Abstract
Radiation-induced coronary heart disease (RICHD) is the second most common cause of morbidity and mortality in patients treated with radiotherapy for breast cancer, Hodgkin’s lymphoma and other prevalent mediastinal malignancies. The risk of RICHD increases with radiation dose. Exposed patients may present decades after treatment with manifestations ranging from asymptomatic myocardial perfusion defects to ostial, triple-vessel disease and sudden cardiac death. RICHD is insidious, with a long latency and a tendency to remain silent late into the disease course. Vessel involvement is often diffuse and is preferentially proximal. The pathophysiology is similar to that of accelerated atherosclerosis, characterised by the formation of inflammatory plaque with high collagen and fibrin content. The presence of conventional risk factors potentiates RICHD, and aggressive risk factor management should ideally be initiated prior to radiation therapy. Stress echocardiography is more sensitive and specific than myocardial perfusion imaging in the detection of RICHD, and CT coronary angiography shows promise in risk stratification. Coronary artery bypass grafting is associated with higher risks of graft failure, perioperative complications and all-cause mortality in patients with RICHD. In most cases, the use of drug-eluting stents is preferable to surgical intervention, bare metal stenting or balloon-angioplasty alone.
INTRODUCTION
Mediastinal radiotherapy (RT) is routinely administered to patients with lymphomas and cancers of the breast and lung, two of the most commonly occurring malignancies. Epidemiological data has identified radiation-induced cardiovascular disease as the most common cause, barring malignancy, of morbidity and mortality in patients who receive this treatment. While contemporary radiation-sparing techniques have reduced the incidence of pathologies affecting the heart’s more radioresistant tissues (ie, pericardium and myocardium), the coronary vasculature and microvasculature continue to be affected, accounting for most of the cardiac mortality attributable to RT.1 Consequently, radiation-induced coronary heart disease (RICHD) is among the most actively studied areas in cardio-oncology today.
The onset of RICHD is insidious, often occurring decades after the initial exposure, resulting in an ischaemic disease burden far in excess of that predicted by conventional risk scores. It represents a pathophysiologically distinct disease entity with a unique histopathology that is less amenable to percutaneous and surgical interventions. Here, we summarise the latest data regarding the epidemiology, clinical characteristics, pathophysiology and management of this disease.
EPIDEMIOLOGY
RICHD most commonly presents years after treatment of breast cancer, Hodgkin’s lymphoma (HL) and certain lung cancers. Its contribution to the burden of ischaemic heart disease (IHD) is well-characterised, with several large, population-based studies estimating excess risk at 25% and 250% among survivors of breast cancer and HL, respectively.2–5 This range reflects the dose-dependence of RICHD, as HL survivors are exposed to far greater cumulative radiation doses. Indeed, the risk of RICHD is linearly related to dose, estimated at 7.5% per Grey Unit (Gy) exposure (table 1).4,5 The risk of RICHD is roughly constant over time, beginning several years after exposure and persisting for at least two to three decades, with over 50% of excess ischaemic events occurring >10 years after RT.
Table 1.
Excess risk of IHD events in survivors of mediastinal malignancies after treatment with radiation therapy
| Darby et al, 20135 | van Nimwegen et al, 20164 | |
|---|---|---|
| Breast cancer survivors treated with RT | HL survivors treated with RT | |
| N | 2168 | 2617 |
| Study period | 1958–2001 | 1965–2013 |
| Primary endpoint | Major IHD event | Major IHD event |
| Mean heart dose, GY, Darbyet al5 |
Risk ratio for IHD events | Mean heart dose, GY, van Nimwegen et al4 |
|
|---|---|---|---|
| <2.0 | 1.10 | 1.14 | 1–5 |
| 2–4 | 1.30 | 2.14 | 5–15 |
| 5–9 | 1.40 | 2.76 | 15–19 |
| ≥10 | 2.16 | 2.79 | 20–24 |
| 3.21 | 25–34 | ||
| 2.54 | 35–45 | ||
| Total excess risk per Gy | |||
| 7.4% (95% CI 2.9 to 14.5) | 7.4% (95% CI 3.0 to 15.8) | ||
HL, Hodgkin’s lymphoma; IHD, ischaemic heart disease; RT, radiotherapy.
CLINICAL CHARACTERISTICS
The archetypal presentation of RICHD is that of a young patient with typical angina, no risk factors for IHD and angiographically severe proximal or ostial disease.6 This pattern of coronary pathology is associated with higher incidences of acute myocardial infarction (MI) and sudden cardiac death. A cohort of 112 HL survivors, treated with very high radiation doses by protocols that are now outdated, experienced 5 sudden and unexplained deaths and 8 IHD mortalities during a mean follow-up interval of 11 years7. Few patients had conventional cardiovascular risk factors, and the mean age was 33 years.
