Abstract
Common dermatological side-effects associated with erlotinib, epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI), include pruritus and skin rash, which are mediated by substance P, leading to the occasional discontinuation of cancer treatment. Aprepitant is an antagonist of neurokinin-1 receptor, through which substance P activates the pruritogens. Thus, aprepitant is expected to offer a promising option for the treatment of erlotinib-induced pruritus. However, the appropriate treatment schedule for aprepitant administration is under consideration. Here, we discuss the need for flexible adjustment of the treatment schedule for aprepitant administration against erlotinib-induced refractory pruritus and skin rush. A 71-year-old female smoker presented with stage IV EGFR-mutated lung adenocarcinoma. She was started on erlotinib at 150 mg/day. However, by 28 days, severe pruritus and acneiform skin rush resistant to standard therapies occurred, resulting in the interruption of erlotinib therapy. After recovery, she was restarted on erlotinib at 100 mg/day. However, severe pruritus and skin rush developed again within 2 weeks. Then, we started the first 3-day dose of aprepitant (125 mg on day 1, 80 mg on day 3, and 80 mg on day 5) based on the results of the previous prospective study, which showed the success rate of 100% with at least the second dose of aprepitant. However, the pruritus and skin rush exacerbated again within 4 weeks. Therefore, we started the second 3-day dose of aprepitant, but in vain. At this point, as the patient-centered medicine, bi-weekly schedule of the 3-day dose of aprepitant was considered and, then, adopted. As the results, the pruritus and skin rush remained well-controlled throughout the subsequent treatment with erlotinib.
Key Words: Lung cancer, Erlotinib, Pruritus, Skin rush, Aprepitant
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), such as gefitinib, erlotinib, and afatinib, show marked responses to advanced lung cancer harboring the sensitizing EGFR mutation [1]. Common dermatological side-effects associated with EGFR-TKIs include pruritus and skin rash. The incidence of pruritus induced by erlotinib is known as 9–13%, leading to the occasional dose modifications or discontinuation of cancer treatment on the basis of the worsened quality of life [2]. Although the pathogenesis of pruritus during the treatment with EGFR-TKIs is not completely understood, substance P is known as an important neuromediator of pruritus [3]. Aprepitant is the first commercially available drug of a new class of neurokinin-1 receptor antagonists for treating chemotherapy-induced nausea and vomiting. The dominant ligand for the neurokinin-1 receptor is substance P. Recent reports described the improvement in erlotinib-induced pruritus after aprepitant administration [4, 5]. However, the appropriate treatment schedule for aprepitant administration is under consideration. Therefore, in the present case report, we discuss the need for flexible adjustment of the treatment schedule for aprepitant administration against erlotinib-induced refractory pruritus and skin rush.
Case Report
A 71-year-old female smoker presented with stage IV lung adenocarcinoma harboring the EGFR exon 21 L858R mutation. She had no history of drug allergies or autoimmune disease. She was started on erlotinib at 150 mg/day for the treatment. Although she had been taking medications over many years, including calcium-channel blocker for hypertension and statin for hyperlipidemia, erlotinib was her only new medication. Then, by 28 days after the start of erlotinib therapy, she presented with severe pruritus of grade 3 according to the National Cancer Institute's Common Toxicity Criteria. The pruritus was resistant to local application of steroid ointment and to standard systemic therapies, including oral steroids and antihistamines. Furthermore, the pruritus was also linked to acneiform skin rush, leading to the interruption of erlotinib therapy for a period of 2 weeks.
