Abstract
Granulomatous mastitis (GM) is a rare benign inflammatory breast disease that affects mostly women of childbearing age with a history of breastfeeding. The etiopathogenesis is still unknown; however, inflammation as the result of a reaction to trauma, metabolic or hormonal processes, autoimmunity, and an infection with Corynebacterium kroppenstedtii have all been implicated. Clinical findings are pain, mass, hyperemia, and inflammation. Because the clinical presentation can mimic infectious mastitis or inflammatory carcinoma, the disease course is often protracted. The diagnosis is made by histopathology. Biopsies show a granulomatous formation in combination with a localized infiltration of multi-nucleated giant cells, epithelioid histiocytes, and plasma cells. Ultrasound, mammography, and magnetic resonance imaging are not specific; however, ultrasound and mammography should be done to exclude other pathologies. Due to the lack of data including randomized controlled studies, the management of GM is controversial. In Western industrialized countries, most authors use a therapy regimen starting with antibiotics and corticosteroids, followed by continuous steroid therapy and surgery in patients with persisting symptoms. More data are needed to define the best therapy. The role of immunotherapy has not yet been ascertained. The implementation of a registry to collect more information on this rare disease is highly recommended.
Key Words: Granulomatous mastitis, Idiopathic, Corticosteroids, Surgery
Introduction
Since granulomatous mastitis (GM) was first described as a benign disease entity in 1972 by Kessler and Wolloch [1], hundreds of cases have been reported from all over the world. Nevertheless, GM is a rare differential diagnosis with an estimated incidence of 2.4 per 100,000 women and 0.37% in the US [2]. The fact that the majority of cases in the US are predominantly in non-white patients suggests that the incidence in Europe (and specifically Germany) is even less [3].
The rarity of the disease causes a lack of valid data. The etiology of GM is still hypothetical, and no consensus on disease management exists [4].
Therefore, the diagnosis and treatment of a patient with GM still remains a challenge for the clinician as well as for the patient who often has to suffer a protracted disease course with a significant impact on quality of life. Different causes of mastitis, and most importantly malignancy, usually have to be excluded before the diagnosis of GM can be considered [5].
In this article, we discuss the etiology, clinical presentation, and diagnosis of GM as well as up-to-date treatment options to give an overview of the current data.
Literature Search
A literature search in PubMed was performed using the search terms ‘granulomatous mastitis’, ‘idiopathic granulomatous mastitis’, ‘granulomatous mastitis and immunology’, and ‘granulomatous mastitis and pathology’ for the years 2000–2018. The search included presentations at national and international conferences on breast diseases such as European Society for Medical Oncology, San Antonio Breast Cancer Symposium, and American Society of Clinical Oncology.
No randomized controlled study was found. Most publications are retrospective studies and case series. 2 systematic reviews, 1 meta-analysis, and 3 presentations were included in this review.
Prevalence and Etiology
GM is a rare benign inflammatory breast disease which was first described by Kessler and Wolloch in 1972 [1].
There is no valid incidence and prevalence data of GM for Europe or Germany. The majority of cases reported in the literature indicate that the disease primarily occurs in women of childbearing age, mostly with a history of breastfeeding. The disease usually occurs around 2 years after breastfeeding at a median age of 30 years [6]. Only 2 reports of male GM [7,8] and a few reports of women developing GM during pregnancy or lactation exist [9,10].
The majority of publications, especially larger case series, originate from the Middle East, Mediterranean countries, Asia, and the US. A higher prevalence of GM among women of Asian, Hispanic, and Arabic origin has been discussed [11,12]. In 2008, in Indianapolis, Indiana, a cluster of 7 GM diagnoses was reported in multigravid Hispanic women.
The Center of Disease Control in Atlanta conducted a case-control study to identify possible risk factors for the disease, but no specific factor was identified [2].
The exact etiology of GM is still unknown, but various hypotheses exist. A possible reason for GM is an inflammatory autoimmune response to epithelial damage, although the trigger for this damage is still unknown. A correlation with breastfeeding and childbirth has also been discussed. An inflammatory reaction might occur in response to extravasated secretions from the lobules [12].
Within GM lesions, Corynebacterium kroppenstedtii has often been cultivated and may therefore play a fundamental role in inducing this disease. Due to the high percentage of evidence of this lipophilic gram-positive rod, GM has also been named ‘cystic neutrophilic granulomatous mastitis’ by some authors [6].