Although angina is common in patients with RICHD, non-anginal chest pain is a common occurrence in the first 2–3 years following chest wall irradiation and/or mastectomy, afflicting nearly half of breast cancer survivors.8 Pericarditis is a known complication of RT and may masquerade as or coexist with angina. Moreover, even in patients with profound obstructive RICHD, silent disease is common, and only half to two-thirds of patients present with typical angina.9,10 Notably, chest pain and other manifestations of RICHD are twice as common following irradiation of the left chest as compared with the right, arguing for a higher index of clinical suspicion in this group.3,11
Angiographically, the disease is characteristically severe, diffuse and disproportionately proximal. The lesions are aptly described as long, smooth, concentric and tubular (figure 1).9 The disease severity is substantiated by a 1993 study of 15 HL survivors who presented with angina; 10 of these patients had obstructive disease of one or more coronary ostia and left main disease was present in 7.10 In a more recent study of HL survivors irradiated at lower doses, CT angiography detected an increased prevalence of left main, proximal left anterior descending (LAD), circumflex and right coronary artery (RCA) disease by factors of 2.8, 1.9, 2.5 and 2.7, respectively.12 Not all RICHDs, however, are confined to the proximal segments, and excess disease burden can be seen in all segments.
Figure 1.
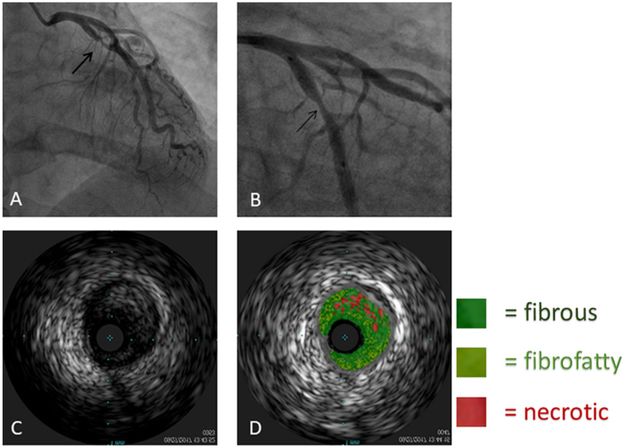
(A) and (B) Angiographic images of the stenotic left circumflex artery (arrow). (C) and (D) Images of intravascular ultrasound with and without virtual histology.
Many patients remain asymptomatic despite the presence of severe obstructive disease (table 2). In a cohort of 294 asymptomatic HL survivors who underwent cardiovascular screening after receiving mean heart doses in excess of 35 Gy, 2.7% of patients had severe three-vessel disease on subsequent angiography, while 7.5% had ≥50% stenosis in at least one vessel.13 As the authors note, where angiography was triggered by abnormal stress testing in their cohort, the rates of ostial and left main disease were similar to those reported among men with definite angina pectoris in the CASS registry in the 1970s.14
Table 2.
Angiographic findings in symptomatic and asymptomatic patients with a history of mediastinal malignancies treated with high-dose radiation exposure
| Coronary angiography (% of cases with the specified disease severity) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, design |
N | Mean study interval, years |
Mean/ median age, years* |
Radiation exposure, Gy (mean) |
≥70% of LAD, LCx, or RCA: |
Ostial vessel ≥50% |
LM≥50% | LM≥70% | |||
| 1- v | 2- v | 3- v | |||||||||
| Orzan et al, 199310 retrospective, observational | Symptomatic | 24 HL/BCA survivors 15 for CAG | 12 | 44 | >45† | 40 | 20 | 13 | 67% | 47% | 43% |
| McEniery et al, 19879 retrospective, case–control | 15 HL/BCA survivors 15 for CAG | 16 | 48 | 41 | 53 | 20 | 7 | 27% | 33% | 20% | |
| Correa et al, 200746 retrospective, case–control | 46 BCA survivors 13 for CAG | 15 | 55 | 60–65† | 31 | 23 | 0 | NA | NA | NA | |
| Heidenreich et al, 200713 prospective, observational | Asymptomatic | 294 HL survivors 40 for CAG | 6.5 | 42 | 44 | 10 | 10 | NA‡ | >28% | NA | 50%‡ |
| Rademaker et al, 200847 retrospective, case series | 9 HL survivors 9 for CCTA | 26 | 45 | 40 | 33 | 0 | 0 | 0 | 9% | 9% | |
| Daniels et al, 201448 prospective, observational | 48 HL survivors for CCTA 8 for CAG | 21 | 47 | 36 | 33 | 0 | 0 | 33% | 0 | 22% | |
Denotes age at time of angiography.
Reported as ‘chest’ or ‘mediastinal dose’; elsewhere, mean heart doses are reported.
