Abstract
Although cocaine abuse and addiction continue to cause serious health and societal problems, an FDA-approved medication to treat cocaine addiction has yet to be developed. Employing a pharmacokinetic strategy, an anti-cocaine vaccine, provides an attractive avenue to address these issues, however, current vaccines have shown varying degrees of efficacy, indicating that further formulation is necessary. As a means to improve vaccine efficacy, we examined the effects of varying anti-cocaine vaccine formulations by combining a Toll-like receptor 9 (TLR9) agonist with a TLR5 agonist in the presence of alum. The TLR9 agonist used was cytosine-guanine oligodeoxynucleotide 1826 (CpG 1826), while the TLR5 agonist was flagellin (FliC). Formulations with the TLR9 agonist elicited superior anti-cocaine antibody titers and blockade of hyperlocomotor effects compared to vaccines without CpG 1826. This improvement was seen regardless of whether the TLR5 agonist, FliC, or the non-adjuvanting Tetanus Toxoid (TT) was used as the carrier protein. Additional insights into the value of FliC as a carrier versus adjuvant was also investigated by generating two unique formats of the protein, wild type and mutated flagellin (mFliC). While the mFliC conjugate retained its ability to stimulate mTLR5, it yielded reduced cocaine sequestration and functional blockade relative to FliC and TT. Overall, this work indicates that activation of TLR9 can improve the function of cocaine vaccines in the presence of TLR5 activation by FliC, with any potential additive effects limited by the inefficiency of FliC as a carrier protein as compared to TT.
Keywords: addiction, adjuvant, carrier protein, cocaine, flagellin, CpG, TLR5, TLR9, vaccine
Graphical abstract
INTRODUCTION
Cocaine abuse continues to represent a major medical and societal hazard in the United States. In recent years, the National Survey on Drug Use and Health (NSDUH) has reported the comparatively high and steady-state levels of domestic cocaine use, which includes 1.5 million users.1 The Drug Abuse Warning Network (DAWN) has reported that 40.3% of drug-related emergency department visits were attributed to cocaine.2 Despite the serious nature of this problem, current treatment protocols are limited to implementing strategies for the alleviation or abatement of cocaine withdrawal symptoms.3
The traditional approach for the treatment of substance abuse disorders has centered upon the preparation small-molecule receptor ligands or endogenous signaling molecule mimetics in an effort to elicit specific changes within the neural circuitry, including dopaminergic, glutamatergic, and GABAergic signaling, among others.4,5 The obvious flaws with such approaches are the abuse potential often associated with the therapeutics themselves and the high risk of side effects related to globally altering neurotransmitter signaling.
In view of such limitations, interest has turned to developing pharmacokinetic (PK) strategies that can maintain free concentrations of an abused drug below a minimally effective threshold at its site of action in the central nervous system (CNS) following use.6 Active vaccination is one method by which this outcome could be achieved; an antigen is administered in the form of a small molecule-protein conjugate, which stimulates the immune system to generate highly specific antibodies to bind free drug in the blood and prevent passage across the blood-brain barrier (BBB) to elicit downstream pharmacodynamic effects.7 While this approach has been effective in many preclinical studies, to date only a single active anti-cocaine vaccine, termed TA-CD, has tested this strategy in clinical trials. Unfortunately, this vaccine did not meet its clinical end point, hence, phase III trials were terminated.6a, 8 Thus, there is a need to refine our understanding of how to elicit improved immune responses to anti-cocaine vaccines.
For the development of an upgraded cocaine vaccine, we saw three fundamental challenges: hapten design, carrier protein, and adjuvant. In the course of our previous studies we have made significant progress in all three domains. With respect to hapten design, we have created a reliable third-generation cocaine hapten, termed GNE, which is relatively easy to synthesize, reliably stable in sera, and can induce high anti-cocaine antibody midpoint titers when conjugated to an immunogenic protein (Figure 1).9
Figure 1.