Recently, hyperprolactinemia provoked by antipsychotic medication was discussed as a non-lactation-related risk factor. Prolactin is described to promote ductal ectasia and milk stagnation as well as having a proinflammatory effect.
Wong et al. [13] reported that 37% of their patients with C. kroppenstedtii-associated mastitis had a psychiatric history needing psychiatric medication, raising the question whether this kind of medication might lead to a higher risk of developing GM. In addition, the authors suggest an underestimation of the exact number of infections with C. kroppenstedtii because routine culture methods using Ziehl-Neelsen or PAS staining do not show the slow-growing corynebacterium. Most publications do not describe specific testing for corynebacteria in the specimens. In addition, assessment of serum prolactin levels is mostly not performed in patients with GM [13].
The role of inflammation in the etiology of GM is further supported by the finding of concurrent erythema nodosum or arthritis [14,15].
Evidence of alpha-1 antitrypsin deficiency is further discussed as a potential risk factor for inducing GM [14]. Alpha-1 antitrypsin deficiency is an autosomal codominant disorder that is most prevalent in Caucasians of European or North American descent. In some cases, it is associated with symptoms of panniculitis. Histopathologic findings of the panniculitis show predominantly lobular inflammation. Currently it is unclear if this concurrence in patients with GM simply results from imprecise histopathologic diagnosis or if it is a rare finding in GM patients.
Presenting Symptoms
The leading symptom of GM is a painful mass. Up to 50% of patients develop erythema and swelling as symptoms of inflammation of the involved breast. Other symptoms are hyperemia, areolar retraction, fistula, and ulceration. Around 37% present with signs of an abscess [16].
The lesion may occur in any quadrant of the breast but is mostly present in the retroareolar region from where it extends radially. Most lesions occur unilaterally.
The mass may clinically mimic a bacterial abscess and/or breast cancer by inducing skin or nipple retraction. Lymphadenopathy is present in up to 15% of patients [17]. The unspecific symptoms are often misleading in the diagnostic process. The time period from the onset of symptoms to the exact diagnosis can therefore be several months [18]. Figure 1 shows a 29-year-old woman before treatment with corticosteroids. The patient presented with signs of breast inflammation, mass, and fistula in the outer upper quadrant of the left breast. Symptoms persisted for about 2 years.
Fig. 1.
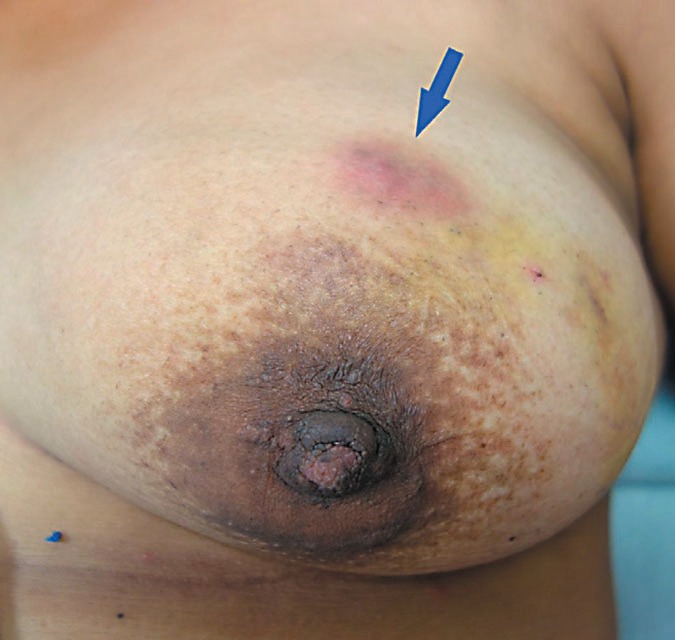
A 29-year-old women with a mass in the outer upper quadrant of the left breast in combination with inflammation and signs of a fistula (arrow). Symptoms persisted for about 2 years. Mammography and ultrasound: BIRADS 4, ACR B. Core needle biopsy was performed. Diagnosis: granulomatous mastitis.
Diagnosis
Histopathology
GM is diagnosed by histopathology only. The disease is characterized by formation of a non-necrotizing granuloma in combination with a localized infiltrate of multi-nucleated giant cells, epithelioid histiocytes, lymphocytes, and plasma cells (fig. 2). Sometimes, organized sterile micro-abscesses occur with neutrophilic infiltrates. Inflammation that extends into adjacent lobules can indicate a higher severity. The involved parenchyma mostly shows loss of acinar structures and damaged ducts [18,19].
