Abstract
Objective
Bone metastases bring greater morbi-mortality to patients with differentiated thyroid carcinoma (DTC). Treatment was limited to radioactive iodine (RAI) and local approaches. Currently, bisphosphonates are included in the therapeutic arsenal. The aim of this study is to evaluate the impact of bone metastases and their treatment with zoledronic acid (ZA) and RAI therapy.
Methods
We retrospectively review 50 DTC patients with structurally evident bone metastases followed in a tertiary cancer center from 1994 to 2018. Clinical-pathologic characteristics, skeletal related events (SRE), and therapeutic approaches were recorded.
Results
Among the 50 patients analyzed, 22 underwent ZA adjuvant therapy and 28 did not. Mortality rate was 44%. Those patients presented SREs more frequently (90.9 vs. 67.9% the survival group, p = 0.05) and also had a greater number of bone lesions (40.9 vs. 10.7% had more than 6 metastatic sites, p = 0.03). The same group of patients was analyzed before and after therapy with ZA and the incidence of SRE decreased from 1.81 (0–8) before therapy to 0.29 (0–7) after therapy (p = 0.006). Comparing similar groups of 22 patients treated with ZA with 28 patients not treated, there was a trend of better overall survival (OS) in the group that received this drug (147 vs. 119 months, p = 0.06) and significantly improvement when bone metastases were RAI avid 155 (125–185) versus 120 (85–157) months, p < 0.01. Conclusion: ZA can successfully diminish the chance of having new SRE and possibly affect OS in DTC patients with bone metastases. The positive impact of RAI adjuvant treatment on OS is directly associated with RAI uptake.
Key Words: Thyroid cancer, Bone metastases, Zoledronic acid, Bisphosphonates, Radioactive Iodine
Background
Bone is a major metastatic site in many malignancies due to its dynamic metabolism, high blood supply, response to tumor adhesion factors, and rich local growth factors [1]. In advanced breast and prostate cancer, long bones may be affected in up to 70–90% of cases [2, 3]. Among differentiated thyroid cancer (DTC), it is the second most frequent site of distant metastases. Incidence may vary from 2 to 13% [4], influenced by the degree of differentiation and histologic subtypes – it is more frequently originated from follicular thyroid cancer than papillary [5]. Apart from indicating a poor prognosis [6], it also causes considerable bone morbidity and impairment to quality of life [7, 8].
The treatment of these patients has always been a challenge and some features are crucial to selecting a proper therapy modality [9]. For unresectable painful bone metastases, there are many palliative measures available such as external beam radiation (EBRT), stereotactic radiation, thermal ablation, and cement injections [10, 11, 12].
Although rarely curative, radioactive iodine therapy (RAI) is recommended for all patients with bone metastases and may benefit individuals with RAI avid bone lesions. However, there is no consensus on the exact activity [13, 14] or on the best regimen (single or multiples doses) [5].
The most recent guidelines recommend the use of bisphosphonates or denosumab in patients with diffuse and/or symptomatic RAI refractory bone metastases [9, 15]. However, this recommendation is based on trials for other solid tumors due to the limited data available for DTC patients [8, 16, 17, 18]. In addition, recent studies in vivo and in vitro [19] showed that an antineoplastic activity could also be attributed to these drugs.
Among this class of drugs, zoledronic acid (ZA) is well established for osteoporosis treatment and widely studied in oncologic settings [20]. It has been suggested that it could impact cell adhesion[21], proliferation [22], migration, and invasion [23].
The aim of this study is to evaluate the impact of RAI adjuvant therapy and routine use of ZA on the morbi-mortality of patients with bone metastases from DTC.
Materials and Methods
After Institutional Review Board approval, medical records of adult patients followed for at least 6 months in a single tertiary oncology referral center between 1994 and 2018 were reviewed.
In our institution, all patients with DTC and distant metastases are routinely referred to RAI. The activity administered is decided by a multidisciplinary tumor board and is empirical between 200 and 300 mCi. Five to 10 days after therapy, patients routinely undergo a whole body scan and if any bone metastasis has iodine uptake, it was classified as iodine avid disease.
Evaluation of Outcomes
Clinical-pathological characteristics, treatment, and postoperative follow-up data (Tg, recurrence/persistence, deaths) were obtained. Patients were classified by the AJCC/TNM-8th edition [24] and ATA risk classification [9].
Distant metastases were assessed by cross-sectional images and considered present when there were highly suspicious images on computed tomography, magnetic resonance imaging, and/or X-Ray. This was true even with no iodine uptake but with high thyroglobulin levels, or if proven by biopsy [9]. An uptake in functional tests not proved in structural exams was not considered.
Treatment with ZA
Patients were referred to ZA adjuvant therapy based on the judgment of their own endocrinologist on an individual basis. They received 4 mg of intravenous infusion of ZA (Zometa®; Novartis Pharma AG, Basel, Switzerland/Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA) in 15 min on an average of once per month. Renal function and serum calcium were documented prior to each dose, which could be suspended/postponed. The incidence of skeletal related event (SRE) was evaluated prior to ZA therapy and compared with how many and how severe the events were from the date of the first dose of ZA until the end of follow-up, using the same patient as his own control. The SREs assessed in this study included: spinal cord compression, pathological fracture, and EBRT or surgery to prevent complications or promote pain control. The occurrence of 2 SRE in the same lesion with a minimum of a 6-month interval was considered the same incident.
