Abstract
Purpose
We hypothesized that effort–reward imbalance (ERI) is associated with an atypical cortisol response. ERI has been associated with higher job stress. Stress triggers cortisol secretion via the hypothalamic–pituitary–adrenal (HPA) axis, and significant deviation from a typical cortisol pattern can indicate HPA axis dysfunction.
Methods
176 police officers participated from the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) Study. ERI was the exposure variable. Outcome variables were saliva-based peak and mean cortisol values, total area under the curve ground (AUCG) and baseline (AUCI); linear regression line fitted to log-transformed cortisol. Regression analyses were used to examine linear trend between ERI and cortisol parameters. Repeated measures analysis examined whether the pattern of cortisol over time differed between low ERI (< median) and high ERI (≥ median).
Results
Mean age was 46 years (SD = 6.6). After adjustment for potential confounders, there was a significant inverse association between ERI and peak cortisol (β = −0.20, p = 0.009), average cortisol (β = −0.23, p = 0.003), and total area under the curve (β = −0.21, p = 0.009). ERI was not significantly associated with AUCI (β = −0.11, p = 0.214); slope of the regression line fitted to the cortisol profile (β = −0.009, p = 0.908). Repeated measures analyses showed that the cortisol pattern did not vary significantly between high and low ERI using the median as a cut point (interaction p value = 0.790).
Conclusions
ERI was inversely associated with the magnitude of awakening cortisol over time, indicating HPA axis dys-regulation and potential future health outcomes.
Keywords: Police, Stress, Effort reward imbalance, Awakening cortisol, HPA axis dysregulation
Introduction
A positive social balance between employer and employee is an important factor in maintaining well-being at work. Siegrist’s (1996) ERI model proposes that a healthful and less stressful work environment depends to some degree on an equitable balance between job demands and rewards. The ERI model posits assumptions concerning relationships with poor health. First, high efforts in combination with low rewards increase the risk of poor health. Second, overcommitment to work may increase the risk of poor health, and, third, ERI together with overcommitment may interact to increase the risk of poor health. These hypotheses are well described in prior literature including the work by Van Vegchel et al. (2005) who reviewed 45 empirical studies on this topic.
ERI and the physiological stress response
Chronic exposure to stress at work including ERI can bring about biological change (Bellingrath et al. 2008). Eller et al. (2012) found that higher ERI was associated with lower awakening cortisol levels in women. Job stress significantly influenced both the awakening cortisol magnitude and the cortisol awakening response (Maina et al. 2009). In a review of studies to identify factors affecting cortisol awakening response (CAR), the rise in cortisol within 20–30 min following awakening, Fries et al. (2009) concluded that CAR was associated with anticipation of demands in the upcoming day. Stress triggers the hypothalamic–pituitary–adrenal (HPA) axis in the body, which responds by secreting cortisol intended to regain homeostasis (McEwen and Wingfield 2003). A problem may exist when stress is chronic, leading to inability of the HPA axis to return bodily functions to homeostasis. This has been termed allostatic overload by McEwen (2008). HPA axis dysfunction can be detected with changes in normal patterns or magnitudes of cortisol secretion (Motzer and Hertig 2004). One method of detecting a cortisol pattern is by examining awakening cortisol response, which in healthy individuals is characterized by a steady peaking at about 20–30 min following awakening and gradual reduction over a short period of time. Any significant deviation from this pattern can be considered an indicator of HPA axis dysfunction. A meta-analysis of 62 studies concluded that job stress and general life stress were positively associated with changes in awakening cortisol measures (Chida and Steptoe 2009). A recent meta-analysis of 32 studies (Eddy et al. 2018) that examined ERI in relation to various physiologic measures of the HPA axis reported an association between greater ERI and increased HPA axis activity.
HPA axis disruption may occur in occupations like policing where stress is evident. Stress-related conditions such as depression, posttraumatic stress, and burnout have been previously reported in police work (Violanti et al. 2017; Burke 2017; Berg et al. 2006; Charles et al. 2011). ERI may be a contributing source of stress in the police environment. Studies conducted on ERI in police work demonstrate lack of an adequate reward structure. For example, low organizational support, high workload, job insecurity, insufficient pay, and excessive paperwork are frequently mentioned (Spielberger et al. 1981). Janzen et al. (2007) found that higher levels of ERI were associated with greater psychological distress among police officers. Basińska and Wiciak (2013) found that ERI was a source of occupational stress in mid-level police managers.
ERI is a known source of occupational stress with an estimated prevalence of 32% and has long been considered a risk factor for poor health (Siegrist 1996, 1998, 2002; van Vegchel et al. 2005; de Jonge et al. 2000; Godin et al. 2005; Kikuchi et al. 2010; Kivimaki et al. 2007; Lehr et al. 2009; Dragano et al. 2017). These studies report strong associations between ERI and a number of health outcomes including increased morbidity, poor self-rated health, mental distress, depression, psychosomatic and physical health complaints, and incident coronary heart disease (CHD). For example, a study in German teachers (Lehr et al. 2009) showed that lack of esteem by supervisors and colleagues has larger impact on depression compared to low salary or job insecurity. A review of 45 empirical studies also concluded that high efforts in combination with low rewards increase the risk of poor health (van Vegchel et al. 2005). Lower levels of support and reward in conjunction with higher levels of effort and overcommitment were found to be associated with depression in special force police officers (Garbarino et al. 2013). Allisey et al. (2016) found that higher levels of reward (social support, recognition, and job security) in relation to efforts were associated with lower levels of absenteeism among police officers. A recent review of 11 European prospective studies reported that, during a 10-year follow-up time, individuals with ERI had 16% higher risk of developing CHD compared to those who did not experience ERI (Dragano et al. 2017).