Fig. 2.
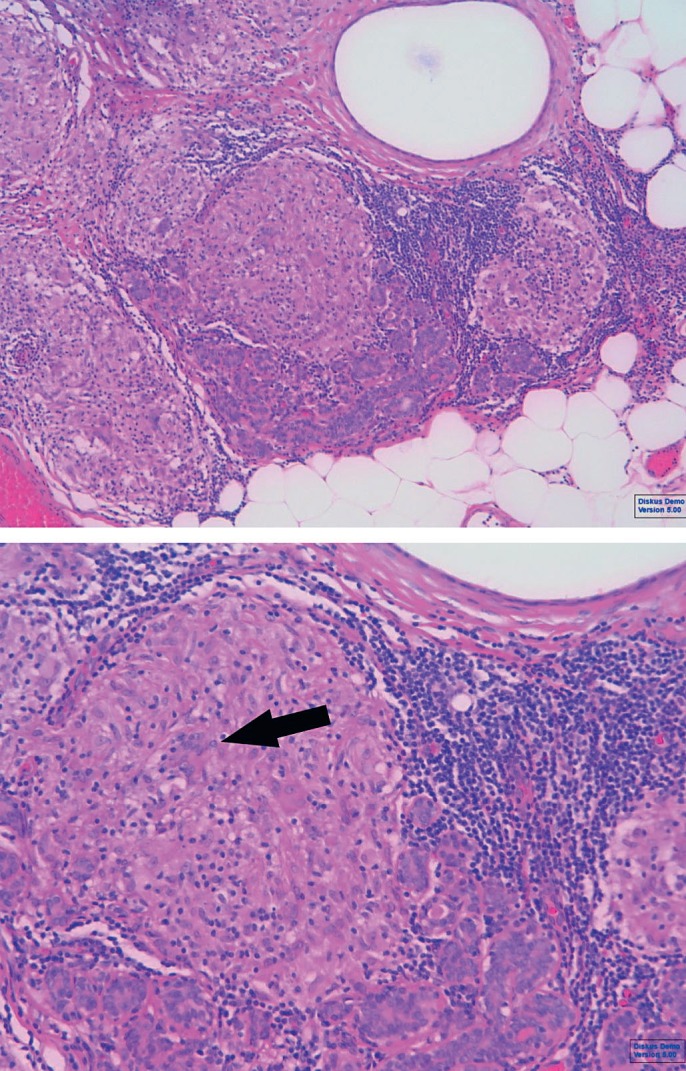
Granulomatous mastitis: sample (hematoxylin and eosin) with non-caseating granuloma consisting of lymphocytes, histiocytes, and giant cells (arrow).
Strictly speaking, GM is a non-infectious disease, although the role of corynebacteria in the development of GM is still discussed. Taylor et al. [20] in 2003 were able to isolate corynebacteria from lipid-filled vacuoles within the granuloma. The authors suggested that the presence of species of corynebacterium is a major causative aspect of GM. After this finding, further case reports were published presenting different species of corynebacterium (C. kroppenstedii, C. tuberculostearicum, and C. freneyi) [21,22], supporting the theory of Taylor et al. [20.] In a Japanese case series, isolated C. kroppenstedii was found in 6 of 19 cases of GM [23]. Some authors define the finding of C. kroppenstedii within GM lesions as indicative of a subgroup of the disease named ‘cystic neutrophilic granulomatous mastitis’ because of its histopathologic presentation of granulomatous and neutrophilic inflammation with cystic spaces or vacuoles [7].
Other clinicians interpreted the finding of corynebacteria in GM lesions as a contaminant by the normal skin flora [17].
The gold standard for diagnosing GM is core needle biopsy of the lesion with a sensitivity of 96%, whereas only 4 out of 19 cases were diagnosed with fine needle aspiration (FNA); the low sensitivity of 21.1% may be caused by insufficient material and nonspecific histopathologic findings (e.g., fat necrosis, abscess) [24].
The challenge for the pathologist and clinician is to differentiate GM from other autoimmune and granulomatous conditions including tuberculosis, sarcoidosis, and Wegener's granulomatosis. Other differential diagnoses are histoplasmosis, actinomycosis, foreign body reaction, fat necrosis, IgG4-RD mastitis, and most importantly inflammatory breast cancer [22]. The correct diagnosis therefore relies on the biopsy tissue, which is of higher quality and quantity if attained by a core needle biopsy as opposed to FNA.