Reported as left main≥70% or three-vessel disease with at least one vessel with ≥70% stenosis.
BCA, breast cancer; CAG, coronary angiography; CCTA, coronary CT angiography; LAD, left anterior descending; LCx, left circumflex coronary artery; LM, left main; NA, not available; RCA, right coronary artery.
PATHOLOGY AND PATHOPHYSIOLOGY
Histopathology
The pathogenesis of RICHD is now well-characterised. Beginning with endothelial effacement, radiation injury proceeds to a state of chronic inflammation, oxidative stress and fibrosis. This process is superficially similar to accelerated atherosclerosis observed in other clinical scenarios but results in plaque that differs not only in location and segment involvement, but also with respect to histology, stability and rapidity of onset. The most robust data on the pathophysiology of RICHD come from animal studies. Though murine models are notoriously resistant to atherosclerosis, RICHD has been successfully simulated in fat-fed, hyperlipidaemic apolipoprotein E knockout mice. Stewart et al observed markedly increased macrophage and neutrophil content in the intima of irradiated mice, and intraplaque haemorrhage was likewise far more common.15 Additionally, dose-dependent increases in the early appearance of intimal fatty streaks and late deposition of fibrin were noted.16 Plaque analysis revealed smaller lipid cores in irradiated mice. Of note, systemic inflammatory markers were not elevated, and out-of-field arteries were unaffected. Earlier studies using canine and rabbit models noted fibrointimal proliferation, rapid appearance of subendothelial foam cells and a high smooth muscle cell (SMC) content.17,18
In humans, the histopathological response to high-dose RT has been examined at necropsy, where lesions were noted to be diffuse in nature with prominent fibrosis. In a study of 10 irradiated HL patients (mean age=26 years), none of whom had known CHD, investigators found >50% narrowing of the great epicardial vessels in 28% of all coronary artery segments.19 Increased fibrotic content in the intima, media and adventitia was noted, and fibrous tissue constituted 70% of total lesion volume, while lipid deposits were a less prominent feature. Marked SMC loss, attenuation of the media and adventitial thickening were also observed. Case reports have also suggested early onset of negative remodelling, but this has not been confirmed in larger series.20
Molecular pathogenesis
Both RICHD and conventional atherosclerosis begin with a pattern of diffuse injury to the coronary artery endothelium. In conventional atherosclerosis, oxidative and shear stresses are thought to be the proximate stimulus for this injury and are exacerbated by conventional risk factors.21 The endothelium responds by becoming inflamed and porous, releasing inflammatory mediators that recruit leucocytes while permitting the passage of low-density lipoproteins through the endothelium. Monocytes then enter the subintima, where they become macrophages and eventually foam cells, thereby propagating inflammation and oxidative stress.
In RICHD, the same processes occur in response to a more discreet stimulus and are accelerated and sustained by a unique pathogenesis. Once in residence, macrophages produce inflammatory signals that instigate phenotypic changes in all layers of the vessel wall. In the intima, the endothelium becomes prematurely senescent, promoting inflammation and endothelial effacement.22,23 Medial SMC differentiate into myofibroblasts, which produce collagen and fibrin,16 and migrate into the intima, eventually encasing the endothelium.24 Deposition of extracellular matrix material in the intima and media, along with chronic inflammation, eventually leads to arterial stenosis (figure 2).
Figure 2.
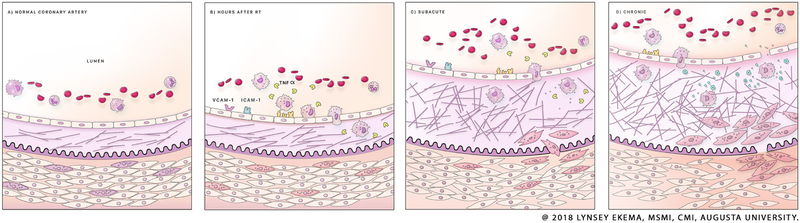
Mechanism of radiation-induced coronary injury. The intima of the healthy vessel is thin, and its collagenous component is well organized, as in frame (A). In the hours after radiation exposure, the endothelium becomes effaced, permitting chemotaxis of macrophages into the intima, where they begin to secrete profibrotic chemokines such as TGF-beta (B). This results in the terminal differentiation of SMCs into myofibroblasts, which enter the intima, where they generate large quantities of type IV collagen (C). The result is a state of chronic fibrosis and progressive stenosis (D), occurring over the months and years after. SMC, smooth muscle cell; TGF, transforming growth factor.
