Abstract
Familial platelet disorder with predisposition to acute myeloid leukemia (FPD/AML) has been well documented in the literature and is a new entity within the latest revised edition of the WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues (OMIM). The disorder arises due to mutations within the RUNX1 gene in chromosome 21; mutations within the Runt-binding domain are the most commonly encountered anomalies that cause decreased platelet count and function. Rare cases of haploinsufficiency have also been shown to cause this disorder. Here, we describe a 12-year-old female with mosaicism for a ring chromosome 21 and monosomy 21 who was born with thrombocytopenia which is now explained by loss of the RUNX1 gene resulting in FPD/AML. We also comment on the structure of the ring and the mechanism of its formation.
Key Words: Acute myeloid leukemia, Familial platelet disorders, Predisposition to AML syndromes, RUNX1 deletion
Established Facts
Molecular defects cause predispositions to cancer. With the latest update of the WHO classification of hematologic malignancies, myeloid neoplasm with germline predisposition has been recognized as a distinct entity and should be incorporated into clinical practice.
Somatic RUNX1 mutations and deletions cause familial platelet disorder and a predisposition to acute myeloid leukemia (OMIM 601399); clinicians should be alert to the need for increased surveillance for malignancy in this population.
Novel Insights
Establishment of the exact molecular content of abnormal chromosomes 21 is critical in order to detect deletions of RUNX1 associated with predisposition syndromes and monitor for thrombocytopenia and developing acute myeloid leukemia. While this need is clear in the hematologic literature, it is not well articulated in the genetic syndromology literature at this point.
Karyotypes may change over time to become genetically more balanced; however, risk of complications from the original karyotype prevails in these patients, and close clinical monitoring is warranted.
This case provides additional proof for the mechanism of ring formation that includes an intermediate isochromosome which is then further rearranged through asymmetrical sister chromatid breaks to form a double ring.
Recently reported in the literature and in the latest edition of the WHO Classification of Tumors of the Hematopoietic and Lymphoid Tissues, the entity of familial disorders with predisposition to acute myeloid leukemia (AML) has been defined. Within this category, loss of function of a single RUNX1 allele not only predisposes the family members to hematologic neoplasms, but also to platelet dysfunction. Herein, a novel mechanism of RUNX1 deletion is described.
Case Report
A 12-year-old female was referred to the pediatric hematology clinic for management of her thrombocytopenia in advance of pending surgery for severe scoliosis. The patient was born with multiple congenital anomalies including intrauterine growth restriction, microcephaly, cleft lip, a broad nasal tip, low-set ears with overfolded helix, prominent antihelix, proximal thumb insertion, and slightly dorsiflexed great toes. At birth, cytogenetic analysis showed mosaicism for cells with 45,XX,-21 and 46,XX,r(21)(p13q22) with the cell lines present in 40 and 60% of the cells, respectively. She also required platelet transfusions. This patient was the subject of a previous publication shortly after her birth that highlighted the thrombocytopenia and the chromosome anomaly which was investigated only by routine chromosome analysis [Cavan et al., 2009].
Her platelet counts have varied from 9–80 k/µL, and analysis of her platelet function demonstrates abnormal function with prolonged closure times for collagen/epinephrine and collagen/ADP. At 1 year of age she developed seizures, and since that time her development has been delayed with severe mental disability including mutism, hearing loss, and continuous drooling. At 10 years of age, she required a tooth extraction that resulted in severe bleeding and the need for multiple platelet transfusions. Currently, in addition to developmental delays, the patient suffers from rapid onset scoliosis of the thoracic spine requiring a chest mold, multiple malpositioned teeth with gingivitis, seizures controlled by medication, and easy bruising/bleeding. She is able to walk independently but with an unsteady gait.
