Abstract
Surgical excision represents the primary treatment for malignant melanoma. On occasion, however, surgery may not be possible, and a different approach is required. Imiquimod is a Toll-like receptor 7 agonist involved in the activation of the innate immune system. We report the case of a 77-year-old female with a large, invasive, malignant melanoma of the malleolar area. Due to the size of the lesion, its location, and the patient's general condition, neither surgery nor radiotherapy were indicated. We offered topical treatment with 5% imiquimod to be applied once/day continuously over a 3-month period, pausing only when intense inflammation on the area of application occurred. Complete clinical and histological resolution of the lesion were observed. This case adds further merit to the growing body of evidence that imiquimod can be used to successfully treat malignant melanoma in cases where no other options are suitable.
Key Words: Melanoma, Topical treatment, Imiquimod, Toll-like receptors
Introduction
Surgery is the main treatment for primary cutaneous malignant melanoma and when isolated metastatic melanoma lesions are present. Some patients, however, may not be suited for surgery for a variety of reasons, such as extension and site of the lesion, the possibility of consequent functional impairment, multiple comorbidities, and patients' personal wishes.
Imiquimod or 1-(2-methyl-propyl)-1H-imidazo[4, 5-c]quinolin-4-amine, is a Toll-like receptor 7 agonist activating the innate immune system [1]. Initially licensed for the treatment of external anogenital warts, over the years its use has been extended to other dermatological conditions such as skin cancers. In melanoma, its use continues to be a subject of debate and controversy.
We report the case of a primary, large melanoma of the ankle, unsuitable for surgery given its size and location, which was successfully treated with a bespoke regimen of topical application of imiquimod.
Case Presentation
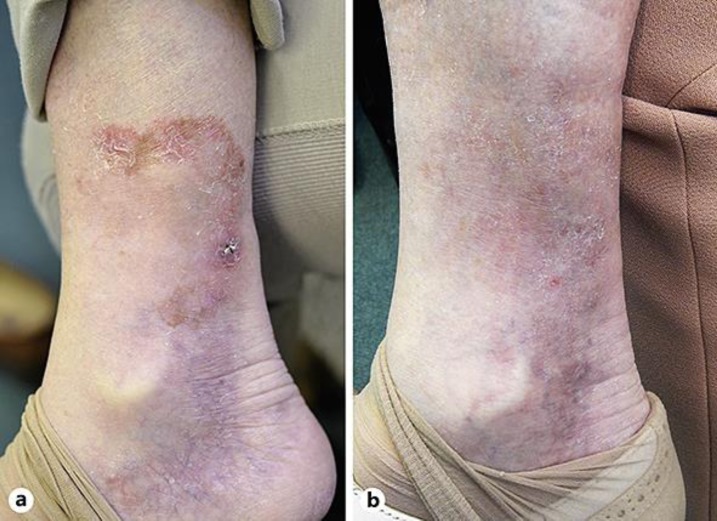
A 77-year-old female was referred for a persistent, slightly erythematous, focally lightly pigmented, solitary 67 × 55 mm patch with rather indistinct borders on the right medial malleolus, which had been present for 2.5 years (Fig. 1a).
Fig. 1.
a Invasive melanoma of the right medial lower leg before treatment. b No clinical evidence of residual melanoma after treatment with 5% topical imiquimod.