PREVENTION
Risk factor modification
The most important preventative measure with respect to RICHD is dose minimisation, and the techniques by which this is accomplished are beyond the scope of this review.25 From the standpoint of cardio-oncology, however, prevention begins prior to initiating treatment and continues indefinitely with serial clinical evaluations, imaging and aggressive risk factor modification. Risk factor modification appears to have the greatest potential for reducing RICHD risk, though it has not been prospectively studied and should begin prior to RT whenever possible. The presence of at least one classic risk factor at time of irradiation reportedly increased the relative risk of IHD events in HL survivors by a factor of 1.6, while hypertension alone nearly doubled this risk.4 A population-based retrospective study reported a twofold increase in the OR of major coronary events in breast cancer survivors where at least one conventional risk factor was present at onset of RT, though this effect did not interact with RT to augment IHD.5 Active smoking and the presence of peripheral vascular disease carried the greatest risk.
Data regarding the effect of conventional risk factors in the years after RT are even more persuasive. An earlier study of HL survivors found that the presence of one or more risk factors at an 11-year follow-up was associated with more than twofold increase in the incidence of IHD events.7 Lastly, sedentary lifestyle should be considered a risk factor for IHD events in irradiated patients, as adherence to national guidelines for vigorous-intensity exercise (≥9 Metabolic equivalent of task (MET) hours/week−1) was associated with relative risk reductions of 25% and 50% in survivors of HL and breast cancer, respectively.26,27
Perhaps most importantly, RT has been reported to increase the relative risk of IHD events in patients with pre-existing coronary disease by up to 60%.3 To attenuate this risk, proper cardio-oncological care should begin before the initiation of RT with risk factor modification and a heart-healthy exercise regimen.
Screening and monitoring
Prospective data regarding the utility and cost-effectiveness of screening for RICHD are limited, as most studies employed modalities better suited to detection of radiation-induced cardiomyopathy, constrictive pericarditis and valvular heart disease.28 Expert opinion on the subject skews in favour of echocardiography for baseline and serial monitoring, though the National Comprehensive Cancer Network recommends stress testing as a reasonable alternative.29 The joint statement of the European Association of Cardiovascular Imaging and the American Society of Echocardiography is the most aggressive in its recommendations, calling for the performance of echocardiography prior to RT, with a repeat study at year 10, and every 5 years thereafter.30 For patients with one or more conventional risk factors, the authors recommend that this test be replaced with stress echocardiography beginning in the fifth year post-RT. For our proposed algorithm, see figure 3.
Figure 3.
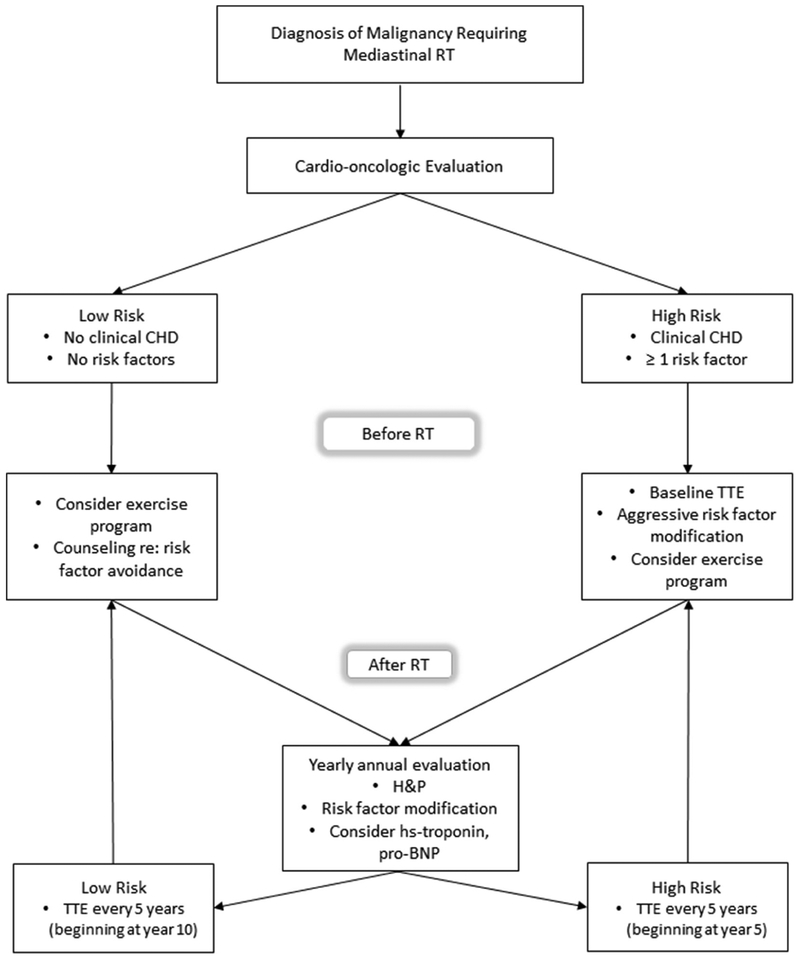
Proposed algorithm for monitoring in patients who have undergone mediastinal radiotherapy. BNP, Brain natriuretic peptide; CHD, coronary heart disease; RT, radiotherapy; transthoracic echocardiogram,TTE, transthoracic echocardiogram.