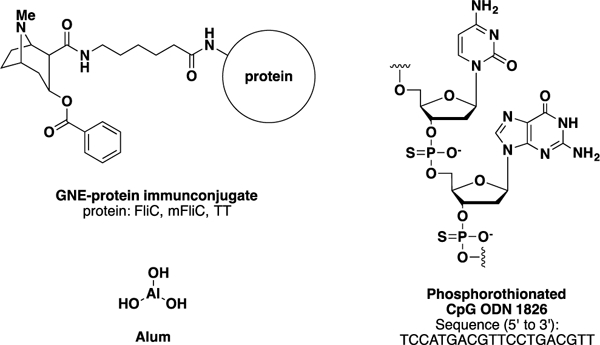
GNE-protein immunoconjugate, CpG ODN 1826 and alum structures
Regarding carrier protein identity, in our pursuit of anti-fentanyl and anti-nicotine vaccines, we have recently disclosed that tetanus toxoid (TT) is a highly effective carrier protein; indeed, our fentanyl-TT immunoconjugate vaccine effectively neutralized a lethal dose of fentanyl.10 Likewise, we have also found that the bacterial protein, flagellin (FliC), is an effective carrier in our anti-cocaine and anti-nicotine vaccine studies.11
As for adjuvant choice, two different TLR activating adjuvants have been recently explored by our laboratory – FliC and CpG 1826. It has been demonstrated that FliC not only acts a carrier protein, but can also stimulate Toll-like receptor 5 (TLR5) and thus induce myeloid differentiation factor 88 (MyD88) to induce a TH2 response with predominant production of IgG1 and no cytotoxic T lymphocytes (CTLs). Moreover, in an independent anti-heroin vaccine study, we found that addition of cytosine-guanine oligodeoxynucleotide 1826 (CpG 1826) to the vaccine elicited superior anti-heroin antibody midpoint titers and opioid affinities compared to formulations without CpG.12 In general, CpG motifs activate TLR9 to promote a TH1-type immune response; CpG 1826 is a B-class ODN that strongly stimulates B-cell immune responses to generate IgG2a and CTLs.13 Our previous studies have also demonstrated the efficacy of adjuvant alum in this context, which is not dependent on TLR activation, but is thought to induce a TH2 response through the depot effect and activation of antigen presenting cells (APCs).14
It has previously been shown that TLR-TLR crosstalk can result in variable changes in cytokine production and immune response, depending on the TLR pair being studied and the specific ligand being used.15 Because the FliC and CpG 1826 adjuvants work through independent TLRs to induce distinct immune responses, it was hypothesized that combining both adjuvants in a single formulation would result in improved efficacy over either adjuvant alone when given in an anti-cocaine vaccine formulation.
Since we have shown that FliC can act as both an adjuvant and carrier in these formulations, we also envisioned the need to generate a construct that would allow us to evaluate the relative importance of these roles – our previous studies indicated that increasing hapten copy number on FliC was associated with decreasing TLR5 agonism.11 To this end, we designed a mutant FliC (mFliC) construct where the lysine residues in the D0 and D1 domains that mediate FliC binding to TLR5 (Figure 2) were eliminated, presumably preventing hapten conjugation from interfering with this critical interaction.
Figure 2.
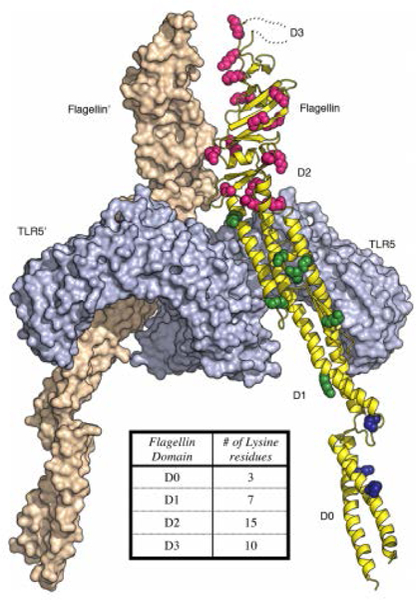
Spatial distribution of lysine residues in flagellin from Salmonella enterica subsp. Enterica serovar Dublin bound to TLR5 (PDB 3V47).
Using the logic presented vide supra, we endeavored to test the relative efficacy of activating TLR5, TLR9, or both, in the context of anti-cocaine vaccination by measuring the relative efficacy of the described mFliC construct, FliC, and TT, in combination with our lead GNE hapten and the adjuvants CpG 1826 and alum.
MATERIALS AND METHODS
Expression and Purification of Recombinant FliC and mFliC.
A variant of FliC, mFliC, was constructed from Salmonella enterica through mutation of the 10 lysine residues within the D0 and D1 domains of the wild-type FliC (as well as one additional lysine residue previously introduced thru cloning) to arginine residues (Figure S1). The gene encoding the fully mutated, C-terminal His-tag protein was ligated into the expression vector pET29a (Novagen) using the restriction sites NdeI and XhoI. FliC and mFliC proteins were overexpressed in E. coli and purified to > 95% homogeneity (Figure S2) according to published procedure.
mTLR5 Reporter Assay.
The ability of FliC and mFliC to stimulate TLR5 was determined using a reporter assay system as previously described. In brief, HEK-Blue mTLR5 cells (Invivogen) were plated in HEK-Detection Medium at a concentration of ~2.5 X 104 cells per well (96-well plates) in the presence or absence of FliC or mFlic. After incubation for ~7 h at 37 °C, absorbance at 620nm was measured correlating to TLR5 activation.
Secondary Structure and MHC-II Binding Predictions.