Histologic techniques for the pathologist include the use of hematoxylin and eosin stains, gram stain, and for the differentiation of sarcoidosis or tuberculosis fast stains and Grocott's methenamine silver. Lacambra et al. [25] showed that tuberculous lesions are characterized by more fibrosis, eosinophils, and necrosis compared to the GM group which is characterized by significantly more plasma cells.
The differential diagnosis of tuberculosis has to be considered by the clinician in patients with signs of lung disease or in those who have a weakened immune system. It is more likely to occur in younger patients with a larger clinical mass at presentation. Fever, arthritis, or erythema nodosum are typical for Lofgren's syndrome and therefore suggestive of sarcoidosis.
Laboratory Findings
Autoimmunity is claimed to underline the etiology of GM; nevertheless, there are no blood-related factors to reliably support this hypothesis. Some reports describe normal titers of rheumatoid factors (RF) and serum complement [26], others show positive RF and in some cases presence of anti-neutrophil cytoplasmic autoantibody (ANCA) and anti-dsDNA antibodies [19] which are connected to Lupus erythematosus. No correlation with ANCA, c-ANCA, interleukin 2 receptor, or angiotensin-converting enzyme is described; however, information on these factors can be useful to exclude other autoimmune diseases [27].
C-reactive protein levels as an unspecific marker of infection are usually normal or slightly elevated up to 1.1–1.5 mg/dl (normal: <0.5 mg/dl), and the levels of carcinoembryonic antigen and cancer antigen (CA) 15-3 are also well within limits [20].
Imaging
The imaging findings of GM overlap with those of malignant lesions. Ultrasound, mammography, and magnetic resonance imaging (MRI) are considered as non-specific in GM [28].
Typical findings on ultrasound are multiple contiguous hypoechoic masses with posterior acoustic shadowing or posterior acoustic enhancement. Advanced cases present with fluid collections and cavities in association with skin fistulas. Most cases present with hypervascularity which can be detected by Doppler imaging [15]. 15–55% of all cases show ipsilaterally enlarged reactive axillary lymph nodes [20,29].
Mammography shows unilateral focal or regional asymmetry as the most frequent pattern, but in 24% it fails to identify an abnormality. Lesions were mammographically occult in 15 out of 45 women, possibly because of an overlying dense breast pattern seen in most women (36 out of 45) [20].
MRI findings are also variable and can show heterogeneous ill-defined masses and non-mass enhancement depending on the severity of the inflammation.
Fazzio et al. [28] describes T2 hyperintense, peripherally enhancing masses with central areas of non-enhancement representing abscess formation, as is typical for advanced cases.
Treatment
Due to the fact that the clinical presentation of the disease is typical for mastitis, most patients get antibiotics in the beginning of their therapy cascade in the form of a blind antibiotic therapy without any microbiological proof of a bacterial infection. GM is per definition a sterile inflammatory disease; therefore, antibiotic therapy usually fails [30,31] (table 1).
Table 1.
Symptoms, treatment options, and outcome of patients with granulomatous mastitis
| Aghajanzadeh et al., 2015 [29] | Elzahaby et al., 2016 [30] | Freemann et al., 2017 [16] | Calis et al., 2017 [17] | Bashir et al., 2017 [31] | |
|---|---|---|---|---|---|
| Patients, n (%) | 206 (100) | 30 (100) | 14 (100) | 19 (100) | 18 (100) |
| Breast mass, n (%) | 169 (82) | 27 (90) | 14 (100) | 5 (26) | 13 (72) |
| Erythema, n (%) | 24 (12) | 7 (50) | 3 (16) | 8 (33) | |
| Pain, n (%) | 24 (12) | 5 (26) | 4 (44) | ||
| Ulceration, n (%) | 37 (18) | ||||
| Received antibiotics, n (%) | 206 (100) | 8 (57) | 13 (68) | 15 (83) | |
| Improved on antibiotics, n (%) | 6 (12) | 3 (21) | |||
| Received steroids, n (%) | 200 (97) | 0 (0) | 8 (33) | ||
| Corticoid regimen | 2–3× 10–20 mg | 2× 16 mg prednisolone | |||
| prednisolone daily | daily for 2 weeks, slow | ||||
| for 2–6 months | tapering over 2 months | ||||
| with tapering | |||||
| Improved on steroids, n (%) | 144 (72) | 5 (66) | |||
| Wide surgical excision, n (%) | 11 (5.3) | 30 (100) | 9 (64) | 5 (26) | 9 (38) |
| Improved after surgical excision, n (%) | 28 (93) | ||||
| Complete mastectomy, n (%) | 1 (7) | 1 (5) |
At the time of clinical presentation, up to one third of all patients with GM show abscess-like symptoms such as pain, erythema, as well as a mass, fluid collection on ultrasound, and reactive lymphadenopathy. Table 1 summarizes therapeutic approaches and success rates in patients with a diagnosis of GM. These patients may undergo abscess puncture, drainage, or incision depending on the size of the lesion. Aspiration can fail because the abscess-like mass often has necrotic tissue in the center that makes the aspirate thick and hard to extract. Typically, the microbiological cultures are negative. Cultures positive for Corynebacterium spp. are of no consequence for the actual therapeutic strategy as so far there is no effective treatment against corynebacteria; also, the agent would have to be active in a lipid environment, but most antimicrobials are hydrophilic with weak distribution to lipid environments.
