Abstract
Background
Anemia is one of the most prevalent complications in patients with chronic kidney disease, which is believed to be caused by the insufficient synthesis of erythropoietin by the kidney. This phase III study aimed to compare the efficacy and safety of CinnaPoietin® (epoetin beta, CinnaGen) with Eprex® (epoetin alfa, Janssen Cilag) in the treatment of anemia in ESRD hemodialysis patients.
Methods
In this randomized, active-controlled, double-blind, parallel, and non-inferiority trial, patients were randomized to receive either CinnaPoietin® or Eprex® for a 26-week period. The primary endpoints of this study were to assess the mean hemoglobin (Hb) change during the last 4 weeks of treatment from baseline along with the evaluation of the mean weekly epoetin dosage per kilogram of body weight that was necessary to maintain the Hb level within 10–12 g/dL during the last 4 weeks of treatment. As the secondary objective, safety was assessed along with other efficacy endpoints.
Results
A total of 156 patients were included in this clinical trial. There was no statistically significant difference between treatment groups regarding the mean Hb change (p = 0.21). In addition, the mean weekly epoetin dosage per kg of body weight for maintaining the Hb level within 10–12 g/dL showed no statistically significant difference between treatment arms (p = 0.63). Moreover, both products had comparable safety profiles. However, the incidence of Hb levels above 13 g/dL was significantly lower in the CinnaPoietin® group.
Conclusion
CinnaPoietin® was proved to be non-inferior to Eprex® in the treatment of anemia in ESRD hemodialysis patients. The trial was registered in Clinicaltrials.gov (NCT03408639).
Key Words: End-stage renal disease, Anemia, Eprex®, CinnaPoietin®, Hemodialysis
Introduction
Anemia is one of the major and prevalent complications of chronic kidney disease (CKD) [1]. The main reason for anemia in patients with CKD is the insufficient synthesis of erythropoietin due to kidney failure. Since the 1980s, erythropoiesis-stimulating agents (ESAs) have been used to treat CKD-associated anemia. Most of the end-stage renal disease (ESRD) patients need recombinant human erythropoietin or blood transfusions to achieve target hemoglobin (Hb) level [2, 3, 4].
Among several types of ESAs, epoetin alfa and beta, 2 short-acting ESAs, have shown the same efficacy in treating CKD-induced anemia. Some studies suggest that subcutaneous (SC) injection of epoetin beta is less painful than epoetin alfa [5, 6]. Other studies have demonstrated that elimination half-life of epoetin beta is longer than epoetin alfa, which is probably due to different glycosylation. As a result, lower doses may be needed to maintain hemoglobin and hematocrit in the target level [7, 8, 9]. However, the Kidney Disease Improving Global Outcomes (KDIGO) guideline along with other evidence suggest that epoetin alfa and beta have the same efficacy and require the same dose to be administered to patients with CKD-induced anemia [10, 11].
CKD is one of the most prevalent diseases in Iran. According to a study in 2009, the overall prevalence of the disease was 18.9% (among 10,063 participants aged over 20) in Tehran, Iran [12]. ESRD defines as under stage 5 of the National Kidney Foundation Kidney Disease Outcomes Quality Initiative classification of CKD. It refers to individuals with an estimated glomerular filtration rate less than 15 mL per minute per 1.73 m2 body surface area, or patients requiring dialysis without considering glomerular filtration rate. The prevalence of ESRD stages 5 has reported 0.1% in Iran [13, 14]. In addition, the financial burden of CKD-related complications is estimated to be very high [15]. With respect to epoetin beta benefits and prevalence of CKD in Iran, CinnaGen has recently produced a biosimilar to the reference epoetin beta product (NeoRecormon®, Roche). Due to lack of rigorous evidence on how efficient epoetin alfa and beta are when compared to each other, we conducted a phase III, double-blind, randomized study to compare the efficacy and safety of epoetin beta biosimilar (CinnaPoietin®) with the efficacy and safety of epoetin alfa (Eprex®) in the treatment of anemia in ESRD patients on hemodialysis.