Methods and Results
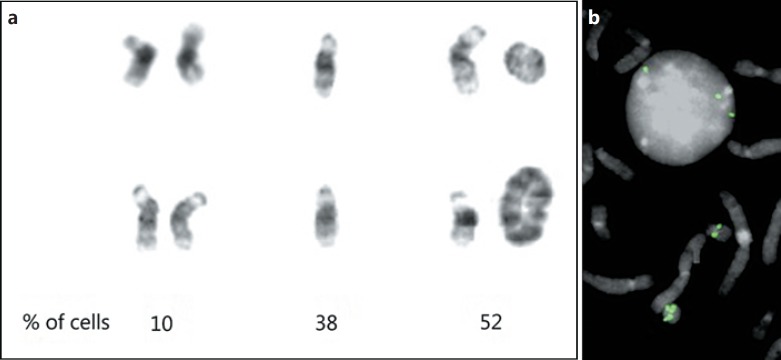
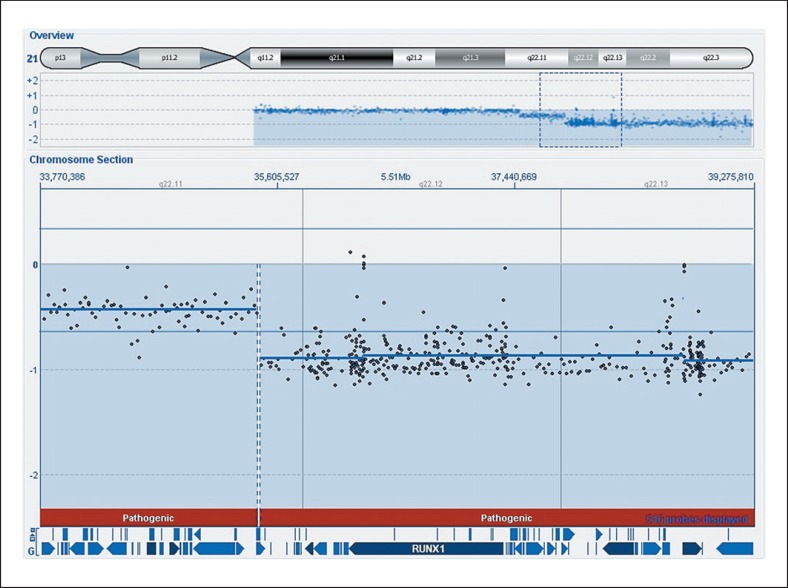
At her recent clinic visit, chromosome and microarray analyses of peripheral blood were ordered. Routine chromosome analysis found that the cells with monosomy 21 and ring chromosome 21 were still present, but an additional clone of 46,XX cells was also observed as well as cells with an isochromosome 21q and rare cells with larger rings (Fig. 1a). Cytological examination of the ring chromosome suggested a double structure, including 2 copies of proximal 21q with loss of distal q, rather than a simple ring. FISH analysis using a probe from band 21q21.1 (Empire Genomics) showed 1 copy on the normal chromosome 21, and 2 copies on the standard ring (Fig. 1b); in 100 cells scored, a double ring was present in a single cell. Microarray analysis using an Oxford Gene Technology CytoSure Constitutional v3 + LOH 4×180k CGH and SNP array with average resolution of 68 kb for targeted (genes) regions, 74–162 kb for the backbone and LOH of 7–10 Mb and CytoSure Interpret v 4.10.41 software was performed. The analysis demonstrated a full copy deletion of the distal portion of the q arm (q22.11qter) which included the RUNX1 gene at 21q22.1 (Fig. 2). Distal 21q demonstrated complete homozygosity, while the proximal portion is biallelic (data not shown). Further, the array clearly demonstrates 3 copy levels of sequences of chromosome 21, with the proximal region of the long arm 21q11.2q22.11 having a log2 (P/R) of −0.077, the regions 21q22.11 to 21q22.12 with a log2 ratio of −0.43, and the distal portion 21q22.12qter present in a single copy. Therefore, on the (double) ring chromosome, the centromere through KRTAP11-1 (nucleotides 1–3,239,434; GRCh38) is present in 2 copies, UBE3AP2 through RN7SL740P (nucleotides 32,410,504–35,436,076) is present in 1 copy, and MRPSS6 through the telomere (nucleotides 35,468,084–48,090,121) is deleted.
Fig. 1.
Chromosomes 21 from the patient cells. a Two normal G-banded chromosomes 21 (left), monosomy 21 (middle), and differently sized rings (right). The ring in the upper right is a double ring structure, as discussed in the text. The numbers below the columns indicate the percentage of cells with that constitution based on cytogenetic analysis of 50 cells. b Nucleus and partial metaphase after hybridization with a FISH probe from 21q21.1 showing 3 FISH signals on the nucleus, a single copy on the normal 21, and 2 copies on the basic ring at the bottom of the image.
Fig. 2.
CGH and SNP-array results indicating 1 copy loss of 21q22.11qter, including the RUNX1 gene, in nearly all cells. The top shows the chromosome 21 ideogram and aCGH results for the entire chromosome 21. Below the boxed region (dashes) is specified with the location of the RUNX1 gene indicated in the blue bar at the bottom. Note the 3 values of the log2 (P/R) indicating 3 different copy numbers, as detailed in the text.