The entire amino acid sequences of FliC and mFliC were used to predict protein secondary structure using the PSIPRED (http://bioinf.cs.ucl.ac.uk/psipred/) method.16 For prediction of MHC-II epitope binding, the eternal software from IEDB (http://www.immunoepitope.org/) was used.17 Full length FliC and mFliC sequences were input on 07/20/2016 to predict binding at mouse H-2-I alleles using the IEDB consensus scoring method.18 Each 15-mer peptide generated by the program was assessed for the presence or absence of a lysine residue and this status was plotted against the consensus percentile prediction from IEDB. A rolling average measuring the likelihood for inclusion of lysine across the predicted binding affinities was generated using a window of 30 neighboring entries and a 6th order polynomial plot.
Preparation of GNE-FliC, GNE-mFliC, and GNE-TT Conjugates.
The cocaine hapten GNE was prepared from cocaine hydrochloride salt (NIDA Drug Supply Program). GNE was then activated with N-hydroxysulfosuccinimide (Sulfo-NHS) under general condensation conditions. Activated GNE was split into three portions and each portion was conjugated to FliC, mFliC or TT. Lyophilized FliC and mFliC were reconstituted in MOPS pH 7.2 buffer prior to conjugation. A solution of TT in 0.15 M sodium chloride with 0.0033% Thimerosal (UMass Biologics) was dialyzed against PBS pH 7.4 buffer using a Slide-A-Lyzer 10 K MWCO dialysis device prior to conjugation. In the conjugation step, each protein was treated with ca. 270 to 300 equivalent of activated GNE, and the reaction mixture was gently shaken at 4 °C for 22 h. After the conjugation, the mixture was dialyzed against PBS pH 7.4 buffer at rt and stored at 4 °C.
Mass Spectrometry Analysis.
In order to quantify hapten copy number (hapten density) of GNE-FliC, GNE-mFliC and GNE-TT, samples were submitted for MALDI-ToF MS analysis and compared with unconjugated protein (Figure S3).
Animals and Vaccinations.
6–8 week old male Swiss Webster mice (n = 6/group) were obtained from Taconic Farms (Germantown, NY). Mice were group-housed in an AAALAC-accredited vivarium containing temperature- and humidity-controlled rooms, with mice kept on a reverse light cycle (lights on: 9PM-9AM). All experiments were performed during the dark phase, generally between 1PM-4PM. General health was monitored by both the scientists and veterinary staff of The Scripps Research Institute, and all studies were performed in compliance with the Scripps Institutional Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. GNE-FliC, GNE-mFliC, and GNE-TT immunoconjugates in PBS pH 7.4 buffer (100 μL, 0.5 mg/mL) were formulated with Alhydrogel® (Invivogen, 100 μL, 10 mg/mL) with or without CpG ODN 1826 (Eurofins MWG Operon, 50 μg). All vaccine conjugate injections were injected subcutaneously (150–200 μL) on days 0, 21 and 42, and 56. No adverse reactions to the vaccines were observed and all mice maintained a healthy weight throughout the vaccine trial. Blood sampling was performed via tail-tip amputation (<1 cm) in order to collect 100–150 μL whole blood on days 28, 49, 70 and via cardiac bleed in order to collect 300–400 μL whole blood on day 72. Samples were then centrifuged at 10000 rpm for 10 min to isolate the serum.
Enzyme-Linked Immunosorbent Assay (ELISA).
PBS pH 7.4 buffer was prepared from 10X powder packet from Fisher Science and used throughout the assay except for the washing step which used deionized water. First, half-area high-binding 96-well microtiter plates (Costar 3690) were coated with 25 μg of GNE-BSA per well at 37 °C overnight, allowing the liquid to evaporate. Following blocking with 5% skim milk in PBS pH 7.4 at rt for 1 h, vaccinated mouse serum was serially diluted 1:1 in 2% BSA in PBS pH 7.4 across the 12 columns starting at 1:100. After a 2 h incubation at rt, the plates were washed 10 times with H2O and donkey anti-mouse IgG horseradish peroxidase (HRP) secondary (Jackson ImmunoResearch) at a 1:10000 dilution in 2% BSA in PBS pH 7.4 was added and incubated at rt for 1 h. After the incubation, 10 times washing was performed and 3,3’,5,5’-tetramethylbenzidine (TMB) substrate (Thermo Pierce) was added. 5 min following TMB addition, the mixture was quenched with 2 M aqueous H2SO4. Plates were allowed to incubate at rt for 20 min before their absorbance was read at 450 nm.
Competitive Radioimmunoassay (Competitive RIA).