Methods
Study Design
This phase III, randomized, multicenter, prospective, 2-armed, parallel, double-blind (patient- and assessor-blinded), active-controlled, and non-inferiority clinical trial was performed in 8 centers in 3 cities (Tehran, Kerman, Shiraz) of Iran. The study procedures were in accordance with the Good Clinical Practice (GCP) guidelines and ethical principles of the Declaration of Helsinki. The study was approved by the Ethics Committees of Tehran University of Medical Sciences (IR.TUMS.REC.1394.969) and Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1394.s50). Written informed consent was obtained from all participants before the study was initiated. The trial has been registered in Clinicaltrials.gov (NCT03408639).
Patients were randomized with 1: 1 ratio to receive either CinnaPoietin® or Eprex®. Patients who had received epoetin previously continued to receive the same dose. Both IV and SC injections were allowed for the patients. The dose was then adjusted based on the patient's response to maintain the Hb level in the target range (10–12 g/dL). Administration dose for patients who were treated with erythropoietin was similar to the previously administered dose (IV or SC without any change). After then, dose adjustment was done based on patients' response. Dose adjustment for patients was performed in the following manner:
Doses should not be increased more frequently than once a month. If the hemoglobin level is increasing and reaches 13 g/dL, the dose should be reduced by approximately 25–50% (50–75% of the previous dose). If the hemoglobin continues to increase, the dose should be temporarily withheld until the hemoglobin begins to decrease; at this point, therapy should be re-initiated at a dose approximately 25–50% below the previous dose. If the hemoglobin increases by more than 1 g/dL in any 2-week period, the dose should be decreased by approximately 25–50%. In addition, patients received Nephrovit® (multivitamin for CKD patients) daily along with monthly injections of vitamin B12 (100 μg) over a period of 26 weeks. Patients were evaluated every 2 weeks for the first 2 months and then at weeks 12, 20, 22, 24, and 26. The first 2 weeks of the trial were considered the washout period, and related data were not included in the statistical analysis.
Patients' background information, medical history, and prior medications were recorded at baseline. Also, medication regimens were recorded in the entire study. Laboratory examinations were performed throughout the trial. Moreover, the incidence of adverse events at each visit was recorded based on patients' reports and laboratory findings.
All injections were administered by experienced nurses at each center. All participants were blinded to the allocated treatments. Both CinnaPoietin® and Eprex® prefilled syringes were relabeled and coded by an independent party to become indiscernible from each other.
Participants
ESRD patients who were on hemodialysis for ≥3 months who met the following criteria were included in this clinical trial: age between 18 to 70 years; Hb level between 8 and 11.5 g/dL, being on adequate hemodialysis, having adequate iron stores, which is defined as serum ferritin ≥200 ng/mL and transferrin saturation ≥20%; complying with medication use, visits, and procedures as judged by the investigator; using an acceptable birth control method for females of childbearing potential; and providing written informed consent.
Patients with any of the following criteria were excluded from the study: uncontrolled hypertension (pre-dialysis diastolic blood pressure ≥100 mm Hg or systolic blood pressure ≥180 mm Hg); anemia secondary to other causes apart from CKD; decompensated liver failure; uncontrolled hyperparathyroidism (iPTH > 800 pg/mL); class III and IV of heart failure according to New York Heart Association classification, unstable angina pectoris, active cardiac disease, stroke and/or cardiac infarction within the last 6 months; history or active blood coagulation disorders, including deep vein thrombosis, pulmonary thromboembolism, and native access thrombosis during the last 6 months; platelet count > 500,000/µL; platelet count < 100,000/µL; white blood cell count < 3,000/µL; white blood cell count > 15,000/µL; recent bleeding (within 3 months before screening), suspected or confirmed occult bleeding; clinical evidence of concurrent systemic infection or inflammatory disease (e.g., diabetic foot, bed sore, vascular access infection, and CRP > 30 mg/L); currently receiving medication for treatment of epilepsy, major surgery within 3 months prior to randomization and during the clinical trial (except vascular access surgery); concomitant immunosuppressive therapy; history of malignancy within the last 5 years (except excised non-melanoma skin cancer); pregnancy or breastfeeding; known history of severe drug-related allergies; known history of drug-related allergy to erythropoietin or its ingredients or hypersensitivity to mammalian-derived products; receiving transplant within 1 year prior to the start of the study; simultaneous participation in another clinical study or having received an investigational medicinal product within 3 months before randomization in this study; having psychiatric, addictive (drugs or alcohol), or any other disorder comprising the ability to give an informed consent; any red blood cell transfusion during the last 3 months; primary hematological disorder; known resistance to recombinant human erythropoietin defined by requiring more than 450 IU/kg/week by IV or 300 IU/kg/week by SC routes, equivalent to approximately 20,000 IU/week by SC route in the absence of iron deficiency; suffering an event of active bleeding in 30 days before the study; and having morbid obesity, defined by a body mass index (BMI) > 37 kg/m2 in women and > 40 kg/m2 in men.