When GM is diagnosed, 2 treatment options are discussed in the literature: a conservative strategy involving medical therapy with corticosteroids versus a surgical approach. In 1980, DeHerthogh et al. [32] first recommended a high-dose corticosteroid therapy with prednisolone 30 mg/day for at least 2 months. In general, this leads to a decrease in the diameter of the lesion but also to a variety of side effects such as weight gain, hyperglycemia, and the risk of Cushing's syndrome. Despite these side effects, this approach became standard of care.
Freeman et al. [16] proposed a lower-dose regimen of 16 mg prednisolone twice a day for 2 weeks and slow tapering over 2 months. According to their data, 2 out of 3 patients failed therapy and suffered from adverse effects of the corticosteroids. A recent German poster presentation [26] demonstrated the success of a high-dose therapy with prednisolone up to 1 mg/kg/day. In 13 patients diagnosed with GM and treated with steroids, no surgery was performed. The duration of steroid application varied between 2 and 6 months, and a recurrence rate of 15% was reported.
There is an ongoing trial addressing the need for further reduction of the corticosteroid exposure of the patient. The trial is investigating the effect of topical 0.1% hydrocortisone butyrate cream twice a day on alternate days versus wide local excision (ClinicalTrials.gov Identifier: NCT02959580).
The application of methotrexate is a further option being discussed in the literature, mainly for patients who have failed corticosteroid therapy. The evidence for this approach is limited and based on only a few case reports [17], and it is questionable if methotrexate is a reasonable therapy option for women of childbearing age [13].
An alternative approach is the wide excision of the lesion. The decision at what stage of the disease surgery is performed depends on the individual clinical appraisal of the patient as does the choice of surgical technique which varies from wide excision to mastectomy [17,18,31]. The decision whether surgery or medical treatment is preferred might as well depend on divergent regional resources, the patient's expectations, and surveillance opportunities. Some authors primarily perform surgery mostly as a wide excision, others start with a conservative regimen and switch to surgery when the medical treatment fails. Most authors perform a wide excision of the granulomatous lesion (table 1).
Neither in the US nor in Germany a consensus or guideline exists regarding the surgical and medical approach. Especially the German ‘Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF)’ and the ‘Arbeitsgemeinschaft Gynäkologische Onkologie (AGO)’ have not defined a guideline for the treatment of GM.
In the literature, there is a great variety of findings related to the risk of recurrence for the different therapeutic approaches [33]. It comes as no surprise that antibiotics seem to have the lowest efficacy in the treatment of this abacterial mastitis with improvement rates ranging from 6 to 21% (table 1). By comparison, corticosteroid therapy has a success rate of between 66 und 72%. In a meta-analysis by Lei et al. [5], a pooled recurrence rate for oral steroid therapy of 20% is reported. Surgery alone or in combination with corticosteroids seems to have the lowest recurrence rates of 6.8 and 4%, respectively [5].
Yilmaz et al. [33] tried to define a scoring system to predict recurrence, which failed due to the relatively low incidence of recurrence in their study with only 8 out of 63 patients.