Statistical Analysis
Statistical analysis was conducted using SPSS version 20.0 for MAC (SPSS, INC, Chicago, IL, USA). The results are expressed as a percentage or mean ± SD. Statistical analysis was performed on the whole series of thyroid carcinomas and considered the different groups of tumors. Fisher's exact test, t test (unpaired, two-tailed), and ANOVA were used when appropriate. For Hazard ratio and confidence intervals binary logistic regression were performed with Cox test.
Factors such as age, gender, histologic category, extrathyroidal extension, vascular invasion, lymph node metastases, AJCC/TNM staging [24], distant metastases, and final status were assessed using univariate and multivariate logistic regression models.
A survival curve was plotted by the Kaplan-Meier method with log-rank statistics. Multivariate survival analysis was performed using Cox regression. In the regression models, all of the variables significantly associated with the specified outcome in the univariate model were included in the multivariate analysis.
Results were considered statistically significant at p < 0.05
Results
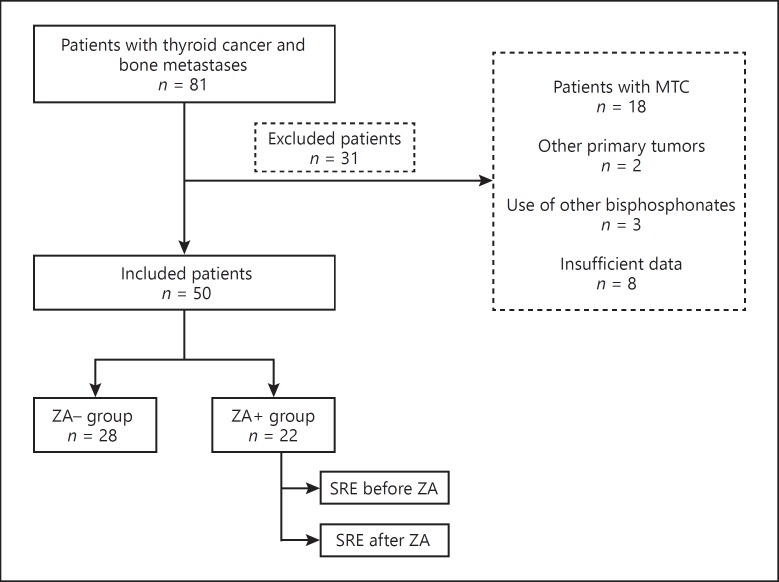
Eighty-one patients with bone metastases and thyroid cancer were identified. Eighteen had metastatic medullary thyroid carcinoma and were excluded from the analysis. Patients with other primary malignancies, history of previous or current use of other bisphosphonates or with insufficient records were also excluded (Fig. 1). The remaining 50 patients evaluated included 25 of PTC (10 follicular variant, 1 tall cell variant, 1 diffuse sclerosing variant and 13 classic PTC), 18 from FTC and 7 with poorly differentiated histopathology. The demographics and clinical characteristics are summarized in Table 1.
Fig. 1.
Flowchart to describe the selection of patients included in the analysis. MTC, medullary thyroid cancer; ZA, zoledronic acid; SRE, skeletal related events.
Table 1.
Characteristics of the patients
| n = 50 | % | |
|---|---|---|
| Age, years | – | |
| Median (range) | 57.6 (26.2–79) | |
| Mean ± SD | 57.1±12.1 | |
| Gender, female Histopathology |
41 | 82 |
| PTC | 25 | 50 |
| FTC | 18 | 36 |
| PDTC/insular carcinoma Size, cm |
7 | 14 |
| Median, range | 4.0 (0.3–14) cm | – |
| Mean ± SD | 4.6±3.1 | |
| N1 Distant metastases (other than bone) |
11 | 22 |
| Lung | 28 | 56 |
| CNS | 4 | 8 |
| Liver | 6 | 12 |
| Stimulated Tg pre RAI, ng/mL | 20,779 (2.0–151,131) | |
| RAI, yes | 45 | 90 |
| RAI Activity, mCi Post therapy scan* |
200 (100–300) 200±100 |
|
| Thyroid bed only | 8 | 16 |
| Lateral neck uptake | 5 | 10 |
| Distant metastases | 32 | 64 |
| Iodine avid bone | 27 | 60 |
| Skeletal-related events at anytime | 39 | 78 |
| Spine compression | 16 | 32 |
| Pathological fracture | 10 | 20 |
| EBRT for bone mets | 25 | 50 |
| SX for bone mets | 12 | 24 |
| Systemic therapy (TKI) | 7 | 14 |
| Symptomatic bone lesion | 35 | 70 |
| Pain | 31 | 62 |
| Paresis | 10 | 20 |
| Paresthesia Number of bone mets |
5 | 10 |
| Median, range | 3 (1–17) | |
| Mean ± SD | 4.4±3.5 | – |
| Final Status, progressive | 33 | 66 |
| Disease-related death Overall survival, months |
22 | 44 |
| Median (range) | 68.1 (8.5–240.6) | – |
| Mean ± SD Follow-up, months |
85.9±59 | |
| Median (range) | 67.8 (7.1–240.6) | |
| Mean ± SD | 85±59.3 | – |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; PDTC, poorly differentiated thyroid cancer; N1, presence of lymph node metastases; CNS, central nervous system; RAI, radioactive iodine; EBRT, external beam radiation therapy; SX, surgery; TKI, tyrosino kinase inhibitor.