In the present study, we examined whether or not ERI in police work is associated with HPA axis functioning as reflected in the magnitude of awakening cortisol and the cortisol awakening response (CAR) or pattern upon awakening. Studies of ERI and CAR among police officers are scarce. To our knowledge, there was one prior study by Izawa et al. (2016) that reported inverse association between ERI and morning cortisol levels indicating an effect on the HPA axis and risk for future cardiovascular disease. Given the limited literature on the association of ERI with awakening cortisol among police officers, the present study will add a body of knowledge to this relationship in high stress occupations.
Methods
Study population and design
Study participants for the current analyses were officers enrolled in the Buffalo Cardio-Metabolic Occupational Police Stress (BCOPS) study (Violanti et al. 2006). The BCOPS study was a cross-sectional study, with prospective component, aimed at investigating the associations of occupational stressors experienced in police work with the psychological and physiological health of police officers. At the baseline examination, a total of 710 police officers who worked at the Buffalo Police Department in New York were invited to participate in the BCOPS study; 464 (65.4%) officers agreed to participate and were examined during the period of June 4, 2004 to October 2, 2009. No specific inclusion criteria were indicated for the study, only that participants be sworn police officers and willing to participate. A written informed consent was collected from each participant. The study was approved by the Internal Review Boards of the State University of New York at Buffalo. Of the 464 officers examined at baseline, 281 were re-examined during the first follow-up study nearly 7 years later (2011–2014). Data during both examinations were collected, using standardized procedures, at the Center for Health Research, School of Public Health and Health Professions, University of Buffalo, State University of New York (Violanti et al. 2006). The cross-sectional analysis for the present study was conducted using data only from the first follow-up study (n = 176 non-retired participants with completed data on variables of interest).
Measures
Standardized instruments and protocols were used to collect data including demographic, lifestyle, physical, biological, occupational, and psychosocial characteristics from each BCOPS study participant. Siegrist’s ERI model (1996) was used to assess effort–reward imbalance (ERI) and served as the exposure variable of interest for the current analyses. Salivary cortisol values collected at awakening were used as outcome variables of interest. Demographic and lifestyle characteristics were used as potential confounders for adjustment of the main association of interest between ERI and awakening cortisol response.
Assessment of ERI
Siegrist’s ERI questionnaire is a standardized and self-report measure of ERI. The ERI scale used in this study consists of a total of 23 items that assess occupational effort (six items), reward (11 items), and overcommitment (six items). ERI was not assessed during the baseline examination of the BCOPS study, but was administered during the first follow-up examination.
Occupational effort is measured by six items that refer to demanding aspects of the current work environment (constant pressure due to heavy workload, many interruptions while performing job, lots of responsibility, pressure to work overtime, job being physically demanding, and over the past few years job being more and more demanding). Participants rated each item using a 5-point Likert type scale (1 disagree, 2 agree but I am not at all distressed, 3 agree and I am somewhat distressed, 4 agree and I am distressed, and 5 agree and I am very distressed) with higher ratings indicating higher efforts. A sum of score based on the six items was computed (theoretically, the total score for effort ranges from 6 to 30). A higher total score assumes that the participant experienced more extrinsic effort at work.
Occupational reward is measured by 11 items that assess three different aspects of reward: financial and status related (four items including poor promotion prospect, position adequately reflecting education, adequate promotion prospect considering effort and achievements, and adequate salary considering effort and achievements), esteem rewards (five items including receiving respect from supervisors, colleagues, adequate support in difficult situations, being treated unfairly, and receiving respect and prestige given effort and achievements), and gratification of job security (two items including experiencing undesirable change in my work situation, and poor job security). Participants rated each item using a 5-point Likert-type scale. The positively worded items (e.g., receiving respect from supervisor) were rated using a scale that ranges from 1 (disagree and I am very distressed) to 5 (agree). The negatively worded items (e.g., being treated unfairly at work) were rated using a scale that ranges from 1 (agree and i am very distressed) to 5 (disagree). Therefore, lower ratings for each item indicates lower rewards. A sum of scores based on ratings of these 11 items was computed (theoretically, the total score for reward ranges from 11 to 55). The lower the total score, the fewer are the occupational rewards received by the person. To identify ERI, the effort-reward ratio is calculated as follows: ERI = k × (E/R), where E is the total score for effort based on six items, R is the total score for reward based on 11 items, and k (k = 6/11) is a correction factor to adjust for the unequal number of items used to assess effort vs. reward. ERI is a continuous variable and an imbalance between ERI is present when the ratio is different from one (ERI ≠ 1), with ERI < 1 indicating high reward but low effort, and ERI > 1 indicating high effort but low reward.