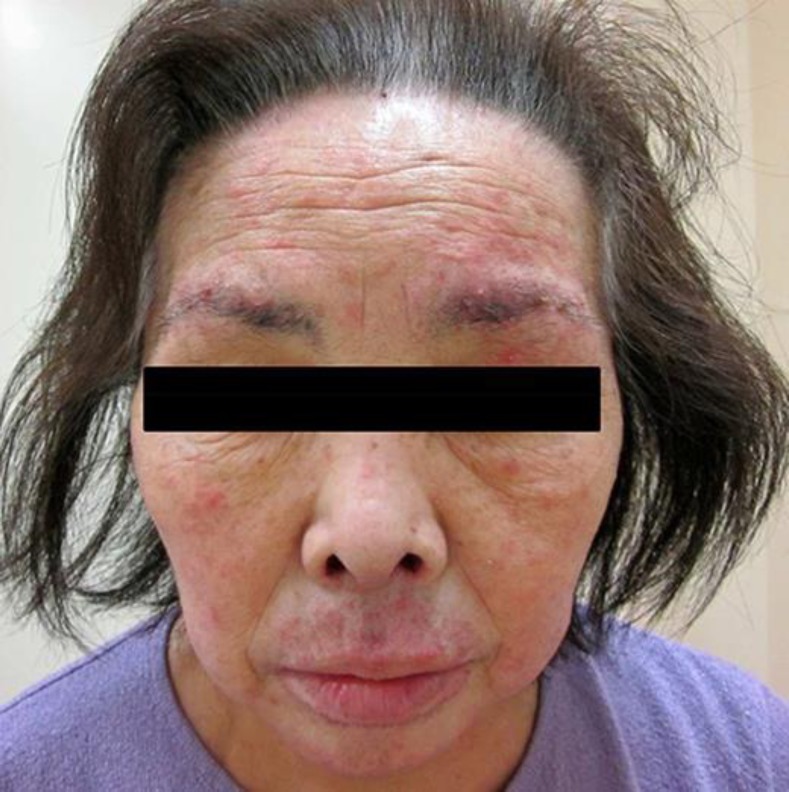
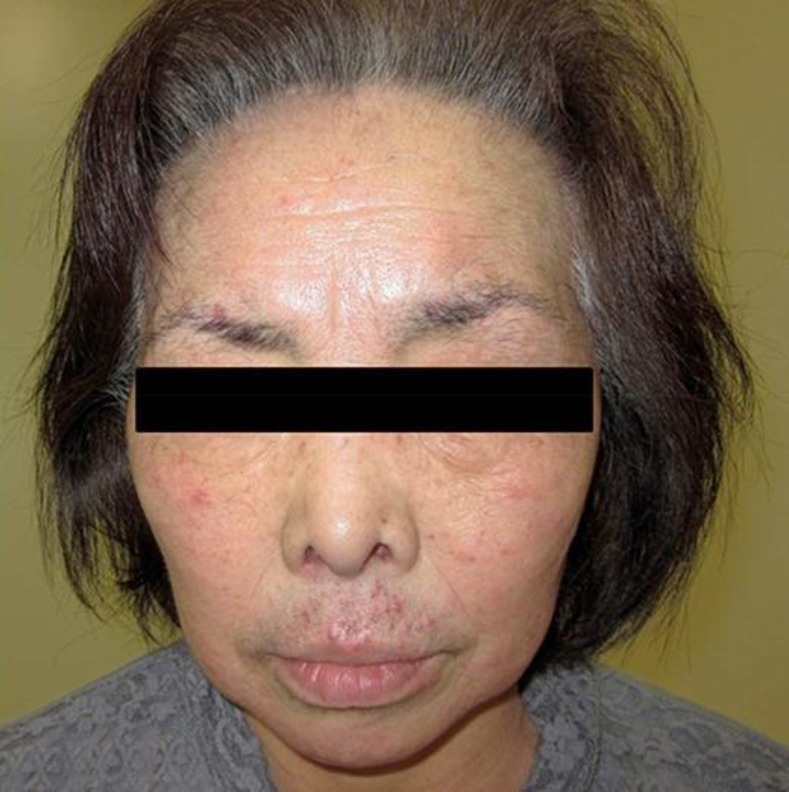
After recovering from these skin side-effects, she was restarted on erlotinib at a reduced dose of 100 mg/day. Oral steroids and antihistamines were continued to prevent recurrence of the pruritus and skin rush. However, within 2 weeks after restarting erlotinib, severe pruritus of grade 3 developed again, followed by acneiform skin rush especially on her face (Fig. 1). An evaluation of the pruritus by means of a visual analogue scale (VAS), in which a score of 0 indicates no pruritus and a score of 10 indicates the worst pruritus imaginable, resulted in a score of 8. Therefore, erlotinib was again discontinued, and she was started on aprepitant at 125 mg on day 1 after discontinuation, 80 mg on day 3, and 80 mg on day 5 with the aim of treating the pruritus and skin rush. This treatment schedule for aprepitant administration was decided after studying the various treatment schedules on the basis of the scientific reports of aprepitant administration [4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16]. Then, the prompt improvement was observed within 5 days after starting the first dose of aprepitant, leading to a score of 2 for the pruritus on the VAS (Fig. 2).