The role of stress echocardiography and myocardial perfusion imaging (MPI) in detecting occult IHD was assessed prospectively in 294 asymptomatic patients at a mean of 15 years after RT.13 Abnormal results were obtained in 14% of these patients, though most abnormalities were not corroborated by follow-up coronary angiography. With regard to the accuracy of specific modalities, the sensitivities of MPI and stress echocardiography were 65% and 75%, respectively, for an angiographic finding of ≥70% vascular stenosis, while 88% of abnormal nuclear imaging findings did not correlate with such lesions. The apparently low positive predictive value of MPI may reflect the presence of microvascular disease in these patients.
The utility of coronary artery calcium (CAC) scoring in RICHD has been evaluated with ambiguous results. A strong correlation between CAC and the presence of verified coronary artery disease was demonstrated in HL survivors at a 22-year follow-up, with an OR of 2.1 for every 200 U increase in CAC volume score.31 As most of these patients were asymptomatic and did not undergo angiography, this likely represents an underestimate. Importantly, the negative predictive value of the study appeared to be excellent, as none of the patients with CACs of 0 reported symptoms or history of IHD. A larger study of breast cancer survivors, however, found no difference in CACs when comparing patients irradiated on the right and left sides after a mean 12-year follow-up period. Whether this incongruity is attributable to the differing RT exposures of the two populations or other factors remains unknown, and prospective studies that include baseline CAC scores are needed.
Coronary CT angiography (CCTA), a technology with excellent negative predictive value for acute coronary syndromes in the general population, has been evaluated for use in RICHD screening with promising results. An increase in the prevalence of coronary artery abnormalities from 15% in the first 5 years after RT to 34% at 10 years post-treatment was noted in a cohort of asymptomatic HL survivors, with confirmed diagnosis of obstructive lesions by coronary angiography in 10% of patients and revascularisation in 6%.32 Another prospective trial of young HL survivors (mean age, 20 years) reported a 16% prevalence of abnormal CCTA results after a mean follow-up interval of only 8 years, with a nearly sevenfold increase in risk in patients receiving mediastinal radiation doses ≥20 Gy.33
Finally, data are emerging regarding the utility of biomarkers in screening for RICHD. Elevated N-terminal pro-B-type natriuretic peptide levels were detected in the months after RT, correlating positively with increasing mean heart dose.34 High-sensitivity troponin T levels appear to rise in a similar manner, correlating with radiation doses to the heart, and to the LAD in particular.35 Serial high-sensitivity C reactive protein values correlated strongly with the incidence of obstructive CAD and valvular disease in a prospective study of HL survivors (OR 2.1) and may be of value in risk stratification.36 A powerful association between abnormal CCTA findings and a novel biomarker, leucocyte telomere length, has been described and merits further investigation.32
MANAGEMENT
Currently, data are unavailable to recommend expansion of the standard of care with respect to the medical management of RICHD. Procedural management, however, places unique demands on cardiologists and surgeons alike. While the increased risks of major IHD events in these patients argue for a more aggressive approach to revascularisation, decision-making as to the manner in which this is accomplished requires careful consideration of the disease factors, including the extent and location of radiation exposure, its propensity to bring about sudden and often silent cardiac events, and the unique difficulties associated with both percutaneous coronary intervention (PCI) and bypass grafting.
Surgical revascularisation
Surgical revascularisation is often complicated by poor tissue healing at the surgical site, radiation-associated lung injury, inadequate bypass targets and the need for compound procedures. These issues surfaced in an early study of coronary artery bypass grafting (CABG) in 47 RT patients, who required a high rate of compound procedures, including 19 single valve replacements and 6 double valve replacements.37 Four surgical site complications were recorded, and perioperative mortality for the study group was 8.5%, while 5-year actuarial survival was only 73%. A larger study published in 2013 reported overall survival rates of 45% for irradiated patients at a mean 7.8 years after surgery, with RT contributing a 3.7-fold increase in relative risk of death.38 Eighty-two per cent of the irradiated patients required compound procedures, which likely contributed to the poor outcomes. Finally, regarding conduit selection, a recent review of 113 CABG cases found no difference in 1-year and 5-year survival in patients receiving internal mammary artery (IMA) grafts.39 This apparent reduction in efficacy may reflect its susceptibility to radiation injury and argues for careful angiographic evaluation and inspection of the IMA before grafting.