Dissociation constants (Kd) and antibody concentrations were determined using competitive RIA. Mouse sera were pooled and diluted into 2% BSA in PBS pH 7.4, giving a concentration that was determined to bind ca 30–50% 3H-cocaine tracer (20,000 dpm, 80 Ci/mmol (PerkinElmer, NET510250UC)). Diluted serum (60 μL) and cocaine tracer (60 μL) were added to the sample chamber of a 5 kDa MWCO 6-well Equilibrium Dialyzer (Harvard Apparatus), and cocaine hydrochloride salt at varying concentrations in 1% BSA in PBS pH 7.4 (120 μL) was added to the buffer chamber. After equilibration on a plate rotator (Harvard Apparatus) at rt for 22–26 h, a sample from each chamber (60 μL) was diluted into Ecolite (+)™ liquid scintillation cocktail (5 mL, MP Biomedicals), and the radioactivity was measured with a Beckman LS 6500 Scintillation Counter. Kd’s and antibody concentrations were calculated according to Müller’s procedure.19
Hyperlocomotion Test.
Mice were allowed to acclimate for one hour in a plastic cage (10.5 × 19 × 8 inch) with a clear ventilated acrylic top. Mice were then quickly removed and injected with either saline, 5, 10, or 20 mg/kg cocaine before being returned to their cages; cages were wiped down with dry paper towels to remove excess debris while mice were being injected. The mice were then returned to the cage to be recorded and tracked by overhead camera using ANY-Maze video tracking software (Stoelting Co). Sessions were run during the middle of the dark cycle in a 4.6 × 4.6 m room with a single 60 W upward-directed light source (35–45 lux), and repeated on mice after a two-day washout period until all mice received all cocaine doses. Distance travelled (m) and time spent immobile (s; 80% pixel consistency for at least 5 s threshold) were measured.
Serum Cocaine Concentration.
Mice (n = 5–6/group) were injected subcutaneously with 10 mg/kg cocaine in a 10 mL/kg volume of physiological sterile saline. 15 min following injection, mice were fully anesthetized, then rapidly decapitated using a sharp guillotine, and trunk blood was collected. The blood samples were then centrifuged at 10000 rpm for 10 min to obtain serum samples. Samples of serum were then frozen at – 80 °C until sample prep for LC-MS analysis. To each 100 μL aliquot of serum, 0.6 M aqueous NaF (100 μL) and cocaine-D3 in acetonitrile (20 μL, 2 μg/mL) were added, and the mixture was vortex mixed for 30 s to equilibrate. After the equilibration, Solid-phase extraction with Oasis® PRiME HLB Extraction Cartridge was conducted. The extracted solution was then evaporated using a GENEVAC®. Serum extractive was reconstituted in 100 μL of acetonitrile, and a 5 μL aliquot of this filtered material was injected into LC-MS system equipped with an Agilent Poroshell 120 SB-C8 column using 5 mM NH4OAc pH 4-acetonitrile mobile phase. Deuterated and non-deuterated masses were extracted in MassHunter and the resulting peaks were integrated. Using the ratio of non-deuterated to deuterated integration values, drug concentrations were determined via a six point standard curve. Standards for the calibration curve were prepared in the same manner as serum samples, using solutions prepared with known non-deuterated concentrations of cocaine.
Computational Analyses.
Computational and Statistical analysis was performed in GraphPad Prism 6 (La Jolla, CA). All values are reported as means ± SEM. In ELISA, absorbance values were normalized to highest absorbance value per sample, and a curve was fit using the log(inhibitor) vs. normalized response - variable slope equation to determine the midpoint titer and standard errors. Titers were compared via one-way ANOVA with Fisher’s LSD post hoc comparisons. Significance was set at α = 0.05 and data is represented as mean ± SEM. In hyperlocomotion tests, the distance traveled and time spent immobile were normalized to baseline and compared, via two-way repeated-measures ANOVA with Bonferroni post-hoc corrected comparisons. Significance was set at α = 0.05 and data is represented as mean ± SEM.
RESULTS
mTLR5 Activation.
In order to assess the relative effects of mFliC as an adjuvant and carrier protein relative to FliC, the ability of these proteins to activate mTLR5 was first measured in vitro using a reporter assay. As hoped, unconjugated mFliC demonstrated modestly improved mTLR5 activation in comparison to FliC. However, following GNE conjugation, this improvement in mTLR5 activation was mitigated (Figure 3). An in silico analysis of secondary structure indicated that the inserted mutations alone were not likely to have altered the overall structure of this mFliC as compared to FliC, though subsequent conjugation of the haptens could perturb the overall structure (Figure S4).
Figure 3.
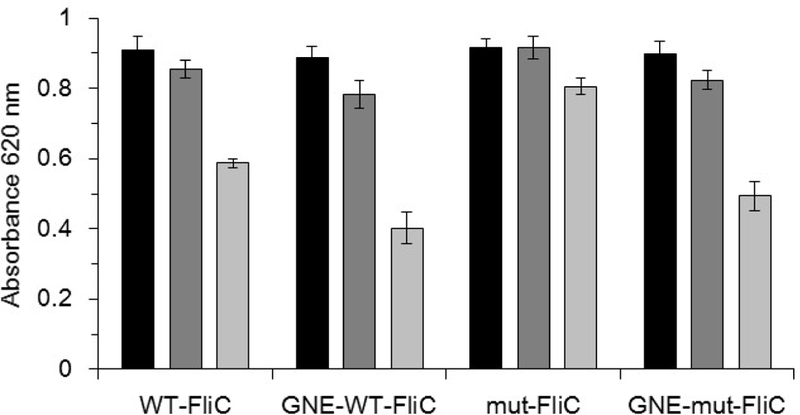
Unconjugated flagellin (WT-FliC and mut-FliC) and GNE-conjugated flagellin (GNE-WT-FliC and GNE-mut-FliC) proteins evaluated in an mTLR5 reporter assay at concentrations of 100, 10, 1 ng/mL. Chemically modified WT-FliC and mut-FliC were determined to possess about 15 and 11 GNE haptens per protein molecule, respectively.
