Abstract
Objectives.
This study evaluated the efficacy of a thiourethane(TU)-modified silane agent in improving properties in filled composites.
Methods.
The TU-silane agent was synthesized by combining 1,3-bis(1-isocyanato-1-methylethyl)benzene and 3-(triethoxysilyl)propyl isocyanate with trimethylol-tris-3-mercaptopropionate (TMP), at 1:2 isocyanate:thiol, leaving pendant thiol and alkoxy silane groups. Barium glass fillers (1 μm average particle size) were functionalized with 5 wt% TU-silane in an acidic ethanol solution. Commercially available 3-(trimethoxysilyl)propyl methacrylate (MA-silane) and (3-mercaptopropyl)trimethoxysilane (SH-silane), as well as no silane treatment (NO-silane), were used as controls. Composites were made with BisGMA-UDMA-TEGDMA (5:3:2), camphorquinone/ethyl-4-dimethylaminobenzoate(0.2/0.8 wt%) and di-tert-butyl hydroxytoluene (0.3 wt%) and 70 wt% silanated inorganic fillers. Polymerization stress (PS) was measured using a cantilever beam apparatus (Bioman). Methacrylate conversion (DC) and rate of polymerization (RP) during photoactivation (800 mW/cm2) were followed in real-time with near-IR. Flexural strength/modulus (FS/FM) were evaluated in three-point bending with 2 × 2 × 25 mm. Statistical analysis: 2-way ANOVA/Tukey’s test (α = 5%).
Results.
DC, Rpmax and E were similar for all groups tested. FS was similar for the TU- and MA-silane, which were statistically higher than the untreated and SH-silane groups. Stress reductions in relation to the MA-silane were observed for all groups, but statistically more markedly for the TU-silane material. This is likely due to stress relaxation and/or toughening provided at the filler interface by the oligomeric TU structure.
Significance.
TU-silane oligomers favorably modified conventional dimethacrylate networks with minimal disruption to existing curing chemistry, in filled composites. For the same conversion values, stress reductions of up to 50% were observed, without compromise to mechanical properties or handling characteristics.
Keywords: Pre-polymerized additives, Resin composite, Polymerization
1. Introduction
Despite the low annual failure rates (1–3%) observed for composite restorations in posterior teeth, with most failures observed being related to secondary caries and fractures of the tooth structure or restorative materials, on average, restorations last about 10 years in service [1–3]. Secondary caries is significantly affected by risk factors associated to the patients [4–6], but the presence of defects at the restoration margins can increase the likelihood of bacterial recolonization and reestablishment of the disease in the region [7–9]. Further, the fact that the composite undergoes polymerization, and consequently shrinkage, confined by the cavity walls leads to the transfer of stresses to the bonded interface, which in turn favor the development of marginal defects [10–12], as well as cracks on the tooth structure [12,13]. Therefore, clinical strategies and material developments have been attempted with the goal of minimizing the deleterious effects associated with polymerization shrinkage of resin-based materials [11,12].
The main modifications in the organic matrix of composites in the last years focused on developing low-shrinking monomers, either via lower molar shrinkage coefficients, as is the case in the epoxide-based material that polymerizes via a ring-opening mechanism (i.e., silorane monomers), or via higher molecular weight monomers, or via the addition of pre-polymerized additives [11,14,15]. This was done with the assumption that reduction in polymerization shrinkage would necessarily lead to reduced polymerization stress and, in turn, better restoration longevity [10]. Clinical studies have demonstrated that this is not always the case [5,10]. In this context, thiourethane oligomers have been proposed as an alternative stress reducing additive, allying the higher molecular weight of the oligomer with thiol chemistry, which affords lower stress through delayed gelation and vitrification [16–19]. The rationale is that the thiol functional groups work as chain transfer agents to promote a radically assisted step-growth polymerization of methacrylates [18,19]. In fact, several prior studies demonstrated the effectiveness of the addition of thiourethane oligomers on polymerization stress reduction and depth of polymerization increase, which in turn relates to the high refractive index of these oligomers [20–23].