Percutaneous revascularisation
The first prospective study of PCI in patients with RICHD versus a non-irradiated cohort compared target-lesion revascularisation (TLR) rates in patients treated with bare metal stents or with balloon-angioplasty alone. All patients were treated with aspirin as the sole antiplatelet agent. TLR rates were markedly increased in the RICHD group (66% vs 16% at 6 months).40 Since the advent of drug-eluting stents (DES) and dual-antiplatelet therapy, however, the data have been more encouraging. A large retrospective study performed in 2014 at the Mayo clinic found no difference in TLR or cardiac mortality at 3 years.41 A 2016 study found that bare metal stenting and/or balloon angioplasty alone was associated with a HR of 2.5 for all-cause mortality, and increased cardiovascular mortality, in patients with RICHD as compared with non-irradiated controls after a mean follow-up of 6.5 years42. There was no such increase in the group receiving DES.
No head-to-head comparison of PCI to CABG has been performed in patients with RICHD, and a tendency still exists to refer lower risk patients with RICHD for CABG.42 This may be attributable in part to the inability of conventional guides to decision-making, such as EuroSCORE, to accurately assess the elevated risks of perioperative complications and graft failure in these patients and to the higher prevalence of comorbid valve disease.38 The greater complexity of ostial PCI, and misconceptions regarding the durability of modern stents, may discourage percutaneous intervention. Nevertheless, while patient selection bias likely contributes to the differences in mortality, the data cited above suggest a mortality advantage in favour of PCI with DES. Studies establishing the efficacy of DES in conventional left main disease should expand its role in RICHD.43 Moreover, studies demonstrating the safety of transcutaneous valvular interventions in low-risk and intermediate-risk patients suggest an alternative to high-risk, compound surgeries,44 and a relevant subgroup analysis of the PARTNERS registry data will soon be possible.45 Finally, our institutional experience with cutting balloon angioplasty suggests a utility for enhancing the patency of ostial stents, which may prove particularly useful in this context.
CONCLUSION
As survivorship lengthens in patients treated for common mediastinal malignancies, cancer survivors will increasingly present with RICHD late in their disease course, often after exposure to radiation doses now considered archaic, with few coronary risk factors yet an uncharacteristically severe burden of high-risk lesions. Many of these patients will present with atypical symptoms or with incidental imaging findings suggesting occult disease. Referral for cardio-oncological care should be made prior to initiation of RT for the purpose of thorough clinical evaluation and initiation of aggressive risk factor modification and a heart healthy exercise programme. Until more robust data become available, decisions regarding baseline screening are best made on a case-by-case basis, making preferential use of stress echocardiography and CCTA. For the purposes of risk stratification, clinicians should consider a history of chest wall irradiation to be an independent risk factor for IHD events. When revascularisation is indicated, decision-making should favour PCI with DES placement as the safest modality in this population, and extension of dual antiplatelet therapy should be considered in patients undergoing bare metal stenting or balloon angioplasty when feasible. Lastly, where the combined presence of critical native vessel disease and valvular abnormalities argue for surgery, the combination of PCI with DES placement and transcutaneous valve repair may merit consideration.
Acknowledgements
The authors thank Lynsey Ekema for her assistance in the preparation of our figures and Ashley Cobb for her assistance in preparation of our tables.
Funding This work was supported by National Institutes of Health grants HL112640, HL126949, HL134354 and AR070029 to NLW.
Footnotes
Correction notice Since this paper was first published online figure 2 has been resupplied. The updated version includes the following copyright line @ 2018 Lynsey Ekema, MSMI, CMI Augusta University.
Competing interests None declared.
Patient consent Not required.
Provenance and peer review Commissioned; internally peer reviewed.
Data sharing statement Not applicable.