Efficacy and Safety Assessment
Assessing the mean Hb level change during the last 4 weeks of treatment from baseline and evaluation of the mean weekly epoetin dosage per kg of body weight during the last 4 weeks of treatment (necessary to maintain the Hb level within 10–12 g/dL during the last 4 weeks of the study) were the primary endpoints of this study. The secondary endpoints of this study were as follows: proportion of patients with any permanent or transient dose change during the main study phase (after wash-out period), proportion of patients with any Hb measurement outside the target range, proportion of patients who needed blood transfusions, proportion of patients who had successful treatment (Hb concentration ≥11.0 g/dL and without any blood transfusion within the preceding 3 months), proportion of patients with maintenance success (maintenance of mean Hb concentration of 11.0 ± 1.0 g/dL for at least 4 consecutive weeks), the percentage of patients with Hb measurements > 10.0 g/dL and hematocrit measurements > 30% during the last 4 weeks, along with the evaluation of safety measures between treatment arms. Safety outcomes in this study included the incidence of Hb levels above 13 g/dL, the proportion of patients with an increase in Hb concentration of > 1.0 g/dL for 4 consecutive weeks, and evaluation of the incidence rate of adverse drug events. By considering the consumed dose in the last 4 weeks of the study, the annual cost of the medicines in both arms was predicted and evaluated as a cost-effectiveness parameter. Any adverse events during the study were recorded by the investigators in the case report forms. Laboratory tests were performed by authorized laboratories and all of the tests were standardized. The mean consumed dose of each epoetin product was analyzed throughout the study [16].
Statistical Analysis
The sample size of 144 patients was considered to provide 80% power with the assumption of a difference of −0.500 between the means for determining non-inferiority by a margin of −1.00. However, by considering drop out, a total of 156 patients were assigned to 2 treatments using the cluster randomization method. The primary efficacy endpoints were analyzed using the per-protocol (PP) analysis. Those patients who did not receive at least one dose of study medication for more than 5 weeks or those whose dose was not modified correctly were excluded from the PP analysis. Dose modification was defined as follows: If the Hb level during a particular visit exceeded 13 g/dL, the dose in subsequent visits must be reduced; otherwise, the patient would be excluded from the PP analysis. If the Hb level at the time of a particular visit was less than 7 g/dL, blood transfusion must be performed; otherwise, the patient would be excluded from the PP analysis.
The secondary efficacy endpoints were assessed using the intention-to-treat approach. The safety analysis was conducted using safety population, meaning those who were randomized and received at least one dose of study medication. The efficacy of treatment was evaluated by employing the unpaired t test, Pearson's chi-square test, and Fisher's exact test. p values less than 0.05 were considered statistically significant. Adverse events were reported as the incidence rate. Moreover, the generalized estimating equations model was used to detect the changes in dose over time. In this study, all statistical computations were done using STATA software 14.0 (Stata Corporation, College Station, TX, USA).