However, it has also been demonstrated that the effect of thiourethane depends on its concentration, and that the addition of this oligomer to resin matrix is somewhat limited due to viscosity concerns [18,19]. Higher concentrations of thiourethane increase the resin viscosity, complicating the addition of fillers and compromising the handling char acteristics of composite. One possible solution to improve the distribution of such oligomers within the composite is to tether them to the filler particles. Other studies have demonstrated that polymer brush functionalization may improve wetting of silicon-containing surfaces, and actually improve their interaction with relatively hydrophobic monomers [24,25]. In the case of particle–particle interactions, which are of interest to the composite application, it is known that the functionalization of the filler surface greatly influences particle packing [26].
Therefore, the present study aimed to investigate the effect of filler silanization with a thiourethane oligomer on polymerization kinetics, mechanical properties and polymerization stress of filled methacrylate-based dental composites, as compared to fillers silanized with a conventional methacrylate silane. The null hypothesis was that the silane type does not affect the outcomes evaluated.
2. Material and methods
2.1. Experimental design
This study was designed as a single factor evaluation of the type/presence of silane in five levels. Non-silanized fillers were treated with methacrylate, thiol or thiourethane silanes, while filler silanized by manufacturer using a methacrylate silane and filler without any surface treatment (non-silanized) were used as positive and negative controls, respectively. The variable responses were polymerization kinetics and stress; flexural strength and elastic modulus of composites assessed by 3-point bending test.
2.2. Synthesis of thiourethane
Except where noted, all reagents used in this study were purchased from Sigma-Aldrich (St. Louis, MO, USA) and used without further purification. The thiourethane silane was synthesized using trimethylolpropane tris(3-mercaptopropionate) (TMP), 1,3-bis(1-isocyanato-1-methylethyl) benzene (BDI) and 3-(triethoxysilyl)propyl isocyanate, at 2.5:1:1 mol ratios. Reagents were mixed in a 100 mL round-bottom flask containing 50 mL dichloromethane, under magnetic stirring at room temperature, using nitrogen gas as the reaction atmosphere for 30 min. After this time, three drops of triethylamine were added, followed by additional magnetically stirring over an ice bath for 24 h. Reaction completion was confirmed by the disappearance of the isocyanate peak in mid-IR (2270 cm−1). The material was then purified by precipitation in hexanes, and vacuum extraction. The complete consumption of isocyanate was verified by 1H Nuclear Magnetic Resonance — NMR (Bruker AMX-400 MHz, Santa Barbara, CA, USA) using dimethyl sulfoxide-d6 as solvent.
2.3. Filler silanization
Non-silanized aluminum–barium–borosilicate glass fillers (ref. 8235; Schott, Elmsford, NY, USA) with 1 μm average particle size were used in this study, and silanized with either nothing (negative control), 3-(trimethoxysilyl) propryl methacrylate (experimental methacrylate control), (3-mercaptopropyl) triethoxysilane (thiol) or the newly synthesized thiourethane silane (TU-silane). The same filler silanized by the manufacturer with a methacrylate silane was used as the commercial methacrylate control. An ethanol solution (80.0 vol%) with milli-Q water was prepared in a polyethylene bottle. The pH was adjusted with 1 M acetic acid at 4.5, stabilized for 24 h at room temperature. The silanization procedure was carried-out by addition of 5.0 g of filler and2.0 vol% of silane to 65 ml of ethanol solution. The solution was stirred for 24 h at room temperature, followed by decantation and filtration, and a final wash with n-hexanes. The filtered fillers were dried in an oven at 37 °C for 48 h, then carefully ground using a glass piston. Particles were then treated in a ball mill to ensure there were no clusters, as assessed by inspection in a stereomicroscope.
In order analyze the efficacy of silanization procedure performed, pellets were produced by mixing less than 1.0% of silanized fillers with KBr under pressure. The pellets were analyzed in mid-IR (Nicolet 6700, Thermo Scientific, Madison, WI, EUA). Additionally, the silanization procedure efficacy was assessed by thermogravimetric analysis (Pyris 7, Perkin-Elmer, Fremont, CA, USA). The weight change of samples as a function of temperature was evaluated with temperature ramping from 50 to 850 °C at 10 °C/min under a nitrogen flow of 20 mL/min.