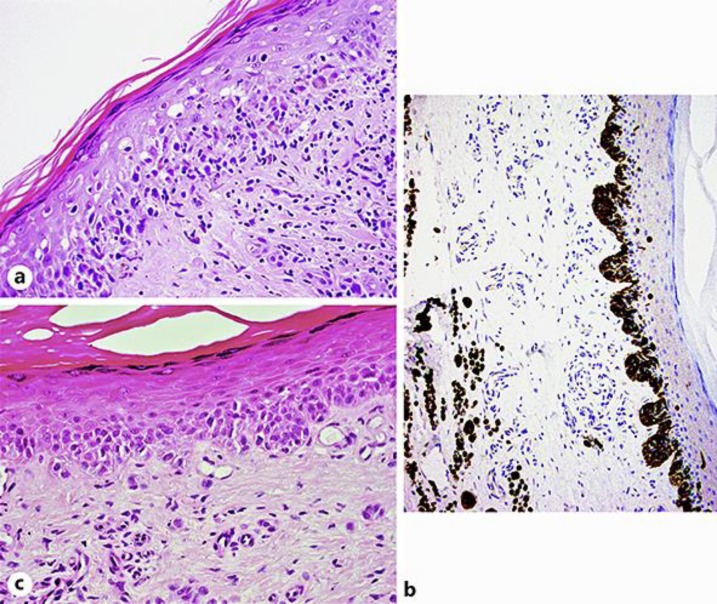
A 4-mm punch biopsy was taken from the centre of the lesion and was reported as an invasive melanoma, Breslow thickness 0.8 mm. Considering the large size of the lesion, further 4 × 4 mm punch biopsies were taken. The resulting pathology report confirmed an invasive malignant melanoma with a Breslow thickness of 0.8 mm with a surrounding area of in situ melanoma. The in situ portion of the lesion was composed by quite plump, large nests of atypical melanocytes pressing on and distorting the basal membrane. Foci of microinfiltration were also observed. The final pathology report, following a second opinion from a different pathology hospital department, was conclusive for an invasive superficial spreading malignant melanoma with Breslow thickness 0.8 mm, at least with a radial in situ component (Fig. 2a–c).
Fig. 2.
a Atypical melanocyte infiltrate in the superficial dermis. Pagetoid spreading is also identified. H&E. ×40. b A dramatic increase of melanocytes is noted at the dermo-epidermal junction. Enlarged, atypical melanocytes are immunohistochemically identified in the mid dermis. Melan-A, ×20. c The periphery of the lesion shows the presence of nests of atypical melanocytes at the dermo-epidermal junction, in keeping with in situ melanoma.
The case was discussed at the local multidisciplinary meeting to decide on further management. Given that a total body CT scan was negative for any metastatic deposit, it was agreed to refer the patient urgently to the plastic surgery team. They concluded that, whereas removal of the entire lesion was feasible, closure of the resulting surgical wound would be impossible. Given the position of the lesion (malleolar area, with complete lack of subcutaneous tissue and nihil elasticity), the extension of the lesion (over a third of the total circumference of the ankle), closure via flap would have been impossible. Closure by graft was not recommended either as the transplanted skin would have had to be applied directly above the periosteum of the malleolar bone and the tendons. Second-intention healing was also considered not to be an option because of the lengthy healing time and impact on the patient (an elderly, tiny, frail lady). On the other hand, the only surgically feasible possibility suggested by the plastic surgeons was, in fact, direct amputation of the lower part of the leg. Due to the lack of adequate subcutis in the area, radiotherapy was also ruled out. Topical immunotherapy was therefore considered. Treatment with topical sensitisers such as diphenylcyclopropenone or diphencyprone would have required initial sensitization and weekly application in a dermatology clinic setting, titrating the dose to the optimal concentration based on the patient's clinical response [2]. For this frail lady, however, regular attendance for the application of the sensitizer represented a challenge.
A trial with 5% imiquimod was therefore discussed. Treatment could be applied at home, with follow-ups in the clinic every few weeks. Faced with all these options, the patient's decision, in agreement with other family members, was not to proceed with any type of surgery. Considered more suitable for her holistic needs, the patient opted for imiquimod treatment. She and her family received explanations on how to apply the cream liberally all over the area every day, only pausing the treatment in the event of florid inflammation. By day 32 from the start of treatment with imiquimod 5% cream, a florid erythema had occurred leading, 6 days later, to superficial necrosis and quite a thick scab. Treatment was paused at day 41. Following resolution of the reaction, 2 weeks later, the patient was invited to restart application, which led to a slightly less intense reaction after 10 days, lasting again for about 2 weeks (during which time application of the medication was again paused). A total of 72 sachets of imiquimod were used over a period of 94 days.