Assessment of salivary cortisol
To assess the awakening cortisol response, subjects were instructed to collect saliva samples immediately after awakening (S1), and 15 (S2), 30 (S3), and 45 (S4) min, thereafter. On the date of the clinical examination, the officers were off work to participate in extensive examination and receive further training. The saliva samples were collected during a single day, on the morning of the day after the clinical examination, after which they performed their regularly scheduled work. Officers were provided with Salivettes (Sarstedt, USA), a commercially available collection device consisting of a dental roll and a centrifuge tube, for the collection of saliva samples. The officers were asked to refrain from taking stimulant medication, smoking, eating and drinking, and brushing their teeth before completing salivary sampling to avoid contamination of saliva with food or blood caused by micro-injuries of the oral cavity. At the designated collection time, the officers removed the dental roll from the centrifuge tube and placed it in their mouth for approximately 2 min allowing for saturation of the roll. The roll was then returned to the tube and samples were returned to the clinic and subsequently sent to the laboratory. Upon delivery, the tubes were centrifuged to provide a non-viscous saliva sample for assay of cortisol. Samples were maintained at −20 °C until being sent to the Technical University of Dresden for analysis of cortisol by a commercially available chemiluminescence immunoassay (IBL, Hamburg, Germany). The four cortisol values from the saliva samples and the corresponding times of collection were used to estimate the following five parameters: the peak cortisol value (maximum of S1, S2, S3, or S4), the mean cortisol value (average of S1, S2, S3, and S4), total area under the curve with respect to ground (AUCG), total area under the curve with respect to baseline (AUCI), and slope of the linear regression line fitted to the log-transformed cortisol data. The first three parameters are considered measures of total hormonal output, while the latter two parameters tend to assess the pattern of awakening cortisol pattern over time. The five cortisol parameters served as outcome variables of interest in the current analyses.
Assessment of covariates
Questionnaires were administered to collect demographic and lifestyle characteristics including age, gender, race/ethnicity, marital status, smoking status, educational status, alcohol consumption, years of police service, police rank, sleep duration, and physical activity level. Height and weight were measured with shoes removed and recorded to the nearest half centimeter and rounded up to the nearest quarter of a pound, respectively. Height and weight were converted to meters and kilograms, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Alcohol consumption was measured from data collected using Food Frequency Questionnaire (FFQ) where, among other things, the officers also reported how often they drank the following amounts of alcoholic beverages: beer (12 oz), red wine (6 oz), white or rose wine (6 oz), and liquor and mixed drinks (1.5 oz). The number of drinks per week was derived as the summed consumption of these amounts from the four types of alcoholic beverages. Hours of sleep in the past week were obtained by asking participants to report average duration of sleep per day during weekdays and weekends separately. Then average sleep duration per day in the past week was derived based on the weighted average over 5 weekdays and 2 weekend days. Hours of physical activity were assessed using the 7-day physical activity recall questionnaire developed in the Stanford Five-City Project (Sallis et al. 1985).
Statistical analysis
Among the 281 BCOPS study participants examined during the first follow-up examination, officers with missing data on ERI (n = 20), those with missing data on waking cortisol values and time of collection at any of the four time points (n = 51), and retired officers (n = 34) were excluded from analyses leaving a final sample size of n = 176 officers for the current analyses. Initial analyses included descriptive results to characterize the demographic and lifestyle characteristics of the study sample. Two statistical approaches were utilized for the main analyses depending on the nature of the research question. In the first approach, analysis of covariance was used for comparing mean levels of cortisol parameters between officers with low vs. high ERI. Officers were classified into the “low ERI” group if the effort/reward ratio < median (i.e., < 0.47) and into the “high ERI” group if the effort/reward ratio ≥ median (i.e., ≥ 0.47). Regression models were used for testing the linear trend between ERI and the cortisol parameters. In the second approach, repeated measures analyses were used to compare patterns of cortisol over time between officers with low vs. high ERI. We fitted a two-way repeated measures ANOVA involving interaction between levels of effort/reward ratio and time of saliva collection (defined as time since awakening in minutes entered into the model as a linear predictor). The statistical significance of the interaction terms was used to determine whether the pattern of cortisol over time depended on the effort/reward ratio. The MIXED procedure in SAS was used to model the repeated measures by applying the autoregressive covariance structure as a model for correlations among measurements made on the same subject. The unadjusted and multivariable adjusted associations were examined. The multivariate model adjusted for age, sex, race, marital status, education, rank, smoking status, BMI, physical activity, alcohol consumption, and sleep duration. A characteristic was considered to be a potential confounder (covariate) for adjustment in the multivariate model based on prior evidence from the literature and whether it was associated with either the exposure or outcome in the current analyses. All statistical analyses were performed using the SAS software version 9.4 (SAS Institute, Inc., Cary, NC), and significance level was set at 5% except for interaction terms (10%).
Results
Demographic and lifestyle characteristics
The demographic and lifestyle characteristics of the sample (n = 176) and their association with ERI are presented in Table 1. The study population consists of 70% males and the majority was white (77%), married (67%), never smokers (63%), and had a rank of patrol officer (57%). The mean age was 46.3 years (SD = 6.6) and on average officers were overweight (mean BMI = 29.3, SD = 4.4). The mean ERI was 0.51 (SD = 0.23, range 0.2–1.47) and only 4% of the study sample (n = 7) had an ERI that exceeded 1. The mean ERI for white officers was significantly higher than the mean ERI for blacks and Hispanics (0.54 ± 0.24 vs. 0.43 ± 0.16, p = 0.004). As expected, ERI was positively correlated with effort score (r = 0.92, p < 0.001) and negatively correlated with reward score (r = −0.68, p < 0.001). Comparison of the analytic sample (n = 176) with remaining members of the original cohort (n = 288) revealed that the two groups were similar with respect to the following baseline characteristics: sex (women 29 vs. 22%), race (whites 78 vs. 80%), marital status (married 71 vs. 77%), and alcohol consumption. However, we found differences between the two groups with respect to rank, education, years of service, and smoking status. The analytic sample consisted of participants with shorter years of service and higher proportion of patrol officers, those with four or more years of college education, and non-smokers.
Table 1.