2.4. Composites formulation
The same resin matrix consisting of 50 wt% BisGMA (bisphenol A diglycidyl dimethacrylate), 30 wt% UDMA (urethane dimethacrylate), and 20 wt% TEGDMA (tri-ethylene glycol dimethacrylate), all from Esstech (Essington, PA, USA), was used in all experiments. The photo-initiation system consisted of 0.2 wt% dl-camphoroquinone and 0.8 wt% tertiary amine (EDMAB [ethyl 4-dimethylaminobenzoate]). 0.1 wt% 2,6-di-tert-butyl-4-methylphenol (BHT) was added as inhibitor. Fillers silanized according to the experimental groups described above were added to the resin at 70 wt% using a mechanical mixer (DAC 150 FVZ SpeedMixer, Flacktek, Lan-drum, SC, USA).
2.5. Degree of conversion and polymerization kinetics
The experimental composites were inserted into a rubber mold (10 mm in diameter and 0.8 mm thick; n = 3) between two glass sides and positioned the chamber of the spectrom eter Nicolet 6700 (Thermo Scientific, Madison, WI, USA). The composites were irradiated for 40 s using the light-curing unit Elipar S10 (3 M ESPE, St. Paul, MN, USA) with irradiance of 1200 mW/cm2. The polymerization kinetics was monitored in near-IR for 5 min with 2 scans/spectrum, 4 cm−1 resolution, 2 Hz data acquisition rate. The degree of conversion (DC) was calculated based on the area of the methacrylate vinyl overtone at 6165 cm−1. The polymerization rate (Rp) was calculated as the first derivative of the conversion vs. time curve. The specimens used for polymerization kinetics analysis were stored for 72 h, and conversion was measured again at that time.
2.6. Three-point bending test
Bar specimens (2 × 2 × 25 mm) were built using silicone molds between two glass sides (n = 5). The composites were light-cured by three overlapping 40 s exposures of 800 mW/cm2 (Elipar S10) on both sides, according to ISO 4049 (Standard I; 2009) and stored dry for 24 h in the dark. After storage, the dimensions of the bars were checked with a digital caliper accurate to 0.01 mm (Mitutoyo Corporation, Tokyo, Japan), and the specimens were positioned in a 3-point bending device coupled to a mechanical testing system (Q-test, MTS, Eden Prairie, WI, USA). The distance between supports was 20 mm and the load was applied to the center of specimen. The diameter of both supports and of the loading rod was 2 mm. The tests were performed at a crosshead speed of 1.0 mm/min until failure and was monitored by the testing machine software (TestWorks v.3.08, MTS Inc., Orono, ME, USA.). The flexural strength (σf) was calculated by the following equation:
| (1) |
where F is the maximum load (N) exerted on the specimen, l is the distance (mm) between the supports, and b is the width (mm) and h the height (mm) at the center of the specimen. The Ef was calculated using the following equation:
| (2) |
where F1 is the load (N) exerted on the specimen and d is the deflection corresponding to the load F1.
2.7. Polymerization stress
Polymerization stress development was followed in real-time for 5 min using the Bioman (n = 5) [27]. The composites were placed over a glass plate treated with a commercial silane coupling agent (Ceramic Primer, 3M-ESPE, St. Paul, MN, USA) held to the device with a bolt, through which the tip of light-curing unit (Elipar S10) was positioned. A 5.6-mm diameter metallic rod treated with metal primer (Z-prime plus, Bisco, Schaumburg, IL) served as the opposite bonding substrate, to produce disk specimens with 0.75 mm thickness (configuration factor = 2.8). The photo-activation was carried-out for 40 s at 800 mW/cm2 (intensity reaching the specimen) and the polymerization stress assessed from the displacement of the cantilever of the apparatus. The load was recorded by strain-gauge load cell and data converted into MPa.