Five patients did not undergo RAI.
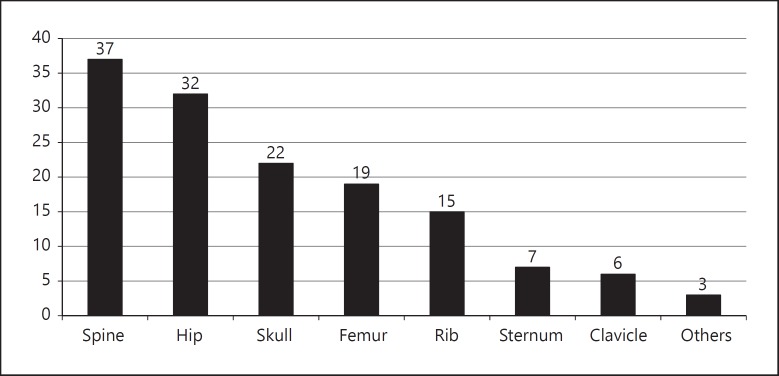
In this study, as a referral cancer center, we found that the bone metastases were usually present at diagnosis and/or were diagnosed during initial follow-up by a positive post therapy scan or staging for high thyroglobulin levels post operation (70%), affecting mostly the spine (74%), hip (64%), and the skull (44%; Fig. 2). The number of bone lesions detected in each patient varies widely in a range of 1–17. In 70% of the cases, the skeletal investigation was guided by the presence of symptoms, in which pain was the most frequent complaint (62%) followed by paresis (20%).
Fig. 2.
Number and location of bone metastases of all patients included.
Likewise, 39 patients (78%) developed at least 1 SRE during the period assessed. As expected, the most common SRE was the need of EBRT in order to control pain or diminish the risk of fracture (50%). In Table 2, the risk factors for ERE are described. As shown, age and sex were not significant in our cohort; however, the number of bone metastases along with its location significantly increased the risk of having at least one ERE. The use of ZA at any time also had an impact and those not treated had a 5 times increased risk of at least 1 ERE.
Table 2.
Risk factors for ERE in DTC patients with bone metastases
| Univariate analysis |
Multivariate analysis |
||||
|---|---|---|---|---|---|
| ERE+, % | p value | HR | 95% CI | p value | |
| Age | |||||
| >55 | 82 | 0.26 | 0.9 | 0.1–4.9 | 0.8 |
| <55 | 66 | ||||
| Gender | |||||
| Female | 82 | 0.09 | 0.68 | 0.09–4.5 | 0.9 |
| Male | 55 | ||||
| Number of sites of bone metastases | |||||
| 1–5 mets | 71 | 0.7 | 1.8 | 0.09–3.4 | 0.07 |
| 6 mets | 100 | ||||
| Spine metastases | |||||
| Yes | 81 | 0.3 | 4.3 | 0.8–23 | 0.08 |
| No | 69 | ||||
| Hip metastases | |||||
| Yes | 88 | 0.03 | 4.1 | 1.0–17.6 | 0.05 |
| No | 61 | ||||
| Femur metastases | |||||
| Yes | 95 | 0.02 | 2.1 | 1.0–73.5 | 0.05 |
| No | 68 | ||||
| Iodine uptake by bone metastases | |||||
| Yes | 78 | 0.41 | 0.5 | 0.08–4.2 | 0.6 |
| No | 72 | ||||
| ZA+ | 64 | 0.04 | 5.4 | 1.1–25.8 | 0.03 |
| ZA– | 89 | ||||
ZA, zoledronic acid therapy.
Regarding other therapies, the vast majority of the patients (45/50) underwent adjuvant therapy with RAI. Four did not due to a very aggressive and progressive disease leading to death very quickly after the diagnosis. One patient was taking amiodarone and is therefore still waiting to undergo RAI. Of the individuals who received RAI, 60% had iodine avid bone metastasis. Although all of the patients included in the study had metastatic disease, only a few (14%) needed systemic therapy with multikinase inhibitors. All of them used sorefenib, as this is the only drug approved in Brazil for progressive iodine-refectory metastatic DTC, and the medication was not interrupted during ZA therapy. Regarding response to sorafenib, 4 patients had stable disease, 2 progressed and 1 did not tolerate the drug due to severe adverse effects.