Demographic and lifestyle characteristics of study participants and their association with ERI
| Characteristics | N | % (± SD)a | ERI (mean ± SD or r)£ | p value |
|---|---|---|---|---|
| Gender | 0.984 | |||
| Male | 123 | 69.9 | 0.51 ± 0.22 | |
| Female | 53 | 30.1 | 0.51 ± 0.25 | |
| Race | 0.004 | |||
| White | 135 | 76.7 | 0.54 ± 0.24 | |
| Black/Hispanic | 41 | 23.3 | 0.43 ± 0.16 | |
| Education | 0.342 | |||
| ≤ High school/GED | 11 | 6.3 | 0.43 ± 0.12 | |
| College < 4 years | 87 | 49.4 | 0.51 ± 0.23 | |
| College 4 + years | 78 | 44.3 | 0.53 ± 0.24 | |
| Marital status | 0.187 | |||
| Single | 20 | 11.4 | 0.60 ± 0.30 | |
| Married | 117 | 66.9 | 0.51 ± 0.20 | |
| Divorced | 38 | 21.7 | 0.48 ± 0.26 | |
| Smoking status | 0.156 | |||
| Current | 14 | 8.0 | 0.60 ± 0.31 | |
| Former | 50 | 28.6 | 0.55 ± 0.25 | |
| Never | 111 | 63.4 | 0.49 ± 0.20 | |
| Rank | 0.944 | |||
| Patrol officer | 100 | 56.8 | 0.51 ± 0.24 | |
| Sergeant, lieutenant, captain | 37 | 21.0 | 0.52 ± 0.23 | |
| Detective/executive/other | 39 | 22.2 | 0.51 ± 0.18 | |
| Age (in years) | 176 | 46.3 ± 6.6 | r = −0.08 | 0.291 |
| Years of service | 176 | 19.1 ± 6.8 | r = −0.13 | 0.087 |
| Body mass index (kg/m2) | 176 | 29.3 ± 4.4 | r = −0.13 | 0.080 |
| Physical activity index/week¥ | 176 | 10.8 ± 13.7 | r=0.10 | 0.199 |
| No. of alcohol drinks/week | 176 | 5.1 ± 9.5 | r=0.05 | 0.488 |
| Sleep h/day | 176 | 6.2 ± 1.1 | r=0.03 | 0.664 |
| Effort score | 176 | 13.4 ± 4.7 | r=0.92 | < 0.001 |
| Reward score | 176 | 49.4 ± 5.4 | r = −0.68 | < 0.001 |
| ERI | 176 | 0.51 ± 0.23 |
Estimates are proportions of participants at each level of categorical variables (e.g., sex) and means ± SD for continuous characteristics such as age
Physical activity index is a weighted average of hours in moderate (weight = 1), hard (weight = 2), and vigorous (weight = 3) activities that include occupational, household, and leisure time activities
Mean values (± SD) of ERI across categorical characteristics or correlation coefficient (r) between ERI and continuous characteristics
p value for the association of ERI with demographic factors, lifestyle variables, effort score, and reward score
ERI and awakening cortisol parameters
The unadjusted and multivariable adjusted associations between ERI and awakening cortisol parameters are shown in Table 2. Results, in both the unadjusted and multivariable adjusted models, indicate a significant negative association between ERI and awakening cortisol parameters that represent total hormonal output (peak, average cortisol, and total area under the curve). On the other hand, associations of ERI with awakening cortisol parameters that represent pattern over time (area under the curve with respect to baseline and slope of the regression line fitted to the awakening profile) were not statistically significant. Regression analyses of ERI and the awakening cortisol parameters showed a negative and significant association between ERI and the following awakening cortisol parameters (Table 2): peak cortisol (β = −0.15, p = 0.044), average cortisol (β = −0.19, p = 0.013), and total area under the curve (β = −0.18, p = 0.018). Further adjustment for demographic and lifestyle variables including age, gender, race, marital status, education, rank, smoking status, BMI, physical activity, alcohol consumption, and sleep duration did not attenuate these associations (Table 2). Following adjustment for these covariates, peak cortisol (β=−0.20, p=0.009), average cortisol (β = −0.23, p = 0.003), and total area under the curve (β = −0.21, p = 0.009) were all inversely and significantly associated with ERI, but area under the curve with respect to increase (β = −0.11, p = 0.214) and slope of the regression line fitted to the cortisol profile (β = −0.009, p=0.908) were not significantly associated with ERI (Table 2).
Table 2.