2.8. Statistical analysis
Data analysis was performed using the SigmaStat v.3.5 statistical software package (Systat Software Inc., Chicago, IL, USA). Data were individually analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. The significance level was set at α = 0.05 for all analyses.
3. Results
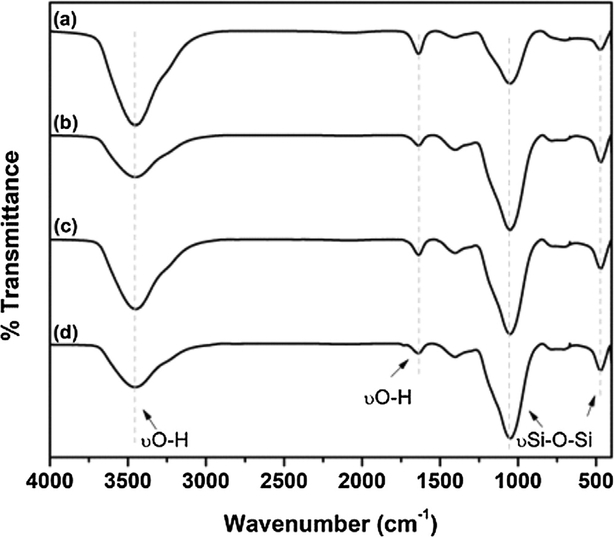
The chemical changes in the filler surface after the silanization treatments are illustrated by mid-IR spectroscopy (Fig. 1). Characteristic absorption bands of nano silica were observed at 1050 and 461 cm−1 due to the asymmetric and symmetric stretching vibration of Si–O–Si structure [28]. A broad sharp band at around 3455 cm−1 and a sharp band at 1636 cm−1, observed in all samples, are associated with hydroxyl groups that are present on nano silica surface [28–30]. Table 1 presents the peak intensity ratios of the main functional group (-OH) changed after silanization treatments. After silanization the peak ratios I3455/1050 and I1636/1050 significantly decreased for the three silane coupling agents.
Fig. 1 -.
Mid-IR spectra of (a) non-silanated, (b) methacrylate,(c) thiol and (d) thiourethane fillers. Note the reduction on functional −OH group and increase of Si–O–Si group indicating the effectiveness of the silanization process.
Table 1 -.
Peak intensity ratios of the main functional group (−OH) measured at Mid-IR according to silanization treatments.
| Treatment | I3455/1050 | I1636/1050 |
|---|---|---|
| Unsilanized | 1.80 | 0.43 |
| Methacrylate | 0.44 | 0.11 |
| Thiol | 0.74 | 0.16 |
| Thiourethane | 0.46 | 0.10 |
I = peak intensity.
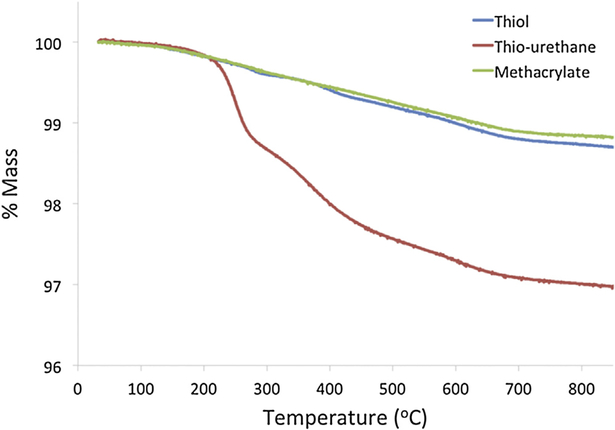
Fig. 2 shows the thermogravimetric curves of silanized fillers. The first weight loss, in temperature from 40 °C to about 120 °C, is attributed to the removal of water and ethanol physically adsorbed and it corresponds to less than 0.05 wt% for all the samples [29]. The more significant weight loss can be observed starting at around 200 °C, and is caused mainly by the degradation of the silane coupling agent compounds on the nano-silica surface [28]. The methacrylate and thiol fillers presented weight loss of 1.18 and 1.30%, respectively, while the thiourethane silanes presented weight loss of 3.03%.
