. doi: 10.1161/CIRCIMAGING.113.000867. [DOI] [PubMed] [Google Scholar]
- 18.Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis-state of the art review. Cardiovasc Diagn Ther. 2016;6:50–63. doi: 10.3978/j.issn.2223-3652.2015.12.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley O, Doyle L, Padera R, Lakdawala N, Dorbala S, Di Carli M, Kwong R, Desai A, Blankstein R. Cardiomyopathy of uncertain etiology: complementary role of multimodality imaging with cardiac MRI and 18FDG PET. J Nucl Cardiol. 2010;17:328–332. doi: 10.1007/s12350-009-9145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dilsizian V, Bacharach SL, Beanlands RS, Bergmann SR, Delbeke D, Dorbala S, Gropler RJ, Knuuti J, Schelbert HR, Travin MI. ASNC imaging guidelines/SNMMI procedure standard for positron emission tomography (PET) nuclear cardiology procedures. J Nucl Cardiol. 2016;23:1187–1226. doi: 10.1007/s12350-016-0522-3. [DOI] [PubMed] [Google Scholar]
- 21.Ishimaru S, Tsujino I, Takei T, Tsukamoto E, Sakaue S, Kamigaki M, Ito N, Ohira H, Ikeda D, Tamaki N, Nishimura M. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J. 2005;26:1538–1543. doi: 10.1093/eurheartj/ehi180. [DOI] [PubMed] [Google Scholar]
- 22.Yamagishi H, Shirai N, Takagi M, Yoshiyama M, Akioka K, Takeuchi K, Yoshikawa J. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18) F-FDG PET. J Nucl Med. 2003;44:1030–1036. [PubMed] [Google Scholar]
- 23.Ahmadian A, Brogan A, Berman J, Sverdlov AL, Mercier G, Mazzini M, Govender P, Ruberg FL, Miller EJ. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol. 2014;21:925–939. doi: 10.1007/s12350-014-9901-9. [DOI] [PubMed] [Google Scholar]
- 24.Soussan M, Brillet PY, Nunes H, Pop G, Ouvrier MJ, Naggara N, Valeyre D, Weinmann P. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–127. doi: 10.1007/s12350-012-9653-3. [DOI] [PubMed] [Google Scholar]
- 25.Tung R, Bauer B, Schelbert H, Lynch JP III, Auerbach M, Gupta P, Schiepers C, Chan S, Ferris J, Barrio M, Ajijola O, Bradfield J, Shivkumar K. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: the potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–2498. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Osborne MT, Hulten EA, Murthy VL, Skali H, Taqueti VR, Dorbala S, DiCarli MF, Blankstein R. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24:86–99. doi: 10.1007/s12350-016-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yazaki Y, Isobe M, Hiroe M, Morimoto S, Hiramitsu S, Nakano T, Izumi T, Sekiguchi M; Central Japan Heart Study Group. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol. 2001;88:1006–1010. doi: 10.1016/S0002-9149(01)01978-6. [DOI] [PubMed] [Google Scholar]
- 28.Crouser ED, Ono C, Tran T, He X, Raman SV. Improved detection of cardiac sarcoidosis using magnetic resonance with myocardial T2 mapping. Am J Respir Crit Care Med. 2014;189:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Orii M, Imanishi T, Akasaka T. Assessment of cardiac sarcoidosis with advanced imaging modalities. Biomed Res Int. 2014;2014:897956. doi: 10.1155/2014/897956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtagh G, Laffin LJ, Beshai JF, Maffessanti F, Bonham CA, Patel AV, Yu Z, Addetia K, Mor-Avi V, Moss JD, Hogarth DK, Sweiss NJ, Lang RM, Patel AR. Prognosis of myocardial damage in sarcoidosis patients with preserved left ventricular ejection fraction: risk stratification using cardiovascular magnetic resonance. Circ Cardiovasc Imaging. 2016;9:e003738. doi: 10.1161/CIRCIMAGING.115.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coleman GC, Shaw PW, Balfour PC Jr, Gonzalez JA, Kramer CM, Patel AR, Salerno M. Prognostic value of myocardial scarring on CMR in patients with cardiac sarcoidosis. JACC Cardiovasc Imaging. 2017;10:411–420. doi: 10.1016/j.jcmg.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uemura A, Morimoto S, Hiramitsu S, Kato Y, Ito T, Hishida H. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J. 1999;138(2 pt 1):299–302. [DOI] [PubMed] [Google Scholar]
- 33.Ahmadian A, Pawar S, Govender P, Berman J, Ruberg FL, Miller EJ. The response of FDG uptake to immunosuppressive treatment on FDG PET/CT imaging for cardiac sarcoidosis. J Nucl Cardiol. 2017;24:413–424. doi: 10.1007/s12350-016-0490-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.