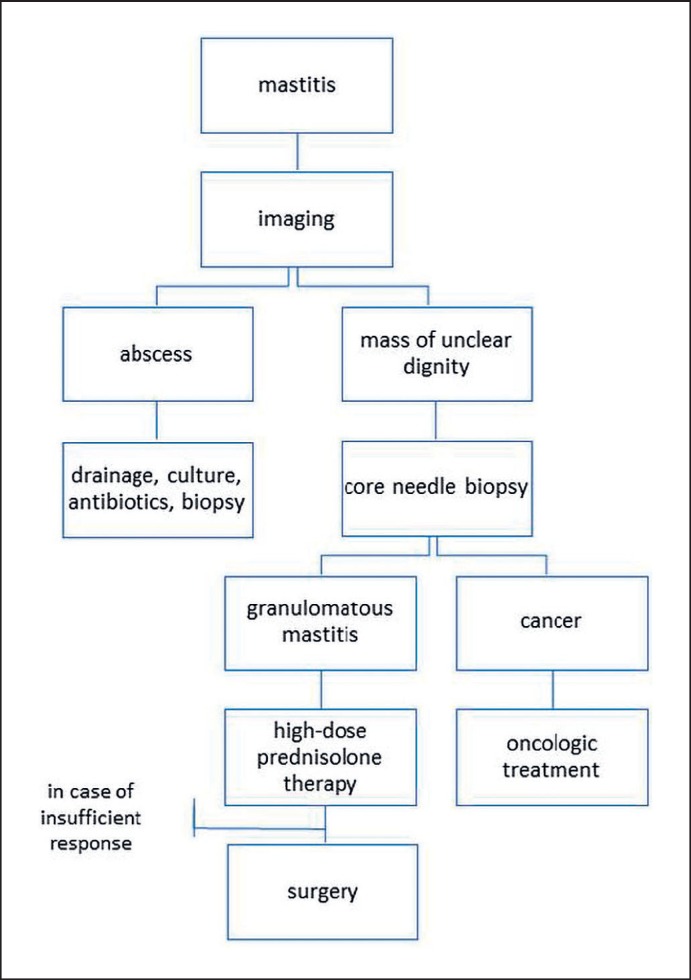
Figure 3 shows a possible algorithm for the management of GM, modified from Freeman et al. [16] and adapted to the local standards of the Breast Unit of ‘Kliniken Essen-Mitte’. This algorithm is based on the relatively good outcome that can be achieved with a conservative therapy with corticosteroids and emphasizes the need to avoid invasive therapies such as wide excision or even mastectomy that may go along with the possibility of scars, asymmetry, unsatisfactory esthetic results, and problems with breastfeeding. The authors believe surgery should be reserved for individual situations and for cases with insufficient response to corticosteroid therapy.
Fig. 3.
Proposed algorithm for the management of granulomatous mastitis, modified from Freeman et al. [16] and adapted to the local standards of the Breast Unit of ‘Kliniken Essen-Mitte’.
Conclusion
If a patient presents with symptoms of chronic mastitis, it is of great importance to keep in mind the possibility of GM as the underlying disease. The clinical presentation and imaging findings of GM overlap with those of malignancy. This makes it necessary to perform a core needle or excisional biopsy to obtain a histopathologic diagnosis.
Comparing the most recent publications on GM to older data, there is no new information on this rare benign breast disease. Therefore, the best therapy is still unclear. Application of high-dose corticosteroids for about 3–6 months depending on the clinical presentation and use of surgery in cases with insufficient response to conservative treatment is the most common approach.
The authors recommend a corticosteroid regimen of 30 mg prednisolone twice a day for 2 weeks, tapering gradually based on clinical findings. Every-2-week visits should take place to evaluate treatment response and possible side effects. Corticosteroids should be administered for a minimum of 8 weeks and a maximum of 6 months to minimize possible side effects [34].
For patients who refuse oral corticosteroid therapy and who present with mild symptoms, a topical application of hydrocortisone acetate 0.5% once a day might be an alternative treatment option. In the patient shown in figure 1, the authors observed a 50% reduction of the breast mass after 3 weeks of therapy with topical hydrocortisone acetate. No side effects occurred [35].
Methotrexate is a treatment option for patients who have relapsed or who do not tolerate high-dose corticosteroid therapy. The authors recommend a low-dose regimen similar to those recommended for patients suffering from chronic rheumatoid diseases: 7.5–25 mg methotrexate as a weekly dose combined with folic acid applied daily or once a week [36]. The most frequently reported adverse reactions include ulcerative stomatitis, leukopenia, nausea, abdominal distress, undue fatigue, chills and fever, dizziness, and decreased resistance to infection.
Due to the lack of data, the authors recommend that the indication must be very strict and that patients should be reevaluated at least every 2–4 weeks, especially if no long-term response is seen.