Fig. 2 -.
Thermogravimetric curves presenting the weight loss of fillers as function of the temperature increase according to the silane type. Since only the organic phase is lost, reductions on filler mass indicates that the fillers were covered by silane. Note the higher weight loss observed for thiourethane silane.
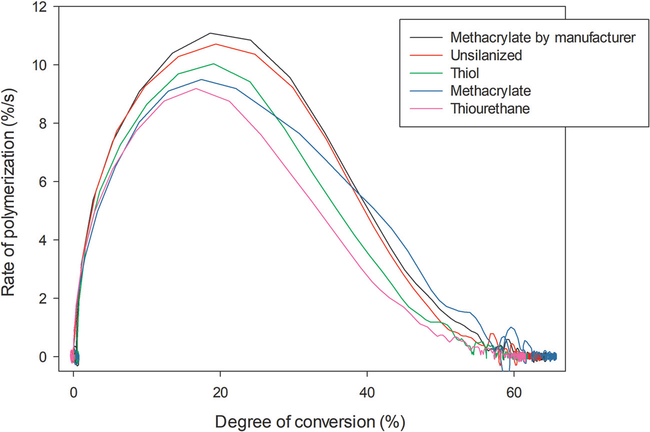
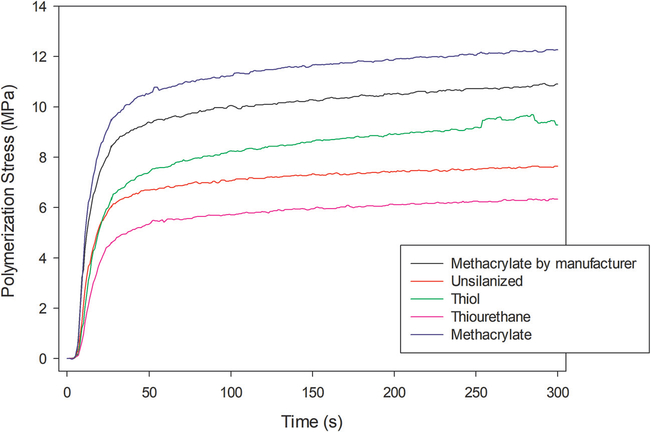
The results of the polymerization kinetics are illustrated in Fig. 3 and Table 2. At the end of the kinetics evaluation (5 min), composites with fillers silanized using thiol or the commercial methacrylate control showed the highest DC, while no difference was observed among the other silanization conditions. The silanization process did not affect the maximum rate of polymerization nor the conversion measured after 72 h. Regarding flexural strength (Table 2), fillers silanized with methacrylate and non-treated (negative control) resulted in composites with the lowest values of flexural strength. In contrast, the silanization process did not affect the elastic modulus of composites (Table 2). The stress development during the polymerization is presented in Fig. 4. The values of maximum polymerization stress are presented in Table 2. Composites containing methacrylate-silanized fillers presented the highest values of stress, whereas the silanization with thiourethane resulted in the lowest values.
Fig. 3 -.
Rate of polymerization (%/s) as a function of degree of conversion for the composites filled with particles silanized with different silanes. Results obtained with 0.8 mm thick specimens, with 1200 mW/cm2 reaching the top of the specimen.
Table 2 -.
Means (standard deviations) for data from conversion kinetics, mechanical properties and polymerization stress analysis. Statistical analyses were performed for each outcome comparing the effect of silanization treatments. For each outcome, distinct letters indicate statistical difference among the silane types at Tukey’s post hoc test (p < 0.05).