REFERENCES
- 1.Darby SC, McGale P, Taylor CW, et al. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol 2005;6:557–65. [DOI] [PubMed] [Google Scholar]
- 2.Boekel NB, Schaapveld M, Gietema JA, et al. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys 2016;94:1061–72. [DOI] [PubMed] [Google Scholar]
- 3.McGale P, Darby SC, Hall P, et al. Incidence of heart disease in 35,000 women treated with radiotherapy for breast cancer in Denmark and Sweden. Radiother Oncol 2011;100:167–75. [DOI] [PubMed] [Google Scholar]
- 4.van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of hodgkin lymphoma. J Clin Oncol 2016;34:235–43. [DOI] [PubMed] [Google Scholar]
- 5.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–98. [DOI] [PubMed] [Google Scholar]
- 6.Veinot JP, Edwards WD. Pathology of radiation-induced heart disease: a surgical and autopsy study of 27 cases. Hum Pathol 1996;27:766–73. [DOI] [PubMed] [Google Scholar]
- 7.Glanzmann C, Kaufmann P, Jenni R, et al. Cardiac risk after mediastinal irradiation for Hodgkin’s disease. Radiother Oncol 1998;46:51–62. [DOI] [PubMed] [Google Scholar]
- 8.Gärtner R, Jensen MB, Nielsen J, et al. Prevalence of and factors associated with persistent pain following breast cancer surgery. JAMA 2009;302:1985–92. [DOI] [PubMed] [Google Scholar]
- 9.McEniery PT, Dorosti K, Schiavone WA, et al. Clinical and angiographic features of coronary artery disease after chest irradiation. Am J Cardiol 1987;60:1020–4. [DOI] [PubMed] [Google Scholar]
- 10.Orzan F, Brusca A, Conte MR, et al. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J 1993;69:496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris EE, Correa C, Hwang WT, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006;24:4100–6. [DOI] [PubMed] [Google Scholar]
- 12.van Rosendael AR, Daniëls LA, Dimitriu-Leen AC, et al. Different manifestation of irradiation induced coronary artery disease detected with coronary computed tomography compared with matched non-irradiated controls. Radiother Oncol 2017;125:55–61. [DOI] [PubMed] [Google Scholar]
- 13.Heidenreich PA, Schnittger I, Strauss HW, et al. Screening for coronary artery disease after mediastinal irradiation for Hodgkin’s disease. J Clin Oncol 2007;25:43–9. [DOI] [PubMed] [Google Scholar]
- 14.Chaitman BR, Bourassa MG, Davis K, et al. Angiographic prevalence of high-risk coronary artery disease in patient subsets (CASS). Circulation 1981;64:360–7. [DOI] [PubMed] [Google Scholar]
- 15.Stewart FA, Heeneman S, Te Poele J, et al. Ionizing radiation accelerates the development of atherosclerotic lesions in ApoE−/− mice and predisposes to an inflammatory plaque phenotype prone to hemorrhage. Am J Pathol 2006;168:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoving S, Heeneman S, Gijbels MJ, et al. Single-dose and fractionated irradiation promote initiation and progression of atherosclerosis and induce an inflammatory plaque phenotype in ApoE(−/−) mice. Int J Radiat Oncol Biol Phys 2008;71:848–57. [DOI] [PubMed] [Google Scholar]
- 17.Amromin GD, Gildenhorn HL, Solomon RD, et al. The synergism of x-irradiation and cholesterol-fat feeding on the development of coronary artery lesions. J Atheroscler Res 1964;4:325–34. [DOI] [PubMed] [Google Scholar]
- 18.Gillette SM, Gillette EL, Shida T, et al. Late radiation response of canine mediastinal tissues. Radiother Oncol 1992;23:41–52. [DOI] [PubMed] [Google Scholar]
- 19.Brosius FC, Waller BF, Roberts WC. Radiation heart disease. Analysis of 16 young (aged 15 to 33 years) necropsy patients who received over 3,500 rads to the heart. Am J Med 1981;70:519–30. [DOI] [PubMed] [Google Scholar]
- 20.van Gameren M, Ligthart JM, Galema TW, et al. Distinct pattern of constrictive remodeling in radiotherapy-induced coronary artery disease. JACC Cardiovasc Interv 2016;9:e121–3. [DOI] [PubMed] [Google Scholar]
- 21.He F, Zuo L. Redox roles of reactive oxygen species in cardiovascular diseases. Int J Mol Sci 2015;16:27770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azimzadeh O, Sievert W, Sarioglu H, et al. Integrative proteomics and targeted transcriptomics analyses in cardiac endothelial cells unravel mechanisms of long-term radiation-induced vascular dysfunction. J Proteome Res 2015;14:1203–19. [DOI] [PubMed] [Google Scholar]
- 23.Philipp J, Azimzadeh O, Subramanian V, et al. Radiation-induced endothelial inflammation is transferred via the secretome to recipient cells in a STAT-Mediated Process. J Proteome Res 2017;16:3903–16. [DOI] [PubMed] [Google Scholar]
- 24.Vos J, Aarnoudse MW, Dijk F, et al. On the cellular origin and development of atheromatous plaques. A light and electron microscopic study of combined X-ray and hypercholesterolemia-induced atheromatosis in the carotid artery of the rabbit. Virchows Arch B Cell Pathol Incl Mol Pathol 1983;43:1–16. [DOI] [PubMed] [Google Scholar]