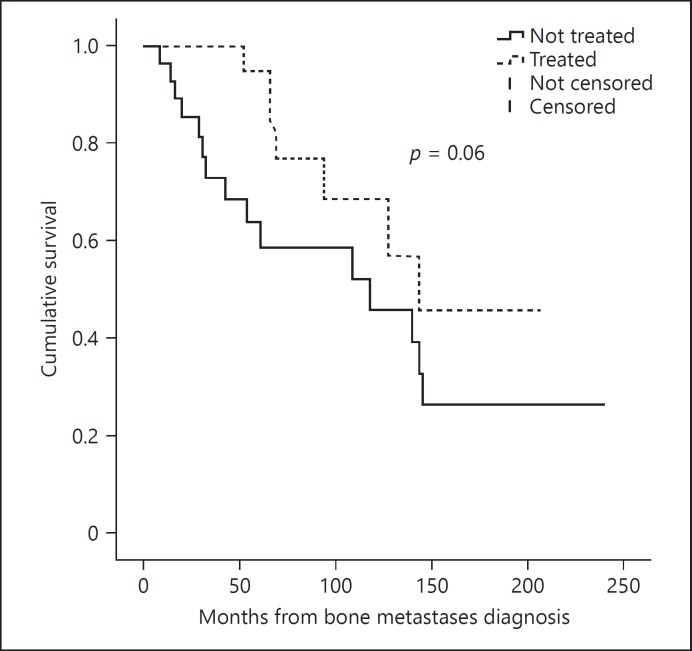
In order to evaluate the impact of ZA, we separated the population into 1 group of patients who received at least 1 dose of ZA (ZA+ group, n = 22), and another group who did not (ZA– group, n = 28). These 2 groups were comparable in terms of age, gender, histopathology, size of the thyroid tumor, extension of bone disease, and the presence of lymph nodes or other distant metastases (Table 3). During this period, multiple cycles of ZA were given in the ZA+ group ranging from 1 to 25 (median 7.5). It seems that the number of cycles tend to be inversely associated with the risk of pathological fracture (p = 0.08). Using the median as a cutoff, we analyzed separately the outcome of patients with more or less than 7 cycles of ZA and demonstrated that in patients with more than 7 cycles, the risk of having a new SRE was diminished by 20% (HR 0.81 IC: 0.67–0.83, p = 0.04). However, the use of the drug was not statistically significant regarding the risk of bone disease progression or disease-related death, but it seems to have a trend toward a better overall survival (OS) in ZA+ 147 (116–179) versus 119 (110–165), p = 0.06 (Fig. 3).
Table 3.
Comparison between the treated and untreated group
| ZA– (n = 28) | ZA+ (n = 22) | p value | |
|---|---|---|---|
| Age, years | 59 | 57.1 | 0.3 |
| Median, range | 31–72 | 26–79 | |
| Mean ± SD | 57.5±10.4 | 56.3±14.3 | |
| Gender, F, n (%) | 21 (75) | 20 (90.9) | 0.13 |
| Histopathology, n (%) PTC |
12 (42.9) | 13 (59.1) | 0.19 |
| FTC | 10 (35.7) | 8 (36.4) | |
| PDTC/insular carcinoma, n (%) | 6 (21.4) | 1 (4.5) | |
| Size, cm Median, range |
4.5 (0.3–14) | 3.4 (1.1–10) | 0.36 |
| Mean ± SD | 4.9±3.3 | 3.8±2.5 | |
| N1, n (%) | 7 (30.4) | 4 (18.1) | 0.73 |
| Distant metastases other than bone, n (%) | 20 (71.4) | 12 (54.5) | 0.20 |
| Lung, n (%) | 18 (64.3) | 10 (45.5) | 0.14 |
| CNS, n (%) | 3 (10.7) | 1 (4.5) | 0.40 |
| Liver, n (%) | 5 (17.9) | 1 (4.5) | 0.16 |
| Other, n (%) | 2 (7.1) | 3 (13.6) | 0.64 |
| Number of bone mets | 3 | 3.5 | 0.35 |
| Median, range | 1–11 | 1–17 | |
| Mean ± SD | 3.9±2.8 | 5±4.2 | |
| Disease-related death, n (%) | 15 (53.6) | 7 (31.8) | 0.1 |
| Follow-up, months Median, range |
50.3 (7.1–240) | 78 (26.6–206) | 0.03 |
| Mean ± SD | 76.5±64.3 | 95±51.8 |
ZA, zoledronic acid; PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; PDTC, poorly differentiated thyroid cancer; N1, presence of lymph node metastases; CNS, central nervous system.
Fig. 3.
OS in patients treated with Zoledronic Acid (ZA+ group) and not treated (ZA– group), (OS) in ZA+ 147 (116–179) versus ZA– 119 (110–165), HR: 0.86 (0.77–7.9), p = 0.06.
In the 22 patients treated with ZA, the number of SRE was significantly lower after ZA treatment for the same patient: median pre ZA 1.81 (0–8) and post treatment 0.29 (0–7), p = 0.006. Spine compression occurred in 8 patients (36.6%) pre ZA, but only 1 post ZA (4.5%), p = 0.02. There was also a diminished number of pathological fractures: 5 patients had 8 fractures pre ZA and 2 patients had 2 fractures post ZA, p = 0.02.
The most frequent adverse event related to ZA infusions was hypocalcemia (32%). None of them was severe and none required intravenous treatment in any patient, but in 6 cases, the management included oral supplementation of calcitriol and calcium, 5 of them after the first cycle of ZA and the other one after the sixth cycle. A slightly elevated serum creatinine (0.8–1.2 mg/dL) was observed in 1 woman and another experienced flu-like symptoms (4.5%); however, all of these adverse events were transient and there was no need to discontinue the treatment in any of these patients. In this study, we did not observe any cases of bisphosphonate-related osteonecrosis of the jaw (BRONJ) or atypical fracture.