MHC-II Binding Predictions.
With this evidence that mFliC retained its adjuvant activity at TLR5 in hand, the functionality of this construct to act as a carrier protein for MHC-II processing was assessed using in silico epitope mapping and binding predictions. Assessment against the mouse allotypes H2-IAb and H2-IAd revealed that the mFliC construct was likely to generate fewer high-affinity peptide fragments containing a lysine-conjugated hapten, as compared to FliC; this was especially apparent for the IAd allotype (Figure 4).
Figure 4.

In silico predicted binding of 15-mer peptides derived from Flic or mFliC to mouse MHC-II allotypes H2-IAb/IAd using the IEDB method. Lower percentiles are indicative of stronger binding. Individual peptides from each protein are shown at top and bottom depending on their lysine inclusion status, which is necessary for hapten presentation. Curves represent rolling average likelihood of lysine inclusion at each predicted binding affinity.
Anti-cocaine Antibody Titers.
Mice were immunized subcutaneously with immunoconjugates of our third generation cocaine hapten, GNE, and -FliC, -mFliC or -TT formulated with alum alone or alum + CpG. Antibody titers were assessed by ELISA against a GNE-BSA conjugate. Within each group, titers remained relatively invariant between bleed 1 and bleed 2 (Figure 3). All vaccines formulated with the TLR9 agonist CpG 1826 induced higher titers than the corresponding formulations without CpG 1826, although this difference was only found to be significant for the TT formulation. Comparing across groups, in the absence of CpG 1826, FliC and mFlic actually demonstrated higher titers than TT; in the presence of CpG the TT group exhibited the most robust immune response overall, while the FliC and mFliC groups demonstrated titers less than half as large.
Anti-cocaine Antibody Concentrations and Kd Value.
To quantify the antibody concentrations generated by vaccination along with their affinity for cocaine, a competitive RIA was conducted (Table 1). Mice in all groups were found to possess antibodies with high affinity for cocaine (10 to 60 nM). Consistent with the ELISA results, the vaccines formulated with CpG induced higher antibody concentration compared to the vaccines without CpG; the GNE-TT+CpG vaccine formulation again elicited the highest antibody concentration overall.
Table 1.
Anti-cocaine antibody concentrations and Kd values measured by competitive RIA against 3H-cocaine in the presence of varying cocaine concentrations (0 – 200 μM). Serum samples from vaccinated mice (n=5 or 6) on 49 days were pooled for analysis. Data are shown as the average of two independent replicates ± SEM. (Anti-cocaine antibody midpoint titers were measured by ELISA using serum from vaccinated mice (n=5 or 6) on day 49 for each study group.)
| Group | GNE-FliC | GNE-FliC+CpG | GNE-mFliC | GNE-mFliC+CpG | GNE-TT | GNE-TT+CpG |
|---|---|---|---|---|---|---|
| Kd (nM) | 25.2 ±4.9 | 31.5 ±22.7 | 20.1 ±0.7 | 12.6 ±18.2 | 25.9 ±35.5 | 54.1 ± 1.5 |
| [Ab] (μg/mL) | 90.1 ±31.5 | 128.4 ±78.5 | 16.8 ±9.7 | 25.5 ±31.6 | 12.0 ±21.87 | 1021.9 ±124.7 |
Hyperlocomotor Activity.
To assess our vaccine efficacy in blocking the pharmacodynamic effects of cocaine, vaccinated mice were subjected to a hyperlocomotion test (Figure 6). As expected, naïve mice exhibited elevated hyperlocomotor activity with increasing doses of cocaine. Interestingly, while FliC or mFliC vaccinated mice also showed robust hyperlocomotor activity when vaccinated without CpG, TT mice vaccinated without CpG exhibited some blockade of the cocaine effect at doses higher than 5 mg/kg.
Figure 6.