The rarity of the disease makes it difficult to initiate studies with larger numbers and evaluate the efficacy of new treatment options that could lead to the prevention of extensive surgery. Furthermore, there is a great need for new approaches to identifying the underlying causes of the disease that include and consider regional accumulation. Here, the implementation of a registry or a multicentric study could help to collect more data.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Kessler E, Wolloch Y. Granulomatous mastitis: a lesion clinically simulating carcinoma. AM J Clin Pathol. 1972;58:642–646. doi: 10.1093/ajcp/58.6.642. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Idiopathic granulomatous mastitis in Hispanic women - Indiana, 2006–2008. MMWR Morb Mortal Wkly Rep. 2009;58:1317–1321. [PubMed] [Google Scholar]
- 3.Huyser M, Kieran J, Myers S. Review of idiopathic granulomatous mastitis in the Southwest Native American population. Presentation at The American Society of Breast Surgeons, 19th Annual Meeting. 2018 Orlando. [Google Scholar]
- 4.Freeman M, Lewis CD, Lower E. Refractory granulomas of breast: benign or malignant disease. J Clin Oncol. 2014;32((suppl)):21. [Google Scholar]
- 5.Lei X, Chen K, Zhu L. Treatments for idiopathic granulomatous mastitis: systematic review and meta-analysis. Breastfeed Med. 2017;12:415–421. doi: 10.1089/bfm.2017.0030. [DOI] [PubMed] [Google Scholar]
- 6.Johnstone KJ, Robson J, Cherian SG. Cystic neutrophilic granulomatous mastitis associated with Corynebacterium including Corynebacterium kroppenstedtii. Pathology. 2017;49:405–412. doi: 10.1016/j.pathol.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Al Manasra AR, Al-Hurani MF. Granulomatous mastitis: a rare cause of male breast lump. Case Rep Oncol. 2016;9:516–519. doi: 10.1159/000448990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moris D, Damaskos C, Davakis S. Is idiopathic granulomatous mastitis a surgical disease? The jury is still out ATM. Ann Transl Med. 2017;5:309. doi: 10.21037/atm.2017.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Rodriguez JA, Patullo A. Idiopathic granulomatous mastitis: a mimicking disease in a pregnant woman: a case report. BMC Res Notes. 2013;6:95. doi: 10.1186/1756-0500-6-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg J, Baute L, Storey L. Granulomatous mastitis in pregnancy. Obstet Gynecol. 2000;96:813–815. doi: 10.1016/s0029-7844(00)01051-6. [DOI] [PubMed] [Google Scholar]
- 11.Patel RA, Strickland P, Sankara IR. Idiopathic granulomatous mastitis: case reports and review of literature. J Gen Intern Med. 2010;25:270–273. doi: 10.1007/s11606-009-1207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheybany F,Sarvghad, M R Naderi HR. Treatment for and clinical characteristics of granulomatous mastitis. Obstet Gynecol. 2015;125:801–807. doi: 10.1097/AOG.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 13.Wong SCY, Poon RWS, Chen JHK. Corynebacterium kroppenstedtii is an emerging cause of mastitis especially in patients with psychiatric illness on antipsychotic medication. Open Forum Infect Dis. 2017;4:ofx096. doi: 10.1093/ofid/ofx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu LX, Ma ZB. Granulomatous lobular mastitis. Chronic Dis Transl Med. 2016;2:17–21. doi: 10.1016/j.cdtm.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gümüs M, Akkurt ZM, Gümüs H. Is erythema nodosum coexisting with lesions of the breast a suggestive sign for idiopathic granulomatous mastitis? Turk J Surg. 2018;34:71–73. doi: 10.5152/turkjsurg.2017.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman CM, Xia BT, Lewis JD. Idiopathic granulomatous mastitis: a diagnostic and therapeutic challenge. Am J of Surg. 2017;214:701–706. doi: 10.1016/j.amjsurg.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Calis H, Karabeyoglu SM. Follow-up of granulomatous mastitis with monitoring versus surgery. Breast Dis. 2017;37:69–72. doi: 10.3233/BD-160259. [DOI] [PubMed] [Google Scholar]