Together, these results indicate that the ring chromosome is a double ring for proximal chromosome 21 formed in a complex manner. The karyotype is therefore appropriately described as: 45,XX,-21[26]/46,XX,ider(21)(q10)r(q22.11q22.12)[19]/46,XX[5].
Discussion
We describe a 12-year-old patient with scoliosis and thrombocytopenia who was known to have mosaicism for monosomy 21 and a ring chromosome 21. Features of mosaic monosomy 21 are present in our patient and have been described previously [Cavan et al., 2009]. Partial monosomy 21 syndrome, either by mosaicism or partial chromosome loss, presents at birth with phenotypic features including intrauterine growth restriction, low birth weight, cleft lip/palate, facial deformities, microcephaly, and profound intellectual disability [Chettouh et al., 1995; Huret et al., 1995].
Despite the known chromosome anomaly, the explanation for her congenital thrombocytopenia was previously unknown. Microarray analysis demonstrated that a majority of the patient's cells is missing one copy of the RUNX1 gene, leading to the diagnosis of familial platelet disorder (FPD). Although haploinsufficiency of RUNX1 is a mechanism for development of FPD/AML, it is not the most common one. Translocations, small intragenic deletions or duplications, and many point mutations have been described in the literature [Michaud et al., 2002; Béri-Dexheimer et al., 2008; Shinawi et al., 2008; Jongmans et al., 2010; van der Crabben et al., 2010; Buijs et al., 2012; Cavalcante de Andrade Silva et al., 2018]. Patients show various phenotypes ranging from normal to severely affected, depending on the mechanism, and the diagnosis may be missed entirely or even misdiagnosed as Fanconi anemia [Jongmans et al., 2010; Owen, 2010; Click et al., 2011]. The product of RUNX1 is a DNA-binding protein that is part of the core-binding factor transcription complex responsible for hematopoietic stimulation and transcription of proteins related to platelet function (including PRKCQ, MYL9, and ALOX12) [Godley, 2014]. Mutations can occur within the Runt DNA-binding domain or the transactivation domain, where the protein core-binding factor beta binds to increase the complex's affinity for DNA [Godley, 2014]. Mutations in these regions tend to decrease binding affinity at both sites and lead to decreased transcription of target proteins.
The development of hematologic malignancy in carriers of RUNX1 mutations approaches 40%, with an earlier age of onset than sporadic cases [West et al., [2014]. The increased risk begins with a baseline-increased sensitivity of bone marrow progenitor cells to G-CSF stimulation, predisposing patients to increased risk for acquiring secondary mutations [Michaud et al., 2002]. The acquisition of secondary mutations is greatly accelerated in FPD/AML individuals and is believed to contribute to the increased risk of leukemia transformation. Recent analysis of a patient with FPD/AML has shown that preleukemic cell populations occur in these patients, and multiple mutations are needed before acute leukemia occurs [Ng et al., 2018]. Interestingly, the preleukemic cells may persist after chemotherapy and only disappear after bone marrow transplantation. Patients most commonly acquire a second alteration in RUNX1 within the normal allele that acts as the primary driver of malignancy by a dominant-negative mechanism [Preudhomme et al., 2009]. Other secondary alterations leading to malignancy have also been reported including mutations in NF1, ASXL1, CBL, CDC25C, DNMT3A, and KRAS [Godley, 2014]. Although malignant transformation most frequently involves the myeloid lineage including AML and myelodysplastic syndrome, other hematologic malignancies have also been reported, such as T-cell acute lymphocytic leukemia and B cell malignancies [Preudhomme et al., 2009; West et al., 2014].
In addition to acquired secondary aberrations of the RUNX1 sequence, the congenital RUNX1 mutation has been shown to affect timing and severity of hematologic malignancy development [Owen, 2010]. Deletions, both small and large, have been shown to lead to earlier disease occurrence and may even be associated with more complex phenotypes compared to patients with point mutations. Cases of intellectual disability in conjunction with FPD/AML have been reported and may be due to large deletions of RUNX1 that include adjacent genes associated with neuronal signaling maintenance, such as ITSN1, RCAN1, CLIC6, and SYNJ1 [Béri-Dexheimer et al., 2008; Owen, 2010; van der Crabben et al., 2010].
FPD/AML caused by RUNX1 mutation or deletion is just one of the multiple genes that cause familial predisposition syndromes. ANKRD26 mutations, although less common, also produce a phenotype similar to RUNX1 mutations [Godley, 2014; Swerdlow et al., 2017]. Mutations in genes such as GATA2 and CEPBA show no clinical manifestations other than hematologic malignancy. In addition, bone marrow failure syndromes and telomere disorders can also be associated with increased risk for hematologic malignancy [Godley, 2014; Swerdlow et al., 2017].