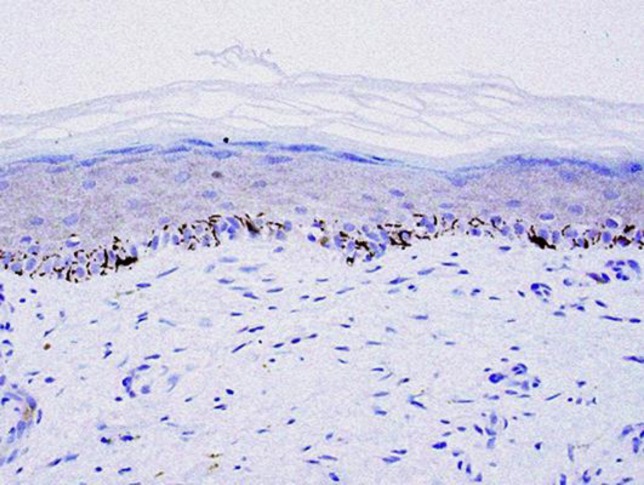
Five weeks after completion of the treatment, three biopsies were taken. These showed only occasional, normal melanocytes in normal skin, sometimes with scattered dendritic processes present. There was no cytological atypia, no pagetoid spreading, and no dermal melanocytes. There was evidence of lichenoid inflammation, superficial dermal fibrosis, and a perivascular chronic inflammatory cell infiltrate (Fig. 3). No residual melanoma was identified, correlating well with the clinical appearance, which was characterized only by mild postinflammatory hyperpigmentation.
Fig. 3.
Biopsies taken after treatment show the presence of melanocytes scattered along the basal membrane. Melan-A ×20. The rete ridges appear flattened. No atypical melanocytes are identified, neither in the dermis nor at the dermo-epidermal junction.
At the 3-month clinical review, there was no melanocytic lesion present. Therefore, further biopsies were not taken. The patient agreed to close clinical follow-up, with no further treatment at that stage. At regular clinical follow-ups, performance status, clinical lymph gland stations inspection, and general skin check under dermoscopy were performed, always with negative results. Over the years, the patient had 2 total body computed tomography scans performed at time 0 and after 12 months, and 3 ultrasound scans of the right popliteal fossa, groins, abdomen, and pelvis at times 0, 18, and 36 months after the treatment. So far, about 4 years after initial presentation, the patient is well with no trace of residual melanoma (Fig. 1b).
Discussion
The first reported instance of imiquimod use for melanoma was in 2000 [3]. Despite its use over the past 17 years and a number of trials, clear guidance as to its efficacy is still lacking. Inconsistency in clinical response has been partially explained by the variable expression of the Toll-like receptor 7, and the rather complex molecular environment surrounding its activation, which is influenced by a balance of pro- and anti-inflammatory factors. It would appear, therefore, that future studies, rather than clinical trials, should in fact focus on the pharmacogenetic factors determining activation or suppression of the innate immune response to possibly explain why imiquimod works in some cases and seems to be completely ineffective in others and obtain a more accurate picture of imiquimod's potential future therapeutic use [4, 5].
Imiquimod has been used in the treatment of primary, recurrent, and metastatic melanoma, even influencing internal metastasis [6]. Imiquimod treatment regimens across reported literature vary greatly, as does the period of follow-up. Numerous instances of imiquimod used in combination with other surgical and nonsurgical therapies have been reported, including intralesional IL-2, intralesional Bacille Calmette-Guerin, and ipilimumab [7].