Results
Patients' Disposition
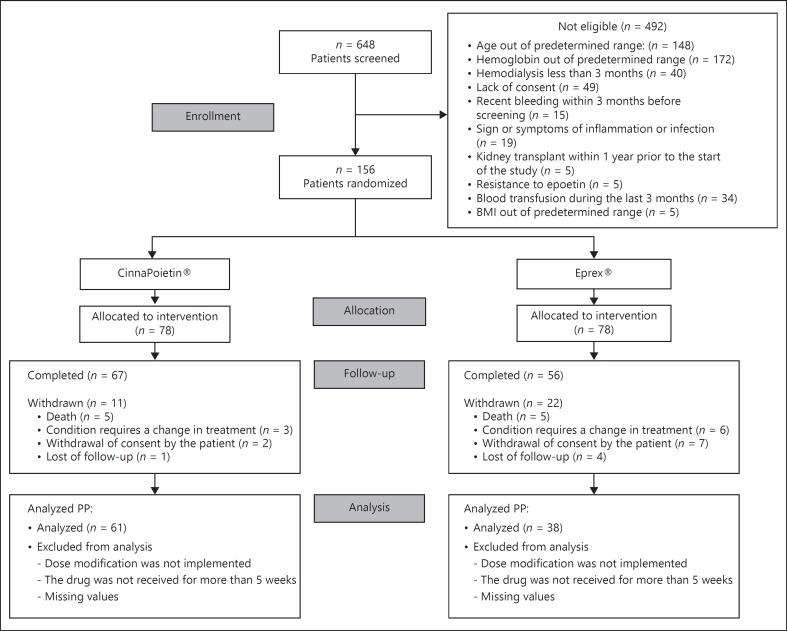
A total of 648 patients were screened across 8 centers in Iran and 156 patients were enrolled in the study. Sixty-seven patients in CinnaPoietin® arm and 56 patients in Eprex® arm completed the 26-week study period. The study profile is shown in Figure 1.
Fig. 1.
Trial profile, PP per-protocol.
The baseline (week 0) characteristics of patients are outlined in Table 1. The route of administration of patients receiving CinnaPoietin® and Eprex® groups during the study is shown in online supplementary Table S1 (for all online suppl. material, see www.karger.com/doi/10.1159/000493097). As shown, more patients received epoetin in the SC route.
Table 1.
Summary of the baseline (week 0) characteristics of the patients enrolled
| Variable | CinnaPoietin® (n = 78) | Eprex® (n = 78) |
|---|---|---|
| Age, years | 55.02±11.38 | 50.01±12.72 |
| Male, n (%) | 45 (57.69) | 45 (57.69) |
| BMI, kg/m2 | 25.5±5.99 | 25.09±4.66 |
| Hgb, g/dL | 10.41±0.89 | 10.64±0.82 |
| Hct, % | 32.37±3.91 | 33.12±3.24 |
| Ferritin, ng/mL | 674.42±410.88 | 626.15±320.47 |
| TSAT, % | 37.38±23.95 | 38.15±20.43 |
| CRP | 10.03±15.09 | 9.88±11.26 |
| History of high blood pressure, n (%) | 34 (43.59) | 14 (17.95) |
| History of diabetes type 1, n (%) | 7 (8.97) | 1 (1.28) |
| History of diabetes type 2, n (%) | 16 (20.51) | 4 (5.13) |
| History of Glomerulopathy, n (%) | 5 (6.41) | 2 (2.56) |
| Medication, n (%) | ||
| Calcium channel blockers | 10 (18.18) | 2 (5.71) |
| Statins | 6 (10.91) | 3 (8.57) |
| ACE/ARBs* | 16 (29.09) | 0 (0.00) |
| Beta blocker | 20 (36.36) | 1 (2.86) |
| Iron sucrose IV | 3 (5.45) | 29 (82.86) |
Angiotensin converting enzyme inhibitors/angiotensin II receptor blockers.
Values are presented as mean ± SD unless stated otherwise.
Efficacy
The mean Hb change in patients was 0.4 ± 1.26 and 0.74 ± 1.39 for CinnaPoietin® and Eprex® groups, respectively, in the PP population (p = 0.21). The two-sided 90% CI of the difference in the mean change of Hb between CinnaPoietin® and Eprex® (-0.79, 0.11) falls within the prespecified non-inferiority margin.
Also, hemoglobin measurement trends of 2 groups during the entire study are shown in online supplementary Figure S1.
The mean weekly epoetin dosage per kg body weight of patients during the last 4 weeks of treatment necessary to maintain the Hb level within 10–12 g/dL in PP population was 117.02 ± 54.5 IU/kg/week and 110.01 ± 49.27 IU/kg/week for CinnaPoietin® and Eprex® groups respectively (p = 0.63).
A table summarizing data on hemoglobin, hematocrit, and iron status in the period of primary outcome analysis (last month) is presented as online Supplementary Table S2.
Thirteen patients in the CinnaPoietin® arm received 400 mg IV iron infusions as the median average monthly dose during the trial and 40 patients in the Eprex® arm received 300 mg IV iron infusions as the median average monthly dose during the study.