Fig. 1.
Within 2 weeks after restarting erlotinib, severe pruritus developed again, followed by acneiform skin rush especially on her face.
Fig. 2.
Within 5 days after starting the first 3-day dose of aprepitant, the prompt improvement of the pruritus and skin rush was observed.
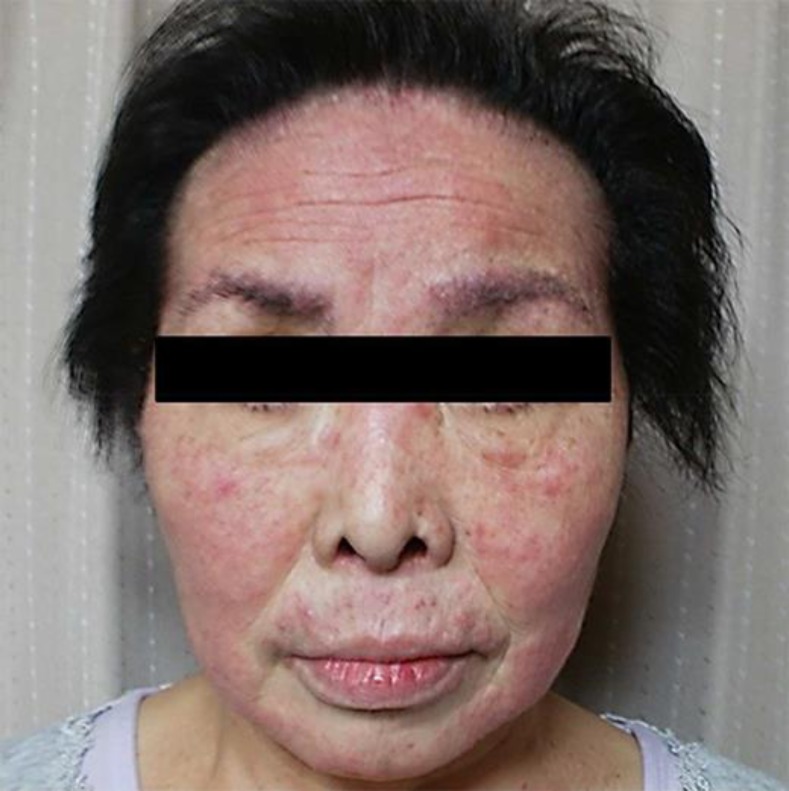
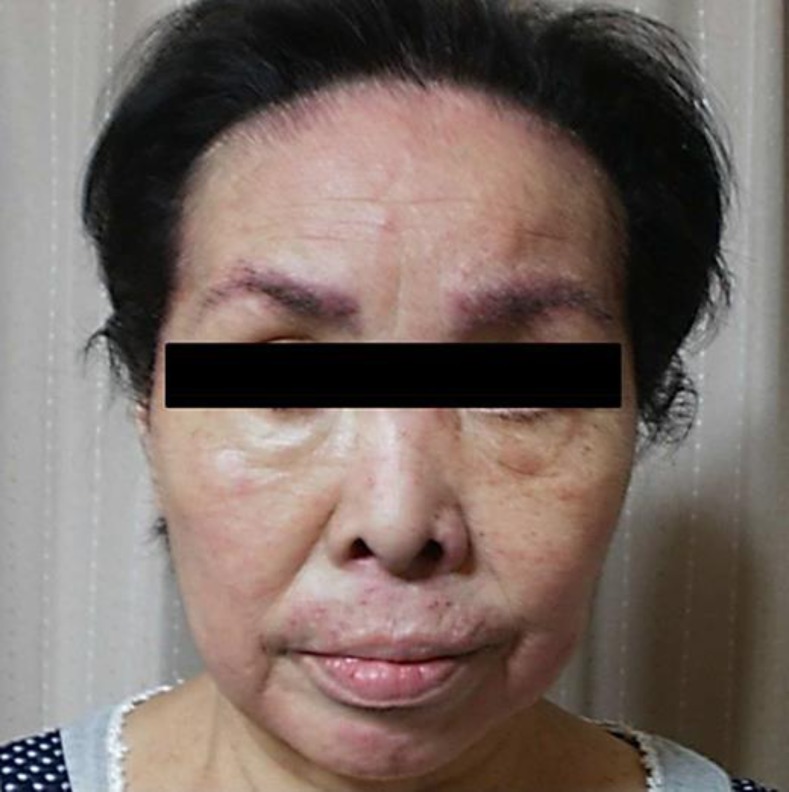
After this recovery, she was restarted on erlotinib at 100 mg/day. However, the pruritus and skin rush gradually exacerbated thereafter, leading to scores of 4 and 8 for the pruritus on the VAS within 2 and 4 weeks, respectively (Fig. 3). Then, by starting the second dose of aprepitant, the prompt improvement was again observed within 5 days, leading to a score of 2 for the pruritus on the VAS. However, the pruritus and skin rush eventually exacerbated within 4 weeks. At this point, bi-weekly schedule for aprepitant administration (125 mg on day 1, 80 mg on day 3, and 80 mg on day 5, every 2 weeks) was considered and, then, adopted. As the results, throughout the subsequent treatment with erlotinib, the pruritus and skin rush remained well-controlled within the scores of 2 and 4 for the pruritus on the VAS (Fig. 4).
Fig. 3.
Within 4 weeks after starting the first 3-day dose of aprepitant, the pruritus and skin rush again exacerbated.
Fig. 4.
After adopting the bi-weekly schedule of the 3-day dose of aprepitant, the pruritus and skin rush remained well-controlled throughout the subsequent treatment with erlotinib.
Discussion
Regarding the mechanism of erlotinib-induced pruritus, erlotinib is known to induce the secretion of stem-cell factor and the subsequent accumulation of dermal mast cells in the lesional skin of patients with erlotinib-induced rash [3]. Substance P, a tachykinin neuropeptide, activates these mast cells through the neurokinin-1 receptor and causes the release of cytokines and chemokines