Unadjusted (± SD) and multivariable adjusted (± SE) mean values of the awakening cortisol parameters by categories of effort reward ratio
| Unadjusted model |
||||
|---|---|---|---|---|
| Low ERI [< median] (n = 88) | High ERI [≥ median] (N = 88) | p value1 | Beta (p value)2 | |
| Waking cortisol parameters | ||||
| Peak cortisol | 26.0 ± 13.0 | 22.0 ± 10.5 | 0.026 | −0.15 (0.044) |
| Average cortisol | 19.5 ± 9.9 | 15.9 ± 8.5 | 0.012 | −0.19 (0.013) |
| Total area under the curve (AUCG) | 1089 ± 1023 | 752 ± 415 | 0.005 | −0.18 (0.018) |
| Area under the curve with respect to baseline (AUCI) | 136 ± 572 | 145 ± 321 | 0.892 | −0.07 (0.386) |
| Slope of regression line fitted to waking cortisol pattern | 0.004 ± 0.03 | 0.011 ± 0.03 | 0.086 | −0.004 (0.956) |
| Multivariable¥ adjusted model |
||||
| Low ERI [< median] (n = 85) | High ERI [> median] (N = 85) | p value3 | Beta (p value)4 | |
| Waking cortisol parameters | ||||
| Peak cortisol | 26.4 ± 1.24 | 21.4 ± 1.24 | 0.006 | −0.20 (0.009) |
| Average cortisol | 19.8 ± 0.98 | 15.6 ± 0.98 | 0.003 | −0.23 (0.003) |
| Total area under the curve (AUCG) | 1102 ± 85 | 725 ± 85 | 0.003 | −0.21 (0.009) |
| Area under the curve with respect to baseline (AUCI) | 152 ± 51 | 151 ± 51 | 0.9861 | −0.11 (0.214) |
| Slope of regression line fitted to waking cortisol pattern | 0.005 ± 0.03 | 0.012 ± 0.03 | 0.072 | −0.009 (0.908) |
Bold values indicate statistical significance
ANOVA p value testing for differences in means between ERI categories (median ERI = 0.47)
The standardized regression coefficient and p value from simple linear regression testing for linear trend between ERI and each waking cortisol parameter
ANCOVA p value testing for differences in means between ERI categories (median ERI = 0.47)
The standardized regression coefficient and p value from multiple linear regression testing for linear trend between ERI and each waking cortisol parameter
Adjustment was made for age, gender, race, marital status, education, rank, smoking status, BMI, physical activity, alcohol consumption, and sleep duration
ERI and awakening cortisol patterns
To test whether differences in the awakening cortisol pattern depended on the level of ERI, a two-way repeated measures ANOVA involving interaction between categories of ERI and time of saliva collection (minutes from baseline) was fitted. The statistical significance of the interaction terms was used to determine whether the pattern of cortisol varied over time varied depending on the level of ERI. The result (Fig. 1) showed that the awakening cortisol profile did not vary significantly between the two groups of officers created using the median ERI as a cut point (Fig. 1, interaction p value = 0.790). The results from repeated measures analyses are consistent with the results from regression analyses of ERI on AUCI and slope presented in Table 2. This is because both AUCI and slope are awakening cortisol parameters that emphasize the changes over time rather than total hormonal output.
Fig. 1.
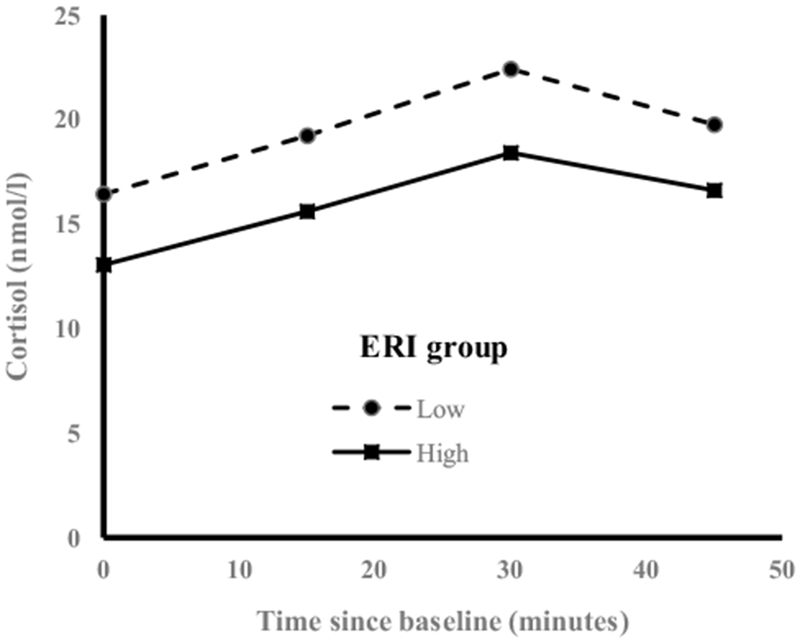
Awakening cortisol pattern by categories of ERI; awakening cortisol profile for officers with ERI < median vs. those with ERI ≥ median (median ERI = 0.47). A two-way repeated measures ANOVA involving interaction between categories of effort/reward ratio and time of saliva collection (defined as time since awakening in minutes entered into the model as a linear predictor) was fit. The interaction p value was 0.790 suggesting there is no difference in profile between ERI groups, but the magnitude of the awakening cortisol output was significantly smaller for officers with high imbalance between effort and reward
Discussion
Results indicated a significant negative association between ERI and magnitude of awakening cortisol. This result was similar to previous work where a high ERI was associated with a diminished cortisol response. Eller et al. (2012) found that higher ERI was associated with lower awakening cortisol levels. Bellingrath et al. (2008) reported suppressed cortisol response among individuals with low reward scores after a dexamethasone suppression test, indicating a less sensitive feedback of the HPA axis. Maina et al. (2009) found lower awakening cortisol associated with high job strain.
Other studies have found contrary results reporting no significant associations between ERI and lowered awakening cortisol (Hanson et al. 2000; Harris et al. 2007; Marchand et al. 2016; Ota et al. 2014). Steptoe et al. (2004) did not find a significant association of dysregulated CAR with ERI. Eller et al. (2011) found significant positive association between ERI and the level of cortisol among men. Karlson et al. (2012) commented that varying conditions of the workplace, type and duration of stress, and methodological factors may account for discordant findings. The meta-analysis by Eddy et al. (2018) specifically reported a positive association between ERI and the following two indices of the HPA axis: the overall volume of cortisol released during the waking period, and the increase in cortisol from awakening to 30 min post-awakening (often referred to as CAR); a result not consistent with the current findings.