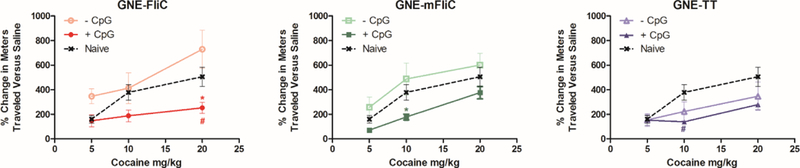
Increases in cocaine-induced hyperlocomotor activity over baseline locomotion on day 43 in vaccinated mice (n=5 or 6) at 5, 10 and 20 mg/kg, measured on days 45, 47 and 49, respectively. # = P<0.05 vs. Naïve. * = P<0.05 vs. – CpG
Furthermore, all the mice immunized with a CpG-containing vaccine showed less hyperlocomotion overall than unvaccinated mice or mice vaccinated with formulations lacking CpG. The amount of time spent immobile for each group also mirrored this pattern of efficacy (Figure S5).
Serum Cocaine Concentrations.
From a pharmacokinetic standpoint, we investigated the effect of our vaccines on the cocaine concentration in the blood near Cmax (Figure 7). The results revealed that the mice administered a CpG-containing vaccine retained significantly more amounts of cocaine in their serum relative to the corresponding mice vaccinated without CpG. Overall, the serum from GNE-TT+CpG and GNE-FliC+CpG vaccinated mice clearly showed higher concentration of cocaine retained in the serum, nearly 15 times greater than control, while the magnitude of the effect was somewhat smaller in GNE-mFlic+CpG vaccinated animals.
Figure 7.

LC/MS measurement of cocaine concentration in the serum of naïve and vaccinated mice (n=5 or 6) given 10 mg/kg cocaine IP. Blood samples were collected at 15 min. Concentrations were derived from integration of peaks with m/z 304.1–304.9, using cocaine-D3 (m/z 307.1–307.9) as an internal standard. * = P<0.05, *** = P<0.001 vs. Naïve.
DISCUSSION
Comparing the results of ELISA, RIA, and pharmacokinetic assay in this study reveals that a GNE-vaccine formulated with the TLR9 agonist CpG 1826 is generally superior to a vaccine without CpG. Interestingly, however, CpG was more effective in improving ELISA titer when it was formulated with the TLR neutral carrier TT as compared to the TLR5 agonists FliC or mFliC. This same pattern was seen while assessing antibody concentrations by RIA. However, the antibodies from mice vaccinated with CpG do tend to have modestly poorer Kd values than antibodies from mice administered vaccine without CpG. Even though CpG formulated vaccines induced antibodies having relatively modest Kd values, the vaccines containing this TLR9 agonist, especially GNE-FliC+CpG and GNE-TT+CpG, were still highly efficacious in blocking cocaine’s effect in the hyperlocomotion test. This is likely because the substantial increase in anti-cocaine antibody concentration due to CpG was sufficient to compensate for the relatively lower antibody affinity for cocaine.
In the hyperlocomotion test, we observed that combining the TLR5 agonist FliC with alum and CpG was highly effective in blunting cocaine’s effect in mice, while the vaccine formulated without the TLR9 agonist showed almost no efficacy. However, this same pattern was also observed for TT, indicating that the influence of TLR9 agonism by CpG is overwhelming any TLR5 effect from FliC in this assay. Alternative TLR agonists could generate a different pattern of response. Consistently, GNE-mFliC+CpG turned out to be better than without CpG, although the vaccine efficacy was poor even with CpG. While the results of our mTLR5 reporter assay indicate that mFliC binds to and activates TLR5, mFliC was still fairly unproductive as a carrier protein, even when compared to FliC. However, as it has been reported that flagellin monomers are much more potent than polymers to stimulate TLR5. We surmise that improved efficacy and TLR5 activation could still be seen by generating a monomeric flagellin protein as the carrier.20 This hypothesis will be tested in future studies.
Overall, the TLR9-activating formulation GNE-TT+CpG exhibited the best efficacy, while the dual TLR9- and TLR5-activating formulation GNE-FliC+CpG had comparable activity in the hyperlocomotion test and analysis of the cocaine concentration in blood at Cmax. Activation of TLR5 alone by FliC or mFliC was insufficient to generate a robust response in this study. The inability of GNE-mFlic+CpG to generate high enough antibody concentrations and behavioral efficacy despite retained TLR5 activation as compared to wild type FliC further supports the concept that effective induction of MHC processing may be a more crucial aspect of FliC function than TLR5 activation. Indeed, the magnitude of change in antibody titer and plasma sequestration was by far the largest for TT, then next largest for FliC, and smallest for mFliC. This pattern generally inverts the pattern of TLR5 agonism for these carrier proteins, while preserving the apparent order of MHC presentation efficiency, indicating that the additive TLR signaling effects in this formulation are not sufficient to overcome the relatively poor efficacy of FliC as a carrier protein.