such as histamine, prostaglandin D2, and leukotriene B4, which mediate pruritus [3]. Actually, injected substance P into the skin of non-atopic patients induces a pruritus response in normal and inflamed skin [17]. Moreover, the mast cells of patients with chronic pruritus have an increased number of neurokinin-1 receptors [18]. On the other hand, aprepitant is known to block the mast-cell degranulation mediated by the neurokinin-1 receptor [3]. Thus, aprepitant is expected to offer a promising option for the treatment of erlotinib-induced pruritus.
Recently, there have been the various successful scientific reports of aprepitant administration for the treatment of refractory pruritus. In these reports, the treatment schedules are not unified, showing 125 mg on day 1, 80 mg on days 3 and 5 [6]; 125 mg on day 1, 80 mg on days 2–3 [4, 7]; 125 mg on day 1, 80 mg on days 2–3, followed by alternating days of 125mg and 80mg thereafter [8]; 125 mg on day 1, 80 mg on days 2–3, every 2 weeks [9, 10]; 80 mg/day [5, 11, 12, 13, 14, 15]; and 80 mg, every 3 days [16]. Notably, Santini et al. have conducted a prospective pilot study for evaluating the efficacy of aprepitant administration in 45 patients, with metastatic solid tumors, treated with EGFR-targeted biological drugs, and with first onset of severe pruritus during the treatment [6]. Among them, 24 (53%) patients had lung cancer, and 16 (36%) patients were treated with erlotinib. Patients were treated with aprepitant at 125 mg on day 1, 80 mg on day 3, and 80 mg on day 5. As the results, 39 (87%) patients showed no recurrence during the study period of 90 days after the first dose of aprepitant. In contrast, 6 (13%) patients showed recurrence after a median follow-up period of 7 weeks from the first dose of aprepitant. However, none of these 6 patients developed a new recurrence after the second dose of aprepitant.