An interesting result in the present study concerned the difference between awakening cortisol magnitude and the morning profile or pattern across levels of ERI. While ERI was significantly and inversely associated with awakening cortisol magnitude, there were no significant differences in awakening cortisol patterns between officers with low and high ERI. Differences in diminished cortisol magnitude may be related to unexamined factors that impact the effort and reward structure in the police occupation. The effects of stress are not limited to work; the effect on the HPA axis is continuous throughout the day and dependent on situational factors (Fries et al. 2009; Hellhammer et al. 2009; Karlson et al. 2012). Situational factors and stressors differ frequently in police work and have varied effects on cortisol levels (Violanti et al. 2017). Additionally, CAR is somewhat unrelated to cortisol levels during the rest of the day (Maina et al. 2009). Further research should consider extending cortisol testing to the entire day or possibly multiple days (Stalder et al. 2015).
Another interesting result which requires additional research was the differences in ERI between whites and people of color. While we do not have direct evidence of this in our present study, there is other research which indicates that 69% of people of color felt stressed because they were victims of racism or discrimination at work in terms of pay and promotion (Lee 2000; Carter and Forsyth 2010). Results in the present study suggest a higher ERI among blacks; however, the sample size is too small to draw any conclusions from this result.
The actions of police officers involve an interaction with the public and are subject to careful scrutiny. Balances of effort and reward may therefore be affected by public opinion of the police. Esteem is an example. In the present day in the USA, there is rather negative image of police and a lack of esteem is evident for the effort put forth by officers (President’s Task force on 21st Century Policing 2015). A negative public opinion may certainly affect components of reward and effort in this profession. Therefore, ERI in policing may be associated with organizational-based rewards and those from public sources. Further research is needed in the application of the ERI model to situations external from the police organization (Hanson et al. 2000).
The decreased magnitude of cortisol secretion associated with ERI may be an important factor in health outcomes. It would be of further interest to assess the psychological and physiological health of cortisol dysregulation among police officers. For example, previous studies have found decreases in waking cortisol magnitude associated with symptoms of posttraumatic stress disorder (PTSD) among police (Huber et al. 2006; Neylan et al. 2005). Changes in cortisol secretion have been associated with cardiovascular and other diseases (Gidron and Ronson 2008). Eller et al. (2005) found an association between CAR and the progression of intima media thickness (IMT), a risk marker of cardiovascular disease. Kuper et al. (2002) found that ERI predicted a higher risk of cardiovascular disease during the Whitehall II study follow-up. There is also evidence that police have an increased risk for cardiovascular disease and early mortality (Vena et al. 1986, 2014; Feuer and Rosenman 1986; Forastiere et al. 1994; Franke and Anderson 1994).
The strengths of this study include the use of a standardized protocol and validated measures for ERI and covariates, albeit self-report. Regarding cortisol measures, participants were given detailed instructions on how to collect samples immediately upon awakening and to record the exact times of collection. The study had an adequate response rate and statistical power. Several limitations should be kept in mind when interpreting the present findings. The study was cross-sectional and does not imply causality or direction of influence. These results should be generalized to other police agencies with care, as departments vary in size and geographic location. It is worth noting that the analytic sample (n = 176) differed from the other members of the original cohort (n = 288) with respect to rank, education, years of service, and smoking status. We have only examined the ratio between effort and reward. Additional work should be considered in terms of the reward structure in police work. Esteem, financial, gratification, and fair treatment are examples. It would be of interest to determine the types of reward that are beneficial in balancing the ERI ratio at work. For example, officers frequently report being treated unfairly in organizational disputes or disciplinary issues. They often feel a lack of procedural justice in providing resolutions to dispute and decision-making (Community Oriented Police Services Newsletter 2013). A sense of esteem and social support by the organization is another example. Support has been found to make officers more resilient to stressful incidents at work (Prati and Pietrantoni 2010).
In conclusion, the examination of ERI and cortisol among police officers indicated that imbalance was associated with a significantly diminished magnitude of cortisol secretion among officers upon awakening. This is one indication of a dysregulated HPA axis function and possible disease states. The multivariate model was adjusted for potential confounders that did not attenuate these findings. The present results provide clues as to the possible association of ERI with biological consequences among police officers and suggests that altering certain components of the workplace that leads to ERI may result in improved health and there is a need for intervention studies in this area.
Acknowledgments
Funding: This study was funded by the National Institute for Occupational Safety and Health, Contract #200-2003-01580. The funders had no involvement in the design, data collection, analysis, interpretation, or writing the manuscript or decision to publish.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Conflict of interest This research was conducted at The State University of New York at Buffalo, Buffalo, NY, USA, and was funded by the National Institute for Occupational Safety and Health (NIOSH), Contract no. 200-2003-01580. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethical approval All procedures performed in this study involving human participants were in accordance with the ethical standards of the University at Buffalo, State University of New York Internal Review Board, and duly approved by the review board.
Informed consent Informed consent was obtained from all participants included in the study.