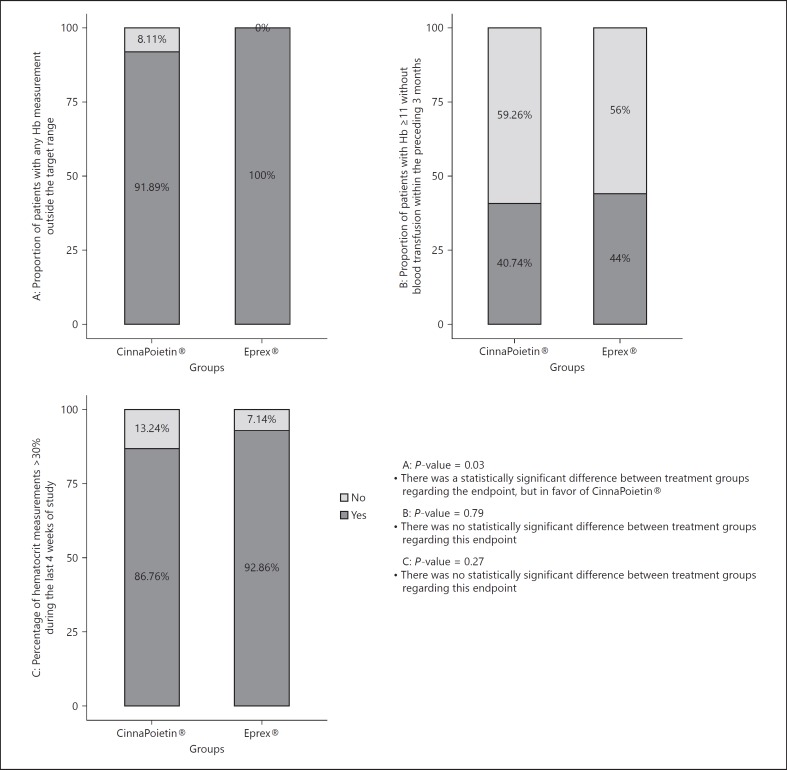
The proportion of patients with any permanent or transient dose change was 90.28% in the CinnaPoietin® group and 95.45% in the Eprex® group (p = 0.33). There was a statistically significant difference between treatment groups in the proportion of patients with any Hb measurement outside the target range (p = 0.03). However, this difference was in favor of CinnaPoietin® (Fig. 2). There was no statistically significant difference between treatment groups in the percentage of hematocrit measurements > 30% in the last 4 weeks (p = 0.27) and the proportion of patients with Hb ≥11 g/dL without blood transfusion within the preceding 3 months (p = 0.79; Fig. 2). Three patients in each group experienced at least one blood transfusion (p = 1.00).
Fig. 2.
Proportion of patients with any Hb measurement outside the target range; proportion of patients with Hb ≥11 without blood transfusion within the preceding 3 months and percentage of hematocrit measurments > 30% during the last4 weeks.
There was a statistically significant difference between treatment groups regarding the proportion of patients who reached maintenance success (maintenance of mean Hb concentration of 11.0 ± 1.0 g/dL) for at least 4 consecutive weeks (p= 0.002). However, this difference was in favor of CinnaPoietin® (online suppl. Fig. S2). There was no statistically significant difference between treatment groups regarding the mean Hb level above 10 g/dL during the last 4 weeks of treatment (p = 0.48; online suppl. Fig. S2).
The final evaluation of the mean weekly epoetin dose during the study demonstrated that it was higher in the Eprex® group (mean 20.87 IU/kg/week more than CinnaPoietin®, with 95% CI (−4.07 to 45.81)), but this difference was not statistically significant (p = 0.1; online suppl. Fig. S3).
Safety
The proportion of patients who experienced at least one Hb measurement above 13 g/dL during the study was 44.29% and 71.19% in CinnaPoietin® and Eprex® groups respectively (p = 0.002). While 61.11% of patients in the CinnaPoietin® and 63.08% of patients in the Eprex® group witnessed at least one case of increase in Hb concentration > 1.0 g/dL for consecutive 4 weeks, there was no statistically significant difference between treatment arms in this regard (p = 0.81). Among all patients, 41 (26.28%) patients experienced at least one adverse event (AE) during the study. There was no significant difference between treatment groups regarding the incidence of AE (p = 0.36). The summary of AEs based on system organ class is presented in Table 2.