On the basis of these results, we adopted the same treatment schedule for aprepitant administration at first, expecting the success rate of 87%, but in vain. Therefore, we started the second dose of aprepitant after 4 weeks from the first dose of aprepitant, expecting the success this time, but in vain again. According to the results of the prospective study, we should have been able to inhibit the recurrence of erlotinib-induced pruritus and skin rush with at least the second dose of aprepitant. However, we realized that the results of the study did not serve as a useful reference for our patient. Furthermore, at last, we recognized the need and importance to flexibly adjust the treatment schedule for aprepitant administration as the patient-centered medicine. Thus, considering that the pruritus and skin rush exacerbated especially after 2 weeks from the start of aprepitant administration, bi-weekly schedule (aprepitant at 125 mg on day 1, 80 mg on day 3, and 80 mg on day 5, every 2 weeks) was adopted for our patient, leading to the maintained success thereafter.
By the way, we did not adopt the treatment schedules for daily aprepitant administration although relatively many case reports have been published [5, 8, 11, 12, 13, 14, 15]. This is because little is known about the risk of erlotinib-aprepitant pharmacokinetic interactions. Aprepitant is an isoenzyme cytochrome P-450 3A4 isoform (CYP3A4) inhibitor [19]. On the other hand, CYP3A4 is involved in the metabolism of erlotinib and is approximately 70% responsible for the clearance [20]. Actually, when aprepitant at a dose of 80mg/day was added during the treatment with erlotinib at a dose of 150 mg/day, trough plasma levels of erlotinib as assessed on liquid chromatography were 1,210, 2,455, and 2,440 ng/mL on days 0, 7, and 14, respectively [5]. The doubling of the trough plasma levels of erlotinib after the initiation of aprepitant supports the hypothesis that aprepitant can significantly decrease the erlotinib clearance through the inhibition of CYP3A4.
In conclusion, to date, the confirmed treatment schedule for aprepitant administration against erlotinib-induced refractory pruritus and skin rush has not yet been established although the various treatment schedules have been tried. In this case report, we have discussed the need for flexible adjustment of the treatment schedule as the patient-centered medicine.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors declare that they have no relevant financial interests.
References
- 1.Nan X, Xie C, Yu X, Liu J. EGFR TKI as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget. 2017 Aug;8((43)):75712–26. doi: 10.18632/oncotarget.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch TJ, Jr, Kim ES, Eaby B, Garey J, West DP, Lacouture ME. Epidermal growth factor receptor inhibitor-associated cutaneous toxicities: an evolving paradigm in clinical management. Oncologist. 2007 May;12((5)):610–21. doi: 10.1634/theoncologist.12-5-610. [DOI] [PubMed] [Google Scholar]
- 3.Gerber PA, Buhren BA, Homey B. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011 Feb;364((5)):486–7. doi: 10.1056/NEJMc1013027. [DOI] [PubMed] [Google Scholar]
- 4.Vincenzi B, Tonini G, Santini D. Aprepitant for erlotinib-induced pruritus. N Engl J Med. 2010 Jul;363((4)):397–8. doi: 10.1056/NEJMc1003937. [DOI] [PubMed] [Google Scholar]
- 5.Mir O, Blanchet B, Goldwasser F. More on aprepitant for erlotinib-induced pruritus. N Engl J Med. 2011 Feb;364((5)):487. doi: 10.1056/NEJMc1013027. [DOI] [PubMed] [Google Scholar]
- 6.Santini D, Vincenzi B, Guida FM, Imperatori M, Schiavon G, Venditti O. Aprepitant for management of severe pruritus related to biological cancer treatments: a pilot study. Lancet Oncol. 2012 Oct;13((10)):1020–4. doi: 10.1016/S1470-2045(12)70373-X. [DOI] [PubMed] [Google Scholar]