The British Association of Dermatologists' revised guidelines advises that imiquimod is unproven for treating lentigo maligna and in situ superficial spreading melanoma and should be used solely in the context of clinical trials. The American Academy of Dermatologists discusses the off-label use of imiquimod and mentions the lack of long-term follow-up, variable treatment regimens, and histologically persistent disease in 25% of the patients. A recent systematic review showed complete clinical clearance in 78.3% of the patients and histological clearance in 77% of the patients [8]. There is a single-phase II trial currently being published (LIMIT-1 study), which shows complete pathological regression in 10 out of 27 patients [9]. The trial did not continue to phase III due to “insufficient justification.” Treatment with imiquimod lasted 12 weeks and was followed by excision. This possibly does not allow for a tailored course of treatment nor for long-term follow-up; however, this is understandable given the lack of data about its efficacy.
Conclusion
Here, we reiterate the importance of topical imiquimod as a potential nonsurgical treatment for selected cases of melanoma. However, given its rather unpredictable efficacy, we recommend that it be considered for use only in situations where clinical or patient factors preclude formal excision. Further studies aimed at clarifying the variance in response to imiquimod would, we believe, have great merit.
Statement of Ethics
Informed consent was obtained from the patient, and the study was ethically conducted in accordance with the Declaration of Helsinki.
Disclosure Statement
The authors report no conflicts of interest or any external sources of funding. No commercial associations or sources of support that might pose a conflict of interest were used for the study.
Author Contributions
We all are responsible and in agreement with the content and writing of the manuscript to which we all contributed significantly.
References
- 1.Kang HY, Park TJ, Jin SH. Imiquimod, a Toll-like receptor 7 agonist, inhibits melanogenesis and proliferation of human melanocytes. J Invest Dermatol. 2009 Jan;129((1)):243–6. doi: 10.1038/jid.2008.184. [DOI] [PubMed] [Google Scholar]
- 2.Read T, Webber S, Thomas J, Wagels M, Schaider H, Soyer HP. Protocol for the TIDAL Melanoma Study: topical imiquimod or diphenylcyclopropenone for the management of cutaneous in-transit melanoma metastases-a phase II single centre, randomised, pilot study. BMJ Open. 2017 Oct;7((10)):e016816. doi: 10.1136/bmjopen-2017-016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed I, Berth-Jones J. Imiquimod: a novel treatment for lentigo maligna. Br J Dermatol. 2000 Oct;143((4)):843–5. doi: 10.1046/j.1365-2133.2000.03787.x. [DOI] [PubMed] [Google Scholar]
- 4.Missall TA, Hurley MY, Fosko SW. Lentiginous melanoma in situ treatment with topical imiquimod: need for individualized regimens. Arch Dermatol. 2010 Nov;146((11)):1309–10. doi: 10.1001/archdermatol.2010.338. [DOI] [PubMed] [Google Scholar]
- 5.Mora AN, Karia PS, Nguyen BM. A quantitative systematic review of the efficacy of imiquimod monotherapy for lentigo maligna and an analysis of factors that affect tumor clearance. J Am Acad Dermatol. 2015 Aug;73((2)):205–12. doi: 10.1016/j.jaad.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 6.Miller AK, Dusing R, Meggison A, Aires D. Regression of internal melanoma metastases following application of topical imiquimod to overlying skin. J Drugs Dermatol. 2011 Mar;10((3)):302–5. [PubMed] [Google Scholar]
- 7.Shi VY, Tran K, Patel F, Leventhal J, Konia T, Fung MA. 100% Complete response rate in patients with cutaneous metastatic melanoma treated with intralesional interleukin (IL)-2, imiquimod, and topical retinoid combination therapy: results of a case series. J Am Acad Dermatol. 2015 Oct;73((4)):645–54. doi: 10.1016/j.jaad.2015.06.060. [DOI] [PubMed] [Google Scholar]
- 8.Tio D, van der Woude J, Prinsen CAC, Jansma EP, Hoekzema R, van Montfrans C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: need for standardization of treatment schedule and outcome measures. J Eur Acad Dermatol Venereol. 2017 Apr;31((4)):616–624. doi: 10.1111/jdv.14085. [DOI] [PubMed] [Google Scholar]