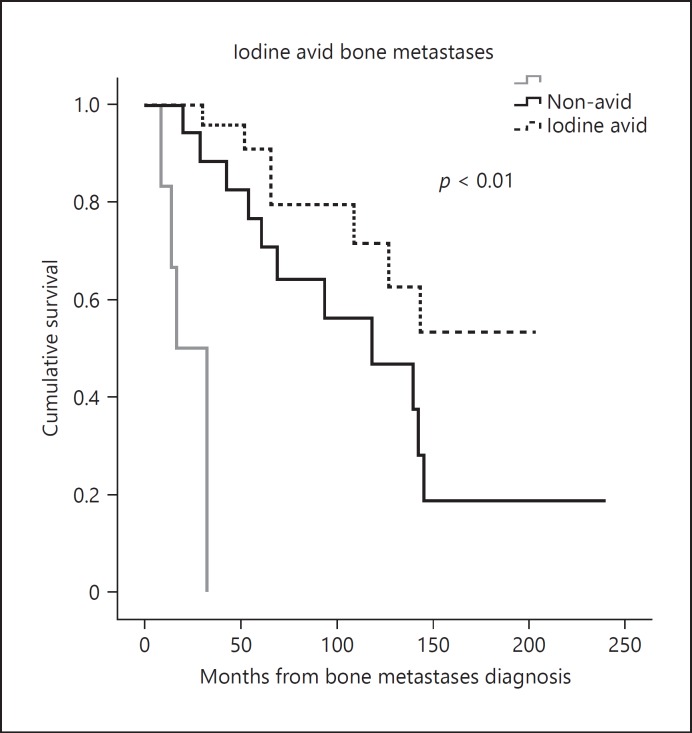
Finally, in terms of disease-related death (Table 4), statistical analysis revealed the presence of concomitant metastases in the brain (p = 0.03), liver (p = 0.05), and the presence of bone metastases that are not RAI avid (p = 0.03) were more frequent in the deceased group. Furthermore, the presence of an SRE experienced at any time point (90.0 vs. 67.9%, p = 0.03) and a higher number of bone metastatic lesions occurred more frequently in patients who died (40.9 vs. 10.7% in patients with ≥6 bone lesions, p = 0.03). During the period assessed in this study, the cumulative survival rate over time was directly associated with the avidity for RAI in bone lesions, with an OS of 155 (125–185) months in patients with iodine avid metastases and 120 (85–157) months in non-avid lesions (p < 0.01; Fig. 4).
Table 4.
Disease-related death risk factors
| Non-survivors (n = 22) | Survivors (n = 28) | p value | |
|---|---|---|---|
| Age, years | 0.09 | ||
| Median, range | 58 (41–79) | 57.6 (26–78) | |
| Mean ± SD | 58±10 | 56±14 | |
| Gender, F, n (%) | 19 (86.4) | 22 (78.6) | 0.37 |
| Histopathology, n (%) | 0.13 | ||
| PTC | 8 (36.4) | 17 (60.7) | |
| FTC | 9 (40.9) | 9 (32.1) | |
| PDTC/insular carcinoma | 5 (22.7%) | 2 (7.1) | |
| Size, cm | 0.08 | ||
| Median, range | 4.5 (1.5–14) | 4.0 (0.3–10) | |
| Mean ± SD | 5.3±3.7 | 3.9±2.4 | |
| N1, n (%) | 4 (18.1) | 7 (25) | 0.43 |
| Distant metastases (other than bone), n (%) | |||
| Lung | 14 (63.6) | 14 (50) | 0.25 |
| CNS | 4 (18.2) | 0 | 0.03 |
| Liver | 5 (22.7) | 1 (3.6) | 0.05 |
| Iodine avid bone mets | 7 (31.8) | 20 (71.4) | 0.01 |
| Skeletal-related events at any time, n (%) | 20 (90.9) | 19 (67.9) | 0.05 |
| Spine compression, n (%) | 8 (36.4) | 7 (25%) | 0.5 |
| Pathological fracture, n (%) | 5 (22.7) | 1 (3.5) | 0.07 |
| Number of bone mets, n (%) | 0.02 | ||
| 1–5 mets | 13 (59.1) | 25 (89.3) | |
| 6 mets | 9 (40.9) | 3 (10.7) | |
| ZA (n = 22) | 7 (31.8) | 15 (53.6) | 0.1 |
| Follow-up, months | 0.34 | ||
| Median, range | 62.5 (7.1–144) | 78 (14–240) | |
| Mean ± SD | 70±47.8 | 96.8±65.5 |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; PDTC, poorly differentiated thyroid cancer; N1, presence of lymph node metastases; CNS, central nervous system; ZA, zoledronic acid.
Fig. 4.
Survival curve of patients with radioactive iodine avid and non-avid bone metastases, OS of 155 months (125–185) in patients with iodine avid metastases and 120 months (85–157) in non-avid lesions (p < 0.01).