References
- Allisey A, Rodwell J, Noblet A (2016) An application of an extended effort–reward imbalance model to police absenteeism behavior. Pers Rev 45:663–668 [Google Scholar]
- Basińska B, Wiciak I (2013) Evaluation of professional demands and financial reward through the perception of police managers. Intern Secur 5:171–184 [Google Scholar]
- Bellingrath S, Weigl T, Kudielka B (2008) Cortisol dysregulation in school teachers in relation to burnout, vital exhaustion, and effort–reward imbalance. Biol Psychol 78:104–113. 10.1016/j.biopsycho.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Berg A, Hem E, Lau B, Ekeberg O (2006) An exploration of job stress and health in the Norwegian police service: a cross sectional study. J Occup Med Toxicol 26:1–9. 10.1186/1745-6673-1-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R (2017) Stress in policing: an overview In: Burke RJ (ed) Stress in policing: sources, consequences, and interventions. Routledge, London, pp 3–28 [Google Scholar]
- Carter RT, Forsyth J (2010) Reactions to racial discrimination: emotional stress and help-seeking behaviors. Psychol Trauma 2(3):183–191 [Google Scholar]
- Charles L, Violanti J, Gu J, Fekedulegn D, Andrew M, Burchfiel C (2011) Sleep duration and biomarkers of metabolic function among police officers. J Occup Environ Med 53:831–837 [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A (2009) Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol 80:265–278 [DOI] [PubMed] [Google Scholar]
- Community Oriented Police Services (COPS) Newsletter (2013) That’s not fair! Policing and perceptions of fairness, vol 6, pp 5–6 [Google Scholar]
- de Jonge J, Bosma H, Peter R, Siegrist J (2000) Job strain, effort–reward imbalance and employee well-being: a large-scale cross-sectional study. Soc Sci Med 50:1317–1327. 10.1177/0093854803254432 [DOI] [PubMed] [Google Scholar]
- Dragano N, Siegrist J, Nyberg ST et al. (2017) Effort–reward imbalance at work and incident coronary heart disease: a multicohort study of 90,164 individuals. Epidemiology 28(4):619–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy P, Wertheim EH, Hale MW, Wright BJ (2018) A systematic review and meta-analysis of the effort–reward imbalance model of workplace stress and hypothalamic-pituitary-adrenal axis measures of stress. Psychosom Med 80(1):103–113 [DOI] [PubMed] [Google Scholar]
- Eller NH, Netterstrom B, Allerup P (2005) Progression in intima media thickness—the significance of hormonal biomarkers of chronic stress. Psychoneuroendocrinology 30:715–723 [DOI] [PubMed] [Google Scholar]
- Eller NH, Kritiansen J, Hansen AM (2011) Long-term effects of psychosocial factors of home and work on biomarkers of stress. Int J Psychophysiol 79:195–202 [DOI] [PubMed] [Google Scholar]
- Eller NH, Nielsen SF, Blond M, Nielsen ML, Hamsen AM, Nettterstrom B (2012) Effort reward imbalance, and salivary cortisol in the morning. Biol Psychol 89:342–348 [DOI] [PubMed] [Google Scholar]
- Feuer E, Rosenman K (1986) Mortality in police and firefighters in New Jersey. Am J Ind Med 9:517–27 [DOI] [PubMed] [Google Scholar]
- Forastiere F, Perucci CA, Miceli M, Rapiti E, Bargagli A, Borgia P (1994) Mortality among urban policemen in Rome. Am J Ind Med 26:785–98 [DOI] [PubMed] [Google Scholar]
- Franke WD, Anderson DF (1994) Relationship between physical activity and risk factors for cardiovascular disease among law enforcement officers. J Occup Med 36:1127–1132 [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C (2009) The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 72:67–73 [DOI] [PubMed] [Google Scholar]
- Garbarino S, Cuomo G, Chiorri C, Magnavita N (2013) Association of work-related stress with mental health problems in a special police force unit. BMJ Open 3:e002791 10.1136/bmjopen-2013-00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidron Y, Ronson A (2008) Psychosocial factors, biological mediators, and cancer prognosis: a new look at an old story. Curr Opin Oncol 20:386–392 [DOI] [PubMed] [Google Scholar]
- Godin I, Kittel F, Coppieters Y, Seigrist J (2005) A prospective study of cumulative job stress in relation to mental health. BMC Public Health 5:67–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson EKS, Maaas CJM, Meijman TF, Godaert GLR (2000) Cortisol secretion throughout the day, perceptions of the work environment, and negative affect. Ann Behav Med 22:316–324 [DOI] [PubMed] [Google Scholar]
- Harris A, Ursin H, Murison R, Eriksen HR (2007) Coffee, stress and cortisol in nursing staff. Psychoneuroendocrinology 32:322–330 [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM (2009) Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–171 [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT (2006) The cortisol awakening response is blunted in psychotherapy inpatients suffering from depression. Psychoneuroendocrinology 31:900–904 [DOI] [PubMed] [Google Scholar]
- Izawa S, Tsutsumi A, Ogawa N (2016) Effort–reward imbalance, cortisol secretion, and inflammatory activity in police officers with 24.h work shifts. Int Arch Occup Environ Health 89:1147–1154. 10.1007/s00420-016-1154-1162 [DOI] [PubMed] [Google Scholar]
- Janzen B, Muhajarine N, Zhu T (2007) Effort–reward imbalance, overcommitment, and psychological distress in Canadian police officers. Psychol Rep 100:525–530 [DOI] [PubMed] [Google Scholar]
- Karlson B, Lindfors P, Riva R, Mellner C, Theorell T (2012) Psychosocial work stressors and salivary cortisol In: Kristenson M, Garvin P, Lundberg U (eds) The role of saliva cortisol measurement in health and disease. Bentham Science Publishers, Bussum, pp 43–66 [Google Scholar]