- 25.Yeboa DN, Evans SB. Contemporary breast radiotherapy and cardiac toxicity. Semin Radiat Oncol 2016;26:71–8. [DOI] [PubMed] [Google Scholar]
- 26.Jones LW, Liu Q, Armstrong GT, et al. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol 2014;32:3643–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones LW, Habel LA, Weltzien E, et al. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol 2016;34:2743–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuomo JR, Sharma GK, Conger PD, et al. Novel concepts in radiation-induced cardiovascular disease. World J Cardiol 2016;8:504–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.National Comprehensive Cancer Network. Practice guidelines in oncology, Hodgkin Lymphoma. Version 2.2015. http://www.nccn.org/professionals/physiciangls/pdf/hodgkins.pdf
- 30.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging 2013;14:721–40. [DOI] [PubMed] [Google Scholar]
- 31.Andersen R, Wethal T, Günther A, et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin’s lymphoma. Am J Cardiol 2010;105:149–52. [DOI] [PubMed] [Google Scholar]
- 32.Girinsky T, M’Kacher R, Lessard N, et al. Prospective coronary heart disease screening in asymptomatic Hodgkin lymphoma patients using coronary computed tomography angiography: results and risk factor analysis. Int J Radiat Oncol Biol Phys 2014;89:59–66. [DOI] [PubMed] [Google Scholar]
- 33.Küpeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J Clin Oncol 2010;28:1025–30. [DOI] [PubMed] [Google Scholar]
- 34.D’Errico MP, Grimaldi L, Petruzzelli MF, et al. N-terminal pro-B-type natriuretic peptide plasma levels as a potential biomarker for cardiac damage after radiotherapy in patients with left-sided breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e239–46. [DOI] [PubMed] [Google Scholar]
- 35.Skyttä T, Tuohinen S, Boman E, et al. Troponin T-release associates with cardiac radiation doses during adjuvant left-sided breast cancer radiotherapy. Radiat Oncol 2015;10:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen MH, Blackington LH, Zhou J, et al. Blood pressure is associated with occult cardiovascular disease in prospectively studied Hodgkin lymphoma survivors after chest radiation. Leuk Lymphoma 2014;55:2477–83. [DOI] [PubMed] [Google Scholar]
- 37.Handa N, McGregor CG, Danielson GK, et al. Coronary artery bypass grafting in patients with previous mediastinal radiation therapy. J Thorac Cardiovasc Surg 1999;117:1136–43. [DOI] [PubMed] [Google Scholar]
- 38.Wu W, Masri A, Popovic ZB, et al. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013;127:1476–84. [DOI] [PubMed] [Google Scholar]
- 39.Brown ML, Schaff HV, Sundt TM. Conduit choice for coronary artery bypass grafting after mediastinal radiation. J Thorac Cardiovasc Surg 2008;136:1167–71. [DOI] [PubMed] [Google Scholar]
- 40.Schömig K, Ndrepepa G, Mehilli J, et al. Thoracic radiotherapy in patients with lymphoma and restenosis after coronary stent placement. Catheter Cardiovasc Interv 2007;70:359–65. [DOI] [PubMed] [Google Scholar]
- 41.Liang JJ, Sio TT, Slusser JP, et al. Outcomes after percutaneous coronary intervention with stents in patients treated with thoracic external beam radiation for cancer. JACC Cardiovasc Interv 2014;7:1412–20. [DOI] [PubMed] [Google Scholar]
- 42.Reed GW, Masri A, Griffin BP, et al. Long-term mortality in patients with radiation-associated coronary artery disease treated with percutaneous coronary intervention. Circ Cardiovasc Interv 2016;9:e003483. [DOI] [PubMed] [Google Scholar]
- 43.Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223–35. [DOI] [PubMed] [Google Scholar]
- 44.Möllmann H, Bestehorn K, Bestehorn M, et al. In-hospital outcome of transcatheter vs. surgical aortic valve replacement in patients with aortic valve stenosis: complete dataset of patients treated in 2013 in Germany. Clin Res Cardiol 2016;105:553–9. [DOI] [PubMed] [Google Scholar]
- 45.Beohar N, Kirtane AJ, Blackstone E, et al. trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the PARTNER Continued Access Registry. JACC Cardiovasc Interv 2016;9:355–63. [DOI] [PubMed] [Google Scholar]
- 46.Correa CR, Litt HI, Hwang WT, et al. Coronary artery findings after left-sided compared with right-sided radiation treatment for early-stage breast cancer. J Clin Oncol 2007;25:3031–7. [DOI] [PubMed] [Google Scholar]
- 47.Rademaker J, Schöder H, Ariaratnam NS, et al. Coronary artery disease after radiation therapy for Hodgkin’s lymphoma: coronary CT angiography findings and calcium scores in nine asymptomatic patients. AJR Am J Roentgenol 2008;191:32–7. [DOI] [PubMed] [Google Scholar]
- 48.Daniëls LA, Krol AD, de Graaf MA, et al. Screening for coronary artery disease after mediastinal irradiation in Hodgkin lymphoma survivors: phase II study of indication and acceptance†. Ann Oncol 2014;25:1198–203. [DOI] [PubMed] [Google Scholar]