CONCLUSION
The current study highlights the impact of combining FliC with CpG 1826 and alum as adjuvants. We have demonstrated that CpG- and alum-adjuvants for cocaine hapten-protein conjugates can elicit robust, high-affinity anti-cocaine antibody production in mice, resulting in our most efficacious anti-cocaine vaccine formulation to date, GNE-TT+CpG. We also wish to emphasize that this is the first study examining dual TLR activation for an anti-cocaine vaccine; it was found that TLR9 activation was effective in improving the activity of TLR5 activating formulations, but not as effective as TLR9 activation alone. We also presented data showing a mutant FliC conjugate that retains TLR5 activation is not as effective as a wild type FliC conjugate with better predicted MHC-II binding and hapten presentation, indicating that the inefficiency of FliC as a carrier protein may be limiting the overall efficacy of such a combination. Future efforts will focus on optimizing the GNE-TT+CpG formulation and administration schedule to generate antibodies with higher affinity for cocaine and on the generation of a monomeric FliC carrier protein. Additional studies with these anti-cocaine vaccines will be undertaken in alternative species to provide better insight to determine the clinical tractability of this approach.
Supplementary Material
Figure 5.

Anti-cocaine antibody midpoint titers measured by ELISA using serum from vaccinated mice (n=5 or 6) on days 28 (bleed 1) and 49 (bleed 2) for each study group. Data are shown as the average of two replicates ± SEM. * = P<0.05 vs. – CpG
ACKNOWLEDGEMENT
The authors thank Beverly Ellis for her assistance with mouse tissue collection. The authors thank Dr. Jonathan Lockner and Dr. Robin Rosenfeld-Gunn for helpful discussions. The authors thank Ke Yang for preliminary studies.
Funding Sources
This work was supported by generous funding from NIDA (DA008590).
ABBREVIATIONS
- FDA
Food and Drug Administration
- TLR9
Toll Like Receptor 9
- TLR5
Toll Like Receptor 5
- FliC
flagellin
- CpG 1826
cytosine-guanine oligodeoxynucleotide 1826
- TT
tetanus toxoid
- BBB
blood-brain barrier
- MyD88
myeloid differentiation factor 88
- NIDA
National Institute on Drug Abuse
- MALDI-ToF
matrix-assisted laser desorption/ionization
- ELISA
enzyme-linked immunosorbent assay
- MWCO
molecular weight cut off
- PBS
phosphate buffered saline
- HRP
horseradish peroxidase
- BSA
bovine serum albumin
- TMB
3,3’,5,5’-tetramethylbenzidine
- RIA
radioimmunoassay
- Cmax
maximum plasma concentration
- Kd
dissociation constant
- TH2
T-helper 2
- CTL
cytotoxic T lymphocytes
- APS
antigen presenting cells
- TH1
T-helper 1
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Supplemental Figures 1-5: Image showing the sequence of mFliC mutated residues; LC trace for purification of mFliC; TLR5 activation assay results; MALDI-ToF traces; Immobility time results for hyperlocomotion (PDF).
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Notes
TSRI manuscript # 29398
REFERENCES
- 1.Administration, S. A.a. M. H. S., Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health NSDUH Series H-44, HHS Publication No. (SMA) 15−4927; Substance Abuse and Mental Health Services Administration: Rockville, MD, 2012. 2015. [Google Scholar]
- 2.Program, N. A. H. D. A., Drug Abuse Warning Network (DAWN), 2011 (ICPSR 34565) HHS Publication No. (SMA) 13–4760, DAWN Series D-39; Rockville, MD, 2013. 2011. [Google Scholar]
- 3.(a)Karila L; Reynaud M; Aubin HJ; Rolland B; Guardia D; Cottencin O; Benyamina A, Pharmacological treatments for cocaine dependence: is there something new? Curr Pharm Des 2011, 17 (14), 1359–68; [DOI] [PubMed] [Google Scholar]; (b)Loftis JM; Huckans M, Substance use disorders: psychoneuroimmunological mechanisms and new targets for therapy. Pharmacol Ther 2013, 139 (2), 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll FI; Howell LL; Kuhar MJ, Pharmacotherapies for treatment of cocaine abuse: preclinical aspects. J Med Chem 1999, 42 (15), 2721–36. [DOI] [PubMed] [Google Scholar]
- 5.Shorter D; Domingo CB; Kosten TR , Emerging drugs for the treatment of cocaine use disorder: a review of neurobiological targets and pharmacotherapy. Expert Opin Emerg Dr 2015, 20 (1), 15–29. [DOI] [PubMed] [Google Scholar]