Mechanisms of ring chromosome formation have been well studied, and most studies show that formation occurs with 2 double-strand breaks on a single chromosome with end-joining of the chromosomal fragment containing the centromere. However, it has been found that some ring chromosomes 21 are derived from intermediate isochromosome structures (of the long arms) which then undergo further rearrangement, namely asymmetric breaks and reunion of the long arms [Wong et al., 1989; McGinniss et al., 1992]. A double ring formed in this manner has 2 copies of the sequences nearest the centromere, 1 copy of sequences between the proximal and distal breakpoints, and lacks the distal sequences entirely. A recent study of multiple ring chromosomes demonstrated that some rings are formed by a deletion/duplication event, and others may be formed by a multistep process including distal arm deletion and a duplication and inversion event leading to chromosomal instability and ultimately ring formation [Rossi et al., 2008]. The phenotype of the individual carrying such a ring depends on the sequences present on the ring and their copy numbers as well as the level of mosaicism in various tissues and may even include features of Down syndrome.
We demonstrate that the ring chromosome 21 in our case actually is a double ring for the proximal portion of the long arm. Whether the ring was ever a single one cannot be determined from the published karyotype [Cavan et al., 2009] and seems unlikely. The fact that the distal portion of chromosome 21, where the critical region for Down syndrome is located (21q22.13), is present in a single copy in most cells may explain the lack of Down syndrome features [Pelleri et al., 2016].
Over time, an additional normal cell line with 2 normal chromosomes 21 has arisen. This is likely explained by a reduplication of the monosomic chromosome, which leads to isodisomy (data not shown) for the normal chromosome 21 in a portion of the cells. In addition, a cell with an isochromosome 21 was found, also resulting in a balanced karyotype. These changes likely persist by cell selection for a more balanced genome, although the isochromosome may be a relic of the original isochromosome formation.
In summary, our patient has mosaicism for karyotypically normal cells, a double ring chromosome 21, and monosomy 21 which resulted in haploinsufficiency for RUNX1 and caused FPD/AML. Close follow-up of this patient for her thrombocytopenia, congenital anomalies, and increased risk for AML and other hematologic malignancies is warranted. Geneticists should be aware of the possibility of predisposition to malignancies in patients with certain constitutional abnormalities.
Statement of Ethics
Informed written consent for the publication of this case report was obtained from the parents. Institution review board approval was not needed for this study as all tests were performed as part of the patient's treatment.
Disclosure Statement
The authors declare no conflicts of interests.
Acknowledgments
We thank Kayesha Cobb, Andrew Miner, and Amy Wu for their expert technical assistance.
References
- 1.Béri-Dexheimer M, Latger-Cannard V, Philippe C, Bonnet C, Chambon P. Clinical phenotype of germline RUNX1 haploinsufficiency: from point mutations to large genomic deletions. Eur J Hum Genet. 2008;16:1014–1018. doi: 10.1038/ejhg.2008.89. [DOI] [PubMed] [Google Scholar]
- 2.Buijs A, Poot M, van der Crabben S, van der Zwaag B, van Binsbergen E. Elucidation of a novel pathogenomic mechanism using genome-wide long mate-pair sequencing of a congenital t(16;21) in a series of three RUNX1-mutated FPD/AML pedigrees. Leukemia. 2012;26:2151–2154. doi: 10.1038/leu.2012.79. [DOI] [PubMed] [Google Scholar]
- 3.Cavalcante de Andrade Silva M, Krepischi ACV, Kulikowski LD, Zanardo EA, Nardinelli L. Deletion of RUNX1 exons 1 and 2 associated with familial platelet disorder with propensity to acute myeloid leukemia. Cancer Genet. 2018;222(223):32–37. doi: 10.1016/j.cancergen.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Cavan BC, Serafica EM, Modequillo MS, Melicor CF. Thrombocytopenia in a Filipino child with mosaic monosomy 21/ring chromosome 21. Acta Med Philipp. 2009;43:60–62. [Google Scholar]