- Kikuchi Y, Nakaya M, Ikeda M, Narita K, Takeda M, Nishi M (2010) Effort–reward imbalance and depressive state in nurses. Occup Med 60:231–233 [DOI] [PubMed] [Google Scholar]
- Kivimaki M, Vahtera J, Elovainio M, Virtanen M, Siegrist J (2007) Effort–reward imbalance, procedural injustice and relational injustice as psychosocial predictors of health: complementary or redundant models? Occup Environ Med 64:659–665. 10.1136/oem.2006.031310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuper H, Sigh-Manoux A, Marmot M (2002) When reciprocity fails: effort–reward imbalance in relation to coronary heart disease and health functioning within the Whitehall II study. Occup Environ Med 59:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J (2000) The salience of race in everyday life: black customers’shopping experiences in black and white neighborhoods. Work Occup 27:353–376 [Google Scholar]
- Lehr D, Hillbert A, Keller S (2009) What can balance the effort? Associations between effort–reward imbalance, over-commitment and affective disorders in German teachers. Int J Occup Environ Health 15:374–384 [DOI] [PubMed] [Google Scholar]
- Maina G, Palmas A, Bovenzi M, Filon FL (2009) Salivary cortisol and psychosocial hazards at work. Am J Ind Med 52:251–260 [DOI] [PubMed] [Google Scholar]
- Marchand A, Juster R,P, Durand P, Lupien S,J (2016) Work stress models and diurnal cortisol variations: the SALVEO study. J Occup Health Psychol 21:182–193. 10.1037/a0039674 [DOI] [PubMed] [Google Scholar]
- McEwen (2008) Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol 583:174–185. 10.1016/j.ejphar.2007.11.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Wingfield JC (2003) The concept of allostasis in biology and biomedicine. Horm Behav 43:2–15 [DOI] [PubMed] [Google Scholar]
- Motzer SA, Hertig V (2004) Stress, stress response, and health. Nurs Clin North Am 39:1–17 [DOI] [PubMed] [Google Scholar]
- Neylan TC, Brunet A, Pole N, Best SR, Metzler TJ, Yehuda R, Marmar CR (2005) PTSD symptoms predict waking salivary cortisol levels in police officers. Psychoneuroendocrinology 30:373–381 [DOI] [PubMed] [Google Scholar]
- Ota A, Mase J, Howteerakul N, Rajatanun T, Suwannapong N, Yatsuya H (2014) The effort–reward imbalance work-stress model and daytime salivary cortisol and dehydroepiandrosterone (DHEA) among Japanese women. Sci Rep 4:6402 10.1038/srep06402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prati G, Pietrantoni L (2010) Risk and resilience factors among Italian municipal police officers exposed to critical incidents. J Police Crim Psychol 25:27–33. 10.1007/s11896-009-9052 [DOI] [Google Scholar]
- President’s Task Force on 21st Century Policing (2015) Final report of the President’s Task Force on 21st Century Policing. Office of Community Oriented Policing Services, Washington, DC [Google Scholar]
- Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS Jr (1985) Physical activity assessment methodology in the Five-City Project. Am J Epidemiol 121:91–106 [DOI] [PubMed] [Google Scholar]
- Siegrist J (1996) Adverse health effects of high-effort/low-reward conditions. J Occup Health Psychol 1:27–41 [DOI] [PubMed] [Google Scholar]
- Siegrist J (1998) Adverse health effects of effort–reward imbalance at work:theory, empirical support, and implications for prevention In: Cooper CL (ed) Theories of organizational stress. Oxford University Press, Oxford, pp 190–204 [Google Scholar]
- Siegrist J (2002) Effort–reward imbalance at work and health. In: Perrewe PL, Ganster DC (eds) Historical and current perspectives on stress and health. JAI Elsevier, Amsterdam, pp 261–291 [Google Scholar]
- Spielberger C, Westberry L, Grier K, Greenfield G (1981) The police stress survey: sources of stress in law enforcement, Human Resources Institute Monograph Series Three, No. 6. University of South Florida, College of Social and Behavioral Sciences, Tampa, FL [Google Scholar]
- Stalder T, Kirshbuam C, Kudielka BM, Adam EK, Pruessner JC, Wust C, Evans P et al. (2015). Assessment of the cortisol awakening response: expert consensus guidelines. Psychoneuroendocrinology. 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Siegrist J, Kisrchbaum C, Marmot M (2004) Effort-reward imbalance, overcommitment, and measures of cortisol and blood pressure over the working day. Psychosom Med 66:323–329. 10.1097/01.psy.0000126198.67070.72 [DOI] [PubMed] [Google Scholar]
- Van Vegchel N, de Jonge J, Bosma H, Schaufeli W (2005) Reviewing the effort–reward imbalance model: drawing up the balance of 45 empirical studies. Soc Sci Med 60:1117–1131 [DOI] [PubMed] [Google Scholar]
- Vena JE, Violanti JM, Marshall J, Fiedler RC (1986) Mortality of a municipal worker cohort: III. Police officers. Am J Ind Med 10:383–397 [DOI] [PubMed] [Google Scholar]
- Vena JE, Charles LE, Gu JK, Burchfiel CM, Andrew ME, Fekedulegn D, Violanti JM (2014) Mortality of a police cohort: 1950–2005. J Law Enforc Leadersh Ethics 1:7–30 [PMC free article] [PubMed] [Google Scholar]
- Violanti JM, Burchfiel CM, Miller DB, Andrew ME, Dorn J, Wactawski-Wende J, Beighley CM, Pierino K, Joseph PN, Vena JE, Sharp DS, Trevisan M (2006) The buffalo cardio-metabolic occupational police stress (BCOPS) pilot study: methods and participant characteristics. Ann Epidemiol 16(2):148–56 [DOI] [PubMed] [Google Scholar]
- Violanti JM, Fekedulegn D, Hartley TA, Charles LE, Miller DB, Burchfiel CM (2017) The impact of perceived intensity and frequency of police work occupational stressors on the cortisol awakening response (CAR): the BCOPS study. Psychoneuroendocrinology 75:124–131 [DOI] [PMC free article] [PubMed] [Google Scholar]