Table 2.
Comparison of adverse events between the 2 groups by organ class
| Organ class | CinnaPoietin® (n = 78), n (%) |
Eprex® (n = 78), n (%) |
|---|---|---|
| Blood and lymphatic system disorders | 0 (0) | 1 (1.28) |
| Cardiac disorders | 3 (3.85) | 6 (7.69) |
| Electrolyte and fluid balance conditions | 1 (1.28) | 0 (0) |
| Eye disorders | 1 (1.28) | 0 (0) |
| General disorders and administration site conditions | 7 (8.97) | 1 (1.28) |
| Hepatobiliary disorders | 1 (1.28) | 0 (0) |
| Infections and infestations | 2 (2.56) | 0 (0) |
| Injury, poisoning and procedural complications | 0 (0) | 2 (2.56) |
| Metabolism and nutrition disorders | 0 (0) | 1 (1.28) |
| Musculoskeletal and connective tissue disorders | 2 (2.56) | 2 (2.56) |
| Nervous system disorders | 1 (1.28) | 1 (1.28) |
| Psychiatric disorders | 1 (1.28) | 0 (0) |
| Reproductive system and breast disorders | 0 (0) | 1 (1.28) |
| Respiratory, thoracic and mediastinal disorders | 3 (3.85) | 0 (0) |
| Skin and subcutaneous tissue disorders | 0 (0) | 3 (3.85) |
| Surgical and medical procedures | 1 (1.28) | 1 (1.28) |
| Vascular disorders | 2 (2.56) | 0 (0) |
| Patients with at least 1 AE, total* | 23 (29.49) | 18 (21.79) |
Statistically not significant (p = 0.36).
The annual cost of anemia treatment in an ESRD patient with CinnaPoietin® is approximately 540 US dollars, whereas with Eprex® it is around 1,330 USD.
Discussion
This clinical trial was conducted to compare the efficacy and safety of CinnaPoietin® (epoetin beta) and Eprex® (epoetin alfa) in their role as short-acting ESAs. The findings suggested that CinnaPoietin® is non-inferior to Eprex® in terms of efficacy. In addition, both products had comparable safety profiles.
The mean change in Hb level during the last 4 weeks was comparable in CinnaPoietin® and Eprex® arms. The mean change in Hb level matched those seen in the B. Gertz et al. [17] study that compared 2 different types of short-acting ESAs (theta and beta) in the treatment of anemia in CKD patients. Another study that compared 2 different types of short-acting ESAs showed that with an equivalence range of ±1 g/dL, epoetin zeta and alfa have similar efficacy levels [16]. This is in accordance with the KDIGO guideline that stated that short-acting alfa and beta ESAs are similar to each other in terms of their efficacy levels [10].
The mean weekly epoetin dosage per kg body weight of patients during the last 4 weeks of treatment necessary to maintain the Hb level within 10–12 g/dL was similar between 2 groups. According to the KDIGO guideline, epoetin alfa or epoetin beta usually starts at the same dose and these 2 types of ESAs have equal efficacy [9]. Few studies compared the dose of epoetin alfa with epoetin beta in patients with CKD. Ostrvica et al. [11] evaluated the efficacy of epoetin alfa and beta for correction of anemia in ESRD patients during 6 months in a prospective study. It was concluded that these 2 ESAs have comparable efficacy in the treatment of renal anemia in hemodialysis patients. Also, patients needed approximately equal doses of epoetin alfa and beta to achieve and maintain the target level of Hb and hematocrit at the end of the study. Nevertheless, this study had a small sample size. In the present study, we included more patients and we concluded that epoetin alfa and beta have comparable efficacy in the treatment of anemia in hemodialysis patients.
In another study, Loughnan et al. [8] compared the efficacy and safety of epoetin beta and epoetin alfa in the maintenance phase of hemodialysis patients. Their results indicated that higher doses of epoetin alfa are required to maintain Hb in the target level. As previously mentioned, the mean dose of epoetin during the whole study was higher in the Eprex® group, although this difference was not significant.