Discussion
This present study showed that in DTC patients with bone metastases, iodine avidity and possibly the use of ZA as adjuvant therapy have an impact on OS. Furthermore, this was the first study to analyze the impact of ZA before and after therapy in each patient as its own control, and was consistent with previous literature in proving that it does diminish SRE when comparing groups and on an individual basis.
Our data, like previous studies [17, 20, 25] showed that ZA reduces the incidence of skeletal complications in this population. Orita et al. [17] showed in a prospective study that ZA was effective at reducing the proportion (56.25 vs. 5.26%, p = 0.017) and delaying the onset (p = 0.042) of spinal cord compression when compared to a historical control group. Other retrospectives studies also showed the positive impact of antiresorptive therapies on SRE in bone metastatic disease from DTC [16, 18] as well as in other neoplasms such as lung and kidney [20, 26]. A randomized trial has evaluated the efficiency of ZA versus placebo in reducing SRE in 773 patients with bone metastases from varied types of tumors including lung, head and neck, and also thyroid cancer metastatic bone disease. They found that ZA delayed the time of the first SRE from 155 to 236 days (p = 0.009) and their annual incidence (1.74 vs. 2.71, p = 0.012), as well as a decreased the risk of developing an SRE by 31% [20]. Similar findings were also reported for advanced renal cell carcinoma, showing a reduction in SREs (74% in the placebo and 37% in treated group, p = 0.0015), a statistical increase in the time of onset, and decreased the risk by 61% (OH: 0.394, p = 0.008) [26].
It is important to note that the reduction in the incidence of SRE in the same patient as his own control has a great impact on metastatic bone disease morbidity. In a retrospective review of 245 DTC patients with bone metastases, Farooki et al. [8] reported that SRE were frequently multiple. He revealed that 78% of the patients experienced at least one SRE, 65% of individuals developed a second event, and 39% sustained 3 or more SREs [8]. Considering the high rate of SRE in this population, the importance of early evaluation and prevention is evident. As shown in this present study, ZA was able to diminish SRE on an individual basis, hence improving the morbidity related to skeletal complications.
The use of ZA has been associated with improvement of OS rates regardless of SREs [27]. Multiple mechanisms have been suggested to explain the better OS of oncologic patients in the use of bisphosphonates and it seems that the advantages go beyond the inhibition of bone resorption. The “seed and soil hypothesis” proposed by Paget in 1889 suggests that oncologic therapies should not only target the tumor cells (seeds) but also make the host microenvironment (soil) less favorable to their growth [28]. In this context, by modulating the skeletal metabolism, these drugs could change bone environment, making it less suitable for cancer cell survival. As a result, it may diminish the possibility of the bone marrow to be a sanctuary for quiescent pathologic cells [29]. Several preclinical studies suggest that these agents may have a direct and indirect antitumor activity. By inhibiting the mevalonate pathway, bisphosphonates can directly reduce cancer cell migration, invasion [23], adhesion [21], and proliferation [22] as well as inducing apoptosis [30]. In addition, an indirect tumor control can be achieved by the inhibition of angiogenesis [31] and improvement of host immune surveillance against cancer cells [32]. These findings may explain some clinical results, especially in breast cancer, that suggest that the benefits of these drugs on OS and DFS might be independent of the presence of bone metastases [33, 34]. Although the antitumor effect of bisphosphonates has been extensively evaluated in other types of cancer, the data concerning DTC is limited. Vitale et al.[18] reported 10 patients with skeletal metastases using Pamidronate and a decrease of more than 50% of the bone lesion in 2 patients and stabilization in 5 patients was observed. The authors suggested that an antitumor effect could be partially responsible for these findings [18]. Our data revealed a trend toward an increase in OS in the group treated with ZA, though not reaching statistical significance (p < 0.06).
Regarding disease-specific death, our findings pointed to other factors that can influence mortality. We showed that the occurrence of any SRE during the follow-up was associated with a worse outcome. Likewise, Farooki et al. [8] reported a significant increase (p < 0.0001) in the mortality of patients with bone metastases from DTC who developed single (68%) or multiple (79%) SREs compared to those who did not (42%) [8]. Although Xu et al.[35] did not see this correlation in their report of 188 patients with medullary thyroid carcinoma and skeletal metastases, studies with other solid tumors like breast cancer show that the presence of SRE was associated with an increased mortality [36].
In the present data, other features were identified more frequently in patients who were non survivors at final follow-up, such as the presence of any bone metastases that were not RAI avid. In fact, any bone lesions that uptake in the post therapy 131I scan significantly improved OS and was associated with a 20% reduction in risk of death. As previously reported by Durante et al.[5] in a cohort of 444 DTC patients with distant metastases (197 patients with bone lesions), the absence of RAI uptake was associated with a significantly reduced OS rate and with a 10-year survival rate of 3 vs. 48%, (p < 0.001) [5]. This was also consistent with the results presented by Schlumberger et al. [37], who evaluated 394 patients with DTC in which 180 had bone metastases, which showed that patients whose metastases concentrated 131I had a higher survival rate, and an estimated risk of complete response of 22.7 (5.0–100, 95% CI, p = 0.001) [37].