| Silanization | Degree of conversion (%) | Maximum rate of polymerization (%/s) | Flexural strength (MPa) | Elastic modulus (GPa) | Maximum polymerization stress (MPa) | |
|---|---|---|---|---|---|---|
| 5 min | 72 h | |||||
| Unsilanized | 62.9 (0.9) B | 78.4 (0.8) | 10.7 (0.6) | 77.8 (14.2) B | 6.5 (0.2) | 7.6 (0.1) D |
| Methacrylate by manufacturer | 64.9 (0.9) A | 79.7 (1.7) | 11.0 (2.0) | 126.4 (77.8) A | 6.4 (1.1) | 10.9 (0.5) B |
| Methacrylate | 61.3 (1.2) B | 76.6 (0.6) | 10.1 (1.6) | 95.6 (6.5) B | 5.6 (0.8) | 12.2 (0.4) A |
| Thiol | 64.5 (0.4) A | 78.5 (0.2) | 10.5 (1.9) | 143.4 (10.5) A | 6.1 (0.5) | 9.4 (0.4) C |
| Thiourethane | 61.2 (0.7) B | 77.8 (3.0) | 9.3 (0.6) | 126.6 (7.4) A | 5.8 (0.6) | 6.3 (0.4) E |
| P-value* | <0.001 | 0.256 | 0.614 | <0.001 | 0.213 | <0.001 |
P-values calculated by one-way ANOVA.
Fig. 4 -.
Development of polymerization stress as a function of time according to the silanization of fillers. The stress was measured for 5 min, using a configuration factor = 2.8, and 40 s of light-curing through a glass plate, with 1200 mW/cm2 reaching the surface of the specimen.
4. Discussion
Prior studies have demonstrated that adding thiourethane oligomers to the organic matrix of dental composites can reduce the polymerization stress and increase the DC, but might compromise the handling properties of the material by significantly increasing its viscosity when added at concentrations higher than 30 wt% [18]. The results of the present study showed that functionalizing the filler particles with thiourethane might be a good strategy to overcome this problem, since no obvious differences were noticed in the handling of the material, while also reducing the polymerization stress without effect to the mechanical properties of composite. Therefore, the hypothesis of study is rejected.
In addition to commercially silanized and non-silanized fillers, fillers silanized in house with methacrylate and thiol silanes were evaluated in the present study as controls. The rationale was to eliminate the variables intrinsic to the silanization synthesis procedure, such as concentration of silane, solvent solution used, agitation time, etc. The commercial controls were added as the industry gold standard. In fact, it was confirmed that composites containing methacrylatefunctionalized fillers either commercially or through the process performed in the present study presented similar results. Thiol-terminated silanes have been used widely in covalent immobilization of proteins or cells to glass or silicone surfaces [31,32]. The association between thiol-terminated silanes with acrylate to produce biomaterials is a more recent approach [33]. Specifically in the case of the present study, thiol silanes were utilized as a model chain-transfer molecule, for which some stress reduction could be expected [32]. This allowed us to decouple the potential effects the thiourethane silanes were hypothesized to have on stress reduction.
The effectiveness of the silanization process was assessed in the present study through two methods. Using mid-IR analysis, the changes on functional −OH group ratios was analyzed and significant reduction on intensity of peaks was observed after the filler silanization. These changes in the ratios confirmed the reduction of hydrophilic chemical structures in silanized fillers in accordance to prior studies [28,30]. Additionally, thermogravimetric analysis demonstrated that the silane mass concentration on the surface of the filler was silane-dependent, with the higher molecular weight thiourethane silane demonstrating the greatest mass loss via this method. Molecules containing only one alkoxy silane functionality, such as the methacrylate and thiol silane controls used in this study, are expected to result in mono-layer filler functionalization, since each molecule can only attach to one site on the surface of the filler [34,35]. This, allied with steric hindrance provided by the flexibility of the silane molecule, favors early saturation and incomplete surface coverage. As for the thiourethane oligomer silane, the high molecular weight and the fact that multiple alkoxy silane functionalities were available to react make it unlikely that a monolayer would be formed. In fact, prior studies demonstrated the ability of thiol-ene to bind to glass and silicone surfaces yielding polymer brush structures [36,37]. Even though the loosely crosslinked structure expected from the thiourethane is unlikely to form a polymer brush heap structure, it is reasonable to assume that the functionalization layer produced with the oligomer was thicker than the one formed with either the experimental controls. This hypothesis seems to be corroborated by the thermogravimetric analysis results, which demonstrated roughly twice the mass loss compared with the experimental controls. It is important to note that, while the increased thickness is very likely, it is also possible that a greater degree of functionalization per unit area of the filler surface was accomplished with the thiourethane silane, once there were more alkoxy silane functionalities to react, as already mentioned.