Krivoshiev et al. [18] compared some parameters related to the efficacy of 2 types of ESAs (epoetin zeta and alfa) in renal anemia. Results of this trial indicated that different types of short-acting ESAs have similar efficacy in ensuring maintenance success and proportion of patients outside the target range. In the current trial, the proportion of patients with any Hb measurement outside the target range in Eprex® was higher than CinnaPoietin®. The difference was statistically significant (p = 0.03) and it was in favor of CinnaPoietin®. Since most of the patients were in the correction phase, the percentage of patients with Hb outside the target range was high in both groups, but this percentage was significantly lower in CinnaPoietin® group. Also, a higher proportion of patients with maintenance success in the CinnaPoietin® group suggests that CinnaPoietin® is more successful than Eprex® in keeping patients in the target range of Hb level (10–12 g/dL).
As indicated in the results, the incidence of Hb levels above 13 g/dL in the Eprex® group was significantly higher than that in the CinnaPoietin® group. Accordingly, we could conclude that CinnaPoietin® was better than Eprex® in maintaining the Hb level of patients in the target level (10–12 g/dL), but in the proportion of patients with an increase in Hb concentration of > 1.0 g/dL for 4 consecutive weeks, there was no statistically significant difference between treatment groups.
Regarding the incidence of adverse events, we did not notice any significant difference between groups and the most common related adverse events during the study were general disorders and administration site conditions; therefore, it seems CinnaPoietin® and Eprex® have comparable safety profile.
In respect to cost comparison, it is estimated that the annual cost for each patient receiving Eprex® is approximately 2.5 times more than that for each patient receiving CinnaPoietin® in Iran. This is an indication that by biosimilar production, the total cost for the health-care system can decrease dramatically, which is in line with the FDA biosimilar action plan, which states that producing biosimilars potentially lower costs for patients and payors and increase access to these novel products [19].
In the present study, we found similar efficacy and safety patterns between CinnaPoietin® and Eprex®. However, further studies with a larger sample size could corroborate our findings. Moreover, comparing local pain after the subcutaneous injection of epoetin alfa and beta can be investigated in another study. We evaluated the dose trend of CinnaPoietin® and Eprex® during the study. However, this study was not powered to assess the dose difference to maintain Hb in the target range of 10–12 g/dL in patients during the entire study and in patients at maintenance phase. This issue along with the clinical judgment of therapy choices and cost-effectiveness assessment can be evaluated in further studies.
Conclusion
This study proved that CinnaPoietin® is non-inferior to Eprex® in terms of efficacy. The comparable safety profile of these products was also demonstrated in the treatment of anemia in ESRD hemodialysis patients. To the best of our knowledge, this is the first phase III, prospective, double-blind, randomized, active-controlled clinical trial that compares the efficacy and safety of epoetin beta and alfa with this adequate sample size.
Ethics Statement
The study procedures were in accordance with the Good Clinical Practice guidelines and ethical principles of the Declaration of Helsinki. The study was approved by the Ethics Committees of Tehran University of Medical Sciences (IR.TUMS.REC.1394.969) and Shiraz University of Medical Sciences (IR.SUMS.MED.REC.1394.s50). Written informed consent was obtained from all the participants before the study initiation.
Disclosure Statement
Dr. Amini is the head of medical department of Orchid Pharmed, which is the CinnaGen partner in conducting clinical trials. Mr. Shahvaroughi Farahani is also an employee of Orchid Pharmed. No other potential conflicts of interest relevant to this article were reported.
Funding Sources
This study was financed by the CinnaGen company.
Author Contributions
M.A. conducted the study according to the accepted protocol and drafted the manuscript. J.A. participated in the design and coordination of the study and revised the manuscript. V.P. participated in the design and coordination of the study and revised the manuscript. A.N. participated in the design and coordination of the study and revised the manuscript. S.O. participated in the design and coordination of the study and revised the manuscript. S.E. participated in the design and coordination of the study and helped to revise the manuscript. H.S. participated in the design and coordination of the study and helped to revise the manuscript. S.A. was the Head of the medical department of the Orchid pharmed company and supervised the clinical trial conduction. A.S. was the Clinical Research Coordinator and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Supplementary data
Acknowledgments
The authors thank all the participants of this study.