In terms of the presentation and distribution of the skeletal lesions, our population was similar to those formerly described. Most of our bone metastases were symptomatic (usually pain) and frequently multiple [8, 16, 36]. The most affected sites were the spine and hip as previous shown by Bernier et al.[38], whose study detected these metastases in 68 and 57% respectively. Likewise, EBRT was also the most common SRE observed by other authors in different neoplasms including DTC [8], MTC [35], and other solid tumors [25].
We recognize the limitations of a retrospective study such as incomplete documentation and differences in the populations analyzed. Another limitation caused by the study design was in the evaluation of the EBRT as an SRE, since in clinical practice it is based on individual clinical judgment, though the classic indication is to prevent fracture or pain control. Also, due the long period of follow-up there may be some inconsistency in diagnostic modalities and therapeutic approach.
Conclusion
Patients with DTC who develop bone metastases often result in greater morbidity. This is objectively measured by the incidence of SREs. The standard adjuvant therapies used in these cases are antiresorptive drugs and RAI, after thyroid surgery. The present data shows that a monthly ZA infusion could successfully prevent a new SRE and possibly affect OS. Even though this evidence is not sufficient to support the benefits of these drugs beyond the improvement in quality of life, it solidifies the need for other trials to evaluate the possible antitumor activity of these agents in thyroid cancer.
Disclosure Statement
No competing financial interests exist for any of the authors.
References
- 1.Roodman G. Mechanisms of bone metastasis. NEJM. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55:61–66. doi: 10.1038/bjc.1987.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bubendorf L, Schopfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser T, Mihatsch M. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 4.Muresan M, Olivier P, Leclère J, Sirveaux F, Brunaud L, Klein M, Zarnegar R, Weryha G. Bone metastases from differentiated thyroid carcinoma. Endocr Relat Cancer. 2008;15:37–49. doi: 10.1677/ERC-07-0229. [DOI] [PubMed] [Google Scholar]
- 5.Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, Caillou B, Ricard M, Lumbroso JD, De Vathaire F, Schlumberger M. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 6.Schlumberger M, Tubiana M, De Vathaire F, Hill C, Gardet P, Travagli JP, Fragu P, Lumbroso J, Caillou B, Parmentier C. Long-term results of treatment of 283 patients with lung and bone metastases from differentiated thyroid carcinoma. J Clin Endocrinol Metab. 1986;63:960–967. doi: 10.1210/jcem-63-4-960. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243–6249. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 8.Farooki A, Leung V, Tala H, Tuttle RM. Skeletal-related events due to bone metastases from differentiated thyroid cancer. J Clin Endocrinol Metab. 2012;97:2433–2439. doi: 10.1210/jc.2012-1169. [DOI] [PubMed] [Google Scholar]
- 9.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid Cancer. Thyroid. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo SS, Fakiris AJ, Teh BS, Cardenes HR, Henderson MA, Forquer JA, Papiez L, McGarry RC, Wang JZ, Li K, Mayr NA, Timmerman RD. Stereotactic body radiation therapy for oligometastases. Expert Rev Anticancer Ther. 2009;9:621–635. doi: 10.1586/era.09.15. Erratum in: Expert Rev Anticancer Ther 2009; 9: 1348. [DOI] [PubMed] [Google Scholar]
- 11.Posteraro AF, Dupuy DE, Mayo-Smith WW. Radiofrequency ablation of bony metastatic disease. Clin Radiol. 2004;59:803–811. doi: 10.1016/j.crad.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Anselmetti GC, Manca A, Ortega C, Grignani G, Debernardi F, Regge D. Treatment of extraspinal painful bone metastases with percutaneous cementoplasty: a prospective study of 50 patients. Cardiovasc Intervent Radiol. 2008;31:1165–1173. doi: 10.1007/s00270-008-9396-3. [DOI] [PubMed] [Google Scholar]
- 13.Andresen NS, Buatti JM, Tewfik HH, Pagedar NA, Anderson CM, Watkins JM. Radioiodine ablation following thyroidectomy for differentiated thyroid cancer: literature review of utility, dose, and toxicity. Eur Thyroid J. 2017;6:187–196. doi: 10.1159/000468927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxon HR, Thomas SR, Hertzberg VS, Kereiakes JG, Chen IW, Sperling MI, Saenger EL. Relation between effective radiation dose and outcome of radioiodine therapy for thyroid cancer. N Engl J Med. 1983;309:937–941. doi: 10.1056/NEJM198310203091601. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network, Inc. 2017 Practice Guidelines in Oncology - Thyroid Carcinoma v.2.2017. www.nccn.org/professionals/physician_gls/PDF/thyroid.pdf (accessed March 10, 2018)
- 16.Orita Y, Sugitani I, Toda K, Manabe J, Fujimoto Y. Zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Thyroid. 2011;21:31–35. doi: 10.1089/thy.2010.0169. [DOI] [PubMed] [Google Scholar]
- 17.Orita Y, Sugitani I, Takao S, Toda K, Manabe J, Miyata S. Prospective evaluation of zoledronic acid in the treatment of bone metastases from differentiated thyroid carcinoma. Ann Surg Oncol. 2015;22:4008–4013. doi: 10.1245/s10434-015-4497-0. [DOI] [PubMed] [Google Scholar]