- 5.Chettouh Z, Croquette MF, Delobel B, Gilgenkrants S, Leonard C. Molecular mapping of 21 features associated with partial monosomy 21: involvement of the APP-SOD1 Region. Am J Hum Genet. 1995;57:62–71. [PMC free article] [PubMed] [Google Scholar]
- 6.Click ES, Cox B, Olson SB, Grompe M, Akkari Y. Fanconi anemia-like presentation in an infant with constitutional deletion of 21q including the RUNX1 gene. Am J Med Genet A. 2011;155A:1673–1679. doi: 10.1002/ajmg.a.34024. [DOI] [PubMed] [Google Scholar]
- 7.Godley LA. Inherited predisposition to acute myeloid leukemia. Semin Hematol. 2014;51:306–321. doi: 10.1053/j.seminhematol.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Huret JL, Léonard C, Chery M, Phillippe C, Schafei-Benaissa E. Monosomy 21q: two cases of del(21q) and review of the literature. Clin Genet. 1995;48:140–147. doi: 10.1111/j.1399-0004.1995.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 9.Jongmans MCJ, Kuiper RP, Carmichael CL, Wilkins EJ, Dors N. Novel RUNX1 mutations in familial platelet disorder with enhanced risk for acute myeloid leukemia: clues for improved identification of the FPD/AML syndrome. Leukemia. 2010;24:242–246. doi: 10.1038/leu.2009.210. [DOI] [PubMed] [Google Scholar]
- 10.McGinniss MJ, Kazazian HH, Jr, Stetten G, Petersen MB, Boman H. Mechanisms of ring chromosome formation in 11 cases of human ring chromosome 21. Am J Hum Genet. 1992;50:15–28. [PMC free article] [PubMed] [Google Scholar]
- 11.Michaud J, Wu F, Osato M, Cottles GM, Yanagida M. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99:1364–1372. doi: 10.1182/blood.v99.4.1364. [DOI] [PubMed] [Google Scholar]
- 12.Ng IKS, Lee J, Ng C, Kosmo B, Chiu L. Preleukemic and second-hit mutational events in an acute myeloid leukemia patient with a novel germline RUNX1 mutation. Biomark Res. 2018;6:16. doi: 10.1186/s40364-018-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen C. Insights into familial platelet disorder with propensity to myeloid malignancy (FPD/AML) Leuk Res. 2010;34:141–142. doi: 10.1016/j.leukres.2009.07.037. [DOI] [PubMed] [Google Scholar]
- 14.Pelleri MC, Cicchini E, Locatelli C, Vitale L, Caracausi M. Systematic reanalysis of partial trisomy 21 cases with or without Down syndrome suggests a small region on 21q22.13 as critical to the phenotype. Hum Mol Genet. 2016;25:2525–2538. doi: 10.1093/hmg/ddw116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preudhomme C, Renneville A, Bourdon V, Philippe N, Roche-Lestienne C. High frequency of RUNX1 biallelic alteration in acute myeloid leukemia secondary to familial platelet disorder. Blood. 2009;113:5583–5587. doi: 10.1182/blood-2008-07-168260. [DOI] [PubMed] [Google Scholar]
- 16.Rossi E, Riegel M, Messa J, Gimelli S, Maraschio P. Duplications in addition to terminal deletions are present in a proportion of ring chromosome: clues to the mechanisms of formation. J Med Genet. 2008;45:147–154. doi: 10.1136/jmg.2007.054007. [DOI] [PubMed] [Google Scholar]
- 17.Shinawi M, Erez A, Shardy DL, Lee B, Naeem R. Syndromic thrombocytopenia and predisposition to acute myelogenous leukemia caused by constitutional microdeletions on chromosome 21q. Blood. 2008;112:1042–1047. doi: 10.1182/blood-2008-01-135970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, editors. ed 4. Lyon: WHO/IARC Press; 2017. WHO classification of tumours of haematopoietic and lymphoid tissues. [Google Scholar]
- 19.van der Crabben S, van Binsbergen E, Ausems M, Poot M, Bierings M, Buijs A. Constitutional RUNX1 deletion presenting as non-syndromic thrombocytopenia with myelodysplasia: 21q22 ITSN1 as a candidate gene in mental retardation. Leuk Res. 2010;34:e8–e12. doi: 10.1016/j.leukres.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 20.West AH, Godley LA, Churpek JE. Familial myelodysplastic syndrome/acute leukemia syndromes: a review and utility for translational investigations. Ann NY Acad Sci. 2014;1310:111–118. doi: 10.1111/nyas.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong C, Kazazian HH, Jr, Stetten G, Earnshaw WC, Van Keuren ML, Antonarakis SE. Molecular mechanism in the formation of a human ring chromosome 21. Proc Natl Acad Sci USA. 1989;86:1914–1918. doi: 10.1073/pnas.86.6.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]