- 7.Borja-Consigliere HA, López-Pestaña A, Vidal-Manceñido MJ, Tuneu-Valls A. Aprepitant in the treatment of refractory pruritus secondary to cutaneous T-cell lymphoma. Actas Dermosifiliogr. 2014 Sep;105((7)):716–8. doi: 10.1016/j.ad.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Vincenzi B, Fratto ME, Santini D, Tonini G. Aprepitant against pruritus in patients with solid tumours. Support Care Cancer. 2010 Sep;18((9)):1229–30. doi: 10.1007/s00520-010-0895-9. [DOI] [PubMed] [Google Scholar]
- 9.Booken N, Heck M, Nicolay JP, Klemke CD, Goerdt S, Utikal J. Oral aprepitant in the therapy of refractory pruritus in erythrodermic cutaneous T-cell lymphoma. Br J Dermatol. 2011 Mar;164((3)):665–7. doi: 10.1111/j.1365-2133.2010.10108.x. [DOI] [PubMed] [Google Scholar]
- 10.Jiménez Gallo D, Albarrán Planelles C, Linares Barrios M, Fernández Anguita MJ, Márquez Enríquez J, Rodríguez Mateos ME. Treatment of pruritus in early-stage hypopigmented mycosis fungoides with aprepitant. Dermatol Ther (Heidelb) 2014 May-Jun;27((3)):178–82. doi: 10.1111/dth.12113. [DOI] [PubMed] [Google Scholar]
- 11.Duval A, Dubertret L. Aprepitant as an antipruritic agent? N Engl J Med. 2009 Oct;361((14)):1415–6. doi: 10.1056/NEJMc0906670. [DOI] [PubMed] [Google Scholar]
- 12.Villafranca JJ, Siles MG, Casanova M, Goitia BT, Domínguez AR. Paraneoplastic pruritus presenting with Hodgkin's lymphoma: a case report. J Med Case Reports. 2014 Sep;8((1)):300. doi: 10.1186/1752-1947-8-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladizinski B, Bazakas A, Olsen EA. Aprepitant: a novel neurokinin-1 receptor/substance P antagonist as antipruritic therapy in cutaneous T-cell lymphoma. J Am Acad Dermatol. 2012 Nov;67((5)):e198–9. doi: 10.1016/j.jaad.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Ständer S, Siepmann D, Herrgott I, Sunderkötter C, Luger TA. Targeting the neurokinin receptor 1 with aprepitant: a novel antipruritic strategy. PLoS One. 2010 Jun;5((6)):e10968. doi: 10.1371/journal.pone.0010968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres T, Fernandes I, Selores M, Alves R, Lima M. Aprepitant: evidence of its effectiveness in patients with refractory pruritus continues. J Am Acad Dermatol. 2012 Jan;66((1)):e14–5. doi: 10.1016/j.jaad.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Song JS, Tawa M, Chau NG, Kupper TS, LeBoeuf NR. Aprepitant for refractory cutaneous T-cell lymphoma-associated pruritus: 4 cases and a review of the literature. BMC Cancer. 2017 Mar;17((1)):200. doi: 10.1186/s12885-017-3194-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen JS, Sonne M, Benfeldt E, Jensen SB, Serup J, Menné T. Experimental itch in sodium lauryl sulphate-inflamed and normal skin in humans: a randomized, double-blind, placebo-controlled study of histamine and other inducers of itch. Br J Dermatol. 2002 May;146((5)):792–800. doi: 10.1046/j.1365-2133.2002.04722.x. [DOI] [PubMed] [Google Scholar]
- 18.Wallengren J. Neuroanatomy and neurophysiology of itch. Dermatol Ther. 2005 Jul-Aug;18((4)):292–303. doi: 10.1111/j.1529-8019.2005.00041.x. [DOI] [PubMed] [Google Scholar]
- 19.Mir O, Coriat R. Aprepitant for pruritus: drug-drug interactions matter. Lancet Oncol. 2012 Oct;13((10)):964–5. doi: 10.1016/S1470-2045(12)70397-2. [DOI] [PubMed] [Google Scholar]
- 20.Rakhit A, Pantze MP, Fettner S, Jones HM, Charoin JE, Riek M. The effects of CYP3A4 inhibition on erlotinib pharmacokinetics: computer-based simulation (SimCYP) predicts in vivo metabolic inhibition. Eur J Clin Pharmacol. 2008 Jan;64((1)):31–41. doi: 10.1007/s00228-007-0396-z. [DOI] [PubMed] [Google Scholar]