- 18.Vitale G, Fonderico F, Martignetti A, Caraglia M, Ciccarelli A, Nuzzo V, Abbruzzese A, Lupoli G. Pamidronate improves the quality of life and induces clinical remission of bone metastases in patients with thyroid cancer. Br J Cancer. 2001;84:1586–1590. doi: 10.1054/bjoc.2001.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clézardin P, Fournier P, Boissier S, Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Curr Med Chem. 2003;10:173–180. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 20.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman J. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, Phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 21.Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, Clezardin P. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracelular matrices. Cancer Res. 1997;57:3890–3894. [PubMed] [Google Scholar]
- 22.Sasaki A, Boyce BF, Story B, Wright KR, Chapman M, Boyce R, Mundy GR, Yoneda T. Bisphosphonate risedronate reduces metastatic human breast cancer burden in bone in nude mice Cancer Res. 1995;55:3551–3557. [PubMed] [Google Scholar]
- 23.Boissier S, Ferreras M, Peyruchaud O. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000;60:2949–2954. [PubMed] [Google Scholar]
- 24.Tuttle RM, Haugen B, Perrier ND. Updated American joint committee on cancer/tumor-node-metastasis staging system for differentiated and anaplastic thyroid cancer (eighth edition): what changed and why? Thyroid. 2017;27:751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen LS, Gordon D, Tchekmedyian S, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng M, Urbanowitz G, Reitsma D, Seaman JJ. Zoledronicacid versus placebo in the treatment of skeletal metastases in patients with lung cancer and other solid tumors: a phase III, double-blind, randomized trial - the zoledronic acid lung cancer and other solid tumors study group. J Clin Oncol. 2003;21:3150–3157. doi: 10.1200/JCO.2003.04.105. [DOI] [PubMed] [Google Scholar]
- 26.Lipton A, Zheng M, Seaman J. Zoledronic acid delays the onset of skeletal-related events and progression of skeletal disease in patients with advanced renal cell carcinoma. Cancer. 2003;98:962–969. doi: 10.1002/cncr.11571. [DOI] [PubMed] [Google Scholar]
- 27.Coleman RE, Lipton A, Costa L, Cook RJ, Lee KA, Saad F, Brown JE, Terpos E, Major PP, Kohno N, Smith M, Body JJ. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features - an exploratory analysis of placebo-controlled trials. J Bone Oncol. 2013;2:70–76. doi: 10.1016/j.jbo.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 29.Rack B, Jückstock J, Genss EM, Schoberth A, Schindlbeck C, Strobl B, Heinrigs M, Rammel G, Zwingers T, Sommer H, Friese K, Janni W. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30:1807–1813. [PubMed] [Google Scholar]
- 30.Hauschka PV, Mavrakos AE, Iafrati MD, Doleman SE, Klagsbrun M. Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem. 1986;26:12665–12674. [PubMed] [Google Scholar]
- 31.Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clézardin P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002;62:6538–6544. [PubMed] [Google Scholar]
- 32.Sato K, Kimura S, Segawa H, Yokota A, Matsumoto S, Kuroda J, Nogawa M, Yuasa T, Kiyono Y, Wada H, Maekawa T. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 33.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Pöstlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rücklinger E, Greil R. ABCSG-12 Trial Investigators, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691. doi: 10.1056/NEJMoa0806285. Erratum in: N Engl J Med 2009; 360: 2379. [DOI] [PubMed] [Google Scholar]
- 34.Gnant M, Clézardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev. 2012;38:407–415. doi: 10.1016/j.ctrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Xu JY, Murphy WA, Jr, Milton DR, Jimenez C, Rao SN, Habra MA, Waguespack SG, Dadu R, Gagel RF, Ying AK, Cabanillas ME, Weitzman SP, Busaidy NL, Sellin RV, Grubbs E, Sherman SI, Hu MI. Bone metastases and skeletal-related events in medullary thyroid carcinoma. J Clin Endocrinol Metab. 2016;101:4871–4877. doi: 10.1210/jc.2016-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong M, Jensen AÖ, Jacobsen JB, Nørgaard M, Fryzek JP, Sørensen HT. Survivalin breast cancer patients with bone metastases and skeletal-related events: a population-based cohort study in Denmark (1999–2007) Breast Cancer Res Treat. 2011;129:495–503. doi: 10.1007/s10549-011-1475-5. [DOI] [PubMed] [Google Scholar]
- 37.Schlumberger M, Challeton C, De Vathaire F, Travagli JP, Gardet P, Lumbroso JD, Francese C, Fontaine F, Ricard M, Parmentier C. Radioactive iodine treatment and external radiotherapy for lung and bone metastases from thyroid carcinoma. J Nucl Med. 1996;37:598–605. [PubMed] [Google Scholar]
- 38.Bernier MO, Leenhardt L, Hoang C, Aurengo A, Mary JY, Menegaux F, Enkaoua E, Turpin G, Chiras J, Saillant G, Hejblum G. Survival and therapeutic modalities in patients with bone metastases of differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2001;86:1568–1573. doi: 10.1210/jcem.86.4.7390. [DOI] [PubMed] [Google Scholar]