- 18.Özel L, Ünal A, Ünal E. Granulomatous mastitis: is it an autoimmune disease? Diagnostic and therapeutic dilemmas. Surg Today. 2012;42:729–733. doi: 10.1007/s00595-011-0046-z. [DOI] [PubMed] [Google Scholar]
- 19.llman JE, Terra SB, Clapp AJ. Granulomatous diseases of the breast and axilla. Radiological findings with pathological correlation. Insights Imaging. 2018;9:59–71. doi: 10.1007/s13244-017-0587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor GB, Paviour SD, Musaad S. A clinicopathological review of 34 cases of inflammatory breast disease showing an association between corynebacteria infection and granulomatous mastitis. Pathol. 2003;35:109–119. [PubMed] [Google Scholar]
- 21.Emre A, Akbulut S, Sertkaya M. Idiopathic granulomatous mastitis: overcoming this important clinical challenge. Int Surg. 2017 Epub ahead of print. [Google Scholar]
- 22.Dobinson HC, Anderson TP, Chambers ST. Antimicrobial treatment options for granulomatous mastitis caused by Corynebacterium species. J Clin Microbiol. 2015;53:2895–2899. doi: 10.1128/JCM.00760-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujii M, Mizutani Y, Sakuma T. Corynebacterium kroppenstedtii in granulomatous mastitis: analysis of formalin-fixed, paraffin-embedded biopsy specimens by immunostaining using low-specificity bacterial antisera and real-time polymerase chain reaction. Pathol Int. 2018;68:409–418. doi: 10.1111/pin.12683. [DOI] [PubMed] [Google Scholar]
- 24.Hovanessian Larsen LJ, Peyvandi B, Klipfel N. Granulomatous lobular mastitis: imaging, diagnosis and treatment. AJR Am J Roentgenol. 2009;193:574–581. doi: 10.2214/AJR.08.1528. [DOI] [PubMed] [Google Scholar]
- 25.Lacambra M, Thai TA, Lam CC. Granulomatous mastitis: the histological differentials. J Clin Pathol. 2011;64:405–411. doi: 10.1136/jcp.2011.089565. [DOI] [PubMed] [Google Scholar]
- 26.Keller K, Meisel C, Petzold A, Wimberger P. Granulomatöse Mastitis - möglicher diagnostischer und therapeutischer Ablauf anhand von Fallbeispielen. Senologie. 2018;15:e23. [Google Scholar]
- 27.Kiene P, Mavrova C, Saronijc B. Diagnostik und Therapie der granulomatösen Mastitis. Senologie. 2018;15:e25. [Google Scholar]
- 28.Fazzio RT, Shah SS, Sandhu NP. Idiopathic granulomatous mastitis: imaging update and review. Insights Imaging. 2016;7:531–539. doi: 10.1007/s13244-016-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aghajanzadeh M, Hassanzadeh R, Alizadeh SS. Granulomatous mastitis: presentations, diagnosis, treatment and outcome in 206 patients from the north of Iran. Breast. 2015;24:456–460. doi: 10.1016/j.breast.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Elzahaby IA, Khater A, Fathi A. Etiologic revelation and outcome of the surgical management of idiopathic granulomatous mastitis; an Egyptian centre experience. Breast Disease. 2016;36:115–122. doi: 10.3233/BD-160238. [DOI] [PubMed] [Google Scholar]
- 31.Bashir MU, Ramcharan A, Alothman SB. The enigma of granulomatous mastitis: a series. Breast Dis. 2017;37:17–20. doi: 10.3233/BD-160261. [DOI] [PubMed] [Google Scholar]
- 32.DeHertogh DA, Rossof AH, Harris AA. Prednisone management of granulomatous mastitis. N Engl J Med. 1980;303:799–800. doi: 10.1056/NEJM198010023031406. [DOI] [PubMed] [Google Scholar]
- 33.Yılmaz TU, Gürel B, Güler SA. Scoring idiopathic granulomatous mastitis: an effective system for predicting recurrence? Eur J Breast Health. 2018;14:112–116. doi: 10.5152/ejbh.2018.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akcan A, Öz AB, Dogan S. Idiopathic granulomatous mastitis: comparison of wide local excision with or without corticosteroid therapy. Breast Care. 2014;9:111–115. doi: 10.1159/000360926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gunduz Y, Altintoprak F, Tatli Ayhan L. Effect of topical steroid treatment on idiopathic granulomatous mastitis: clinical and radiologic evaluation. Breast J. 2014;20:586–591. doi: 10.1111/tbj.12335. [DOI] [PubMed] [Google Scholar]
- 36.Akbulut S, Arikanoglu Z, Senol A. Is methotrexate an acceptable treatment in the management of idiopathic granulomatous mastitis? Arch Gynecol Obstet. 2011;284:1189–1195. doi: 10.1007/s00404-010-1825-2. [DOI] [PubMed] [Google Scholar]