- 6.(a)Haney M; Gunderson EW; Jiang HP; Collins ED; Foltin RW, Cocaine-Specific Antibodies Blunt the Subjective Effects of Smoked Cocaine in Humans. Biol Psychiat 2010, 67 (1), 59–65; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b)Shen XY; Orson FM; Kosten TR, Vaccines against drug abuse. Clin Pharmacol Ther 2012, 91 (1), 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a)Janda KD; Treweek JB, Vaccines targeting drugs of abuse: is the glass half-empty or half-full? Nat Rev Immunol 2012, 12 (1), 67–72; [DOI] [PubMed] [Google Scholar]; (b)Moreno AY; Janda KD, Current challenges for the creation of effective vaccines against drugs of abuse. Expert Rev Vaccines 2011, 10 (12), 1637–1639. [DOI] [PubMed] [Google Scholar]
- 8.(a)Kosten TR; Rosen M; Bond J; Settles M; St Clair Roberts J; Shields J; Jack L; Fox B, Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 2002, 20 (7–8), 1196–1204; [DOI] [PubMed] [Google Scholar]; (b)Martell BA; Mitchell E; Poling J; Gonsai K; Kosten TR, Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiat 2005, 58 (2), 158–164; [DOI] [PubMed] [Google Scholar]; (c)Martell BA; Orson FM; Poling J; Mitchell E; Rossen RD; Gardner T; Kosten TR, Cocaine Vaccine for the Treatment of Cocaine Dependence in Methadone-Maintained Patients A Randomized, Double-blind, Placebo-Controlled Efficacy Trial. Arch Gen Psychiat 2009, 66 (10), 1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai XQ; Whitfield T; Moreno AY; Grant Y; Hixon MS; Koob GF; Janda KD, Probing the Effects of Hapten Stability on Cocaine Vaccine Immunogenicity. Mol Pharmaceut 2013, 10 (11), 4176–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a)Bremer PT; Kimishima A; Schlosburg JE; Zhou B; Collins KC; Janda KD, Combatting Synthetic Designer Opioids: A Conjugate Vaccine Ablates Lethal Doses of Fentanyl Class Drugs. Angew Chem Int Edit 2016, 55 (11), 3772–3775; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b)Jacob NT; Lockner JW; Schlosburg JE; Ellis BA; Eubanks LM; Janda KD, Investigations of Enantiopure Nicotine Haptens Using an Adjuvanting Carrier in Anti-Nicotine Vaccine Development. J Med Chem 2016, 59 (6), 2523–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lockner JW; Eubanks LM; Choi JL; Lively JM; Schlosburg JE; Collins KC; Globisch D; Rosenfeld-Gunn RJ; Wilson IA; Janda KD, Flagellin as Carrier and Adjuvant in Cocaine Vaccine Development. Mol Pharmaceut 2015, 12 (2), 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bremer PT; Schlosburg JE; Lively JM; Janda KD, Injection Route and TLR9 Agonist Addition Significantly Impact Heroin Vaccine Efficacy. Mol Pharmaceut 2014, 11 (3), 1075–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(a)Kobayashi H; Horner AA; Takabayashi K; Nguyen MD; Huang E; Cinman N; Raz E, Immunostimulatory DNA pre-priming: a novel approach for prolonged Th1-biased immunity. Cell Immunol 1999, 198 (1), 69–75; [DOI] [PubMed] [Google Scholar]; (b)Millan CLB; Weeratna R; Krieg AM; Siegrist CA; Davis HL, CpG DNA can induce strong Th1 humoral and cell-mediated immune responses against hepatitis B surface antigen in young mice. P Natl Acad Sci USA 1998, 95 (26), 15553–15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen YM; Shi Y, Alum: an old dog with new tricks. Emerg Microbes Infec 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghosh TK; Mickelson DJ; Solberg JC; Lipson KE; Inglefield JR; Alkan SS, TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. Int Immunopharmacol 2007, 7 (8), 1111–21. [DOI] [PubMed] [Google Scholar]
- 16.McGuffin LJ; Bryson K; Jones DT, The PSIPRED protein structure prediction server. Bioinformatics 2000, 16 (4), 404–405. [DOI] [PubMed] [Google Scholar]
- 17.Singh R; Singh S; Sharma PK; Singh UP; Briles DE; Hollingshead SK; Lillard JW, Helper T Cell Epitope-Mapping Reveals MHC-Peptide Binding Affinities That Correlate with T Helper Cell Responses to Pneumococcal Surface Protein A. Plos One 2010, 5 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a)Wang P; Sidney J; Dow C; Mothe B; Sette A; Peters B, A systematic assessment of MHC class II peptide binding predictions and evaluation of a consensus approach. Plos Comput Biol 2008, 4 (4); [DOI] [PMC free article] [PubMed] [Google Scholar]; (b)Wang P; Sidney J; Kim Y; Sette A; Lund O; Nielsen M; Peters B, Peptide binding predictions for HLA DR, DP and DQ molecules. Bmc Bioinformatics 2010, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller R, Calculation of average antibody affinity in anti-hapten sera from data obtained by competitive radioimmunoassay. J Immunol Methods 1980, 34 (4), 345–52. [DOI] [PubMed] [Google Scholar]
- 20.Smith KD; Andersen-Nissen E; Hayashi F; Strobe K; Bergman MA; Barrett SL; Cookson BT; Aderem A, Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nat Immunol 2003, 4 (12), 1247–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