References
- 1.McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, Tse TF, Wasserman B, Leiserowitz M. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM. Epoetin alfa use in patients with ESRD: an analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis. 2005;46:481–488. doi: 10.1053/j.ajkd.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 3.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 4.Palmer SC, Navaneethan SD, Craig JC, Johnson DW, Tonelli M, Garg AX, Pellegrini F, Ravani P, Jardine M, Perkovic V, Graziano G. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2016;164:472–478. doi: 10.7326/0003-4819-153-1-201007060-00252. [DOI] [PubMed] [Google Scholar]
- 5.Veys N, Vanholder R, Lameire N. Pain at the injection site of subcutaneously administered erythropoietin in maintenance hemodialysis patients: a comparison of two brands of erythropoietin. Am J Nephrol. 1992;12:68–72. doi: 10.1159/000168420. [DOI] [PubMed] [Google Scholar]
- 6.Veys N, Dhondt A, Lameire N. Pain at the injection site of subcutaneously administered erythropoietin: phosphate-buffered epoetin alpha compared to citrate-buffered epoetin alpha and epoetin beta. Clin Nephrol. 1998;49:41–44. [PubMed] [Google Scholar]
- 7.Halstenson CE, Macres M, Katz SA, Schnieders JR, Watanabe M, Sobota JT, Abraham PA. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther. 1991;50:702–712. doi: 10.1038/clpt.1991.210. [DOI] [PubMed] [Google Scholar]
- 8.Loughnan A, Ali GR, Abeygunasekara SC. Comparison of the therapeutic efficacy of epoetin beta and epoetin alfa in maintenance phase hemodialysis patients. Ren Fail. 2011;33:373–375. doi: 10.3109/0886022X.2011.559675. [DOI] [PubMed] [Google Scholar]
- 9.Deicher R, Hörl WH. Differentiating factors between erythropoiesis-stimulating agents. Drugs. 2004;64:499–509. doi: 10.2165/00003495-200464050-00004. [DOI] [PubMed] [Google Scholar]
- 10.KDIGO clinical practice guideline for anemia in chronic kidney disease Kidney Int. 2012:2–279. [Google Scholar]
- 11.Ostrvica E, Mesic E, Ostrvica D, Delic J, Delic-Custendil S, Hukic F. Effectiveness of treating the renal anemia in chronic hemodialyzed patients by epoietin alpha and beta. Med Arh. 2010:64–4. [PubMed] [Google Scholar]
- 12.Hosseinpanah F, Kasraei F, Nassiri AA, Azizi F. High prevalence of chronic kidney disease in Iran: a large population-based study. BMC Public Health. 2009:9–44. doi: 10.1186/1471-2458-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Kidney Foundation Kidney Disease Quality Outcomes Initiative (K/DOQI) http://www.kidney.org/professionals/kdoqi/guidelines.cfm (last accessed August 11, 2018)
- 14.Sepanlou SG, Barahimi H, Najafi I, Kamangar F, Poustchi H, Shakeri R, Hakemi MS, Pourshams A, Khoshnia M, Gharravi A, Broumand B. Prevalence and determinants of chronic kidney disease in northeast of Iran: results of the Golestan cohort study. PLoS One. 2017;12:e0176540. doi: 10.1371/journal.pone.0176540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nafar M, Mousavi SM, Mahdavi-Mazdeh M, Pour-Reza-Gholi F, Firoozan A, Einollahi B. Burden of chronic kidney disease in Iran. Iran J Kidney Dis. 2008;2:183–192. [PubMed] [Google Scholar]
- 16.EMA. Scientific Discussion EMA2007 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000872/WC500054374.pdf (cited January 22, 2018)
- 17.Gertz B, Kes P, Essaian A, Bias P, Buchner A, Zellner D. Epoetin theta: efficacy and safety of subcutaneous administration in anemic pre-dialysis patients in the maintenance phase in comparison to epoetin beta. Curr Med Res Opin. 2012;28:1101–1110. doi: 10.1185/03007995.2012.688736. [DOI] [PubMed] [Google Scholar]
- 18.Krivoshiev S, Todorov VV, Manitius J, Czekalski S, Scigalla P, Koytchev R. Comparison of the therapeutic effects of epoetin zeta and epoetin alpha in the correction of renal anaemia. Curr Med Res Opin. 2008;24:1407–1415. doi: 10.1185/030079908x297402. [DOI] [PubMed] [Google Scholar]
- 19.BIOSIMILARS ACTION PLAN Balancing Innovation and Competition. US FDA. 2018 Jul; [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data