In comparison to the unsilanized fillers, it was expected that the silanization would increase the mechanical properties of composites as result of the chemical bonding between the silanized filler and the organic matrix [28]. In general, unsilanized fillers act as voids within the organic matrix, and can facilitate crack propagation [38]. In fact, composites containing fillers silanized commercially presented higher flexural strength than composites containing non-silanized fillers. However, when compared with the composites containing fillers silanized with methacrylate using the present method, the flexural strength increased by only about 20 MPa, and that was not statistically significant. This points to a less efficient silanization process accomplished in our laboratory, which is not surprising given the thorough optimization that is involved in industrial processes. This is exactly the reason why we produced the controls for this study in house. Again compared with the unsilanized fillers and the fillers silanized with methacrylates in house, composites containing fillers silanized with thiol or thiourethane silanes presented greater flexural strength, perhaps not surprisingly, again because of the covalent interaction provided with the organic matrix. However, the same process was used to silanize the fillers with methacrylates, and while that treatment did not increase the flexural strength in relation to the unsilanized control, the treatment with the thiols and thiourethanes did lead to increased flexural strength values. Those values were actually similar to those observed for composites containing filler particles silanized by the optimized method assumed to be used by the commercial manufacturer. Therefore, assuming that the efficiency of the method was similar for all silanes used in this study (it likely was not exactly the same), other factors need to be considered in explaining the increased flexural strength achieved by the thiol and thiourethane silanes in relation to the unsilanized control. One of them may be the fact that the thiol functionality forms a thiol-carbon bond via chain-transfer [16], and that bond is more flexible than carbon–carbon bonds that are formed via copolymerization of the methacrylate on the filler surface and on the organic matrix. This may have provided a toughening mechanism to absorb part of the energy of the crack propagating through the composite. In addition, the step-growth character of this reaction yields a more homogeneous polymer network with improved mechanical properties [16,17,39]. Both these factors help explain the increased flexural strength reached by filler silanized with thiol or thiourethane silanes.
Furthermore, thiol-methacrylate reactions delay the gelation and vitrification stages of polymerization via chain-transfer reactions, with consequent higher conversion and lower stress [17–23]. However, in the present study, silanization with thiols or thiourethanes did not affect RPmax, DC at RPmax nor ultimate DC. A prior study found that the addition of thiourethane directly in the resin matrix resulted in delayed gelation and vitrification and increased final DC [19]. However, the overall concentration of thiourethane in the material evaluated in that study was around 6 wt% since 20% of this oligomer was added to resin matrix of a composite filled at 70 wt%. Conversely, the thiourethane content in the composite evaluated in the present study was around 2.1 wt% considering that around 3% of the mass of the filler was covered according to TGA data. This seem to indicate that at this concentration, the thiourethane did not have enough pendant thiol bonds to significantly affect the polymerization kinetics, as is demonstrated on the polymerization rate as a function of conversion curves. Even though this property was not measured in this study, this also likely means the crosslinking density of the composites containing thiourethane fillers was similar to the methacrylated ones, which can be further speculated to have resulted in the similar values of elastic modulus observed in this study.
The most interesting finding of this study is that, despite the significantly lower thiourethane concentration compared to the studies where the oligomer was added directly to the matrix, and the absence of any effect on polymerization kinetics, composites containing fillers silanized with thiourethane still presented the lowest values of stress, approximately half of the value presented by the composites containing fillers functionalized with methacrylates in house and 40% lower than the commercial methacrylate silane. It is interesting to note that, in spite of the similar modulus, the materials containing the fillers silanized with methacrylates in house presented lower stress compared to the commercial methacrylate silane. This could be due to a less efficient coverage of the filler particle by the silane molecule with the in-house procedure. Even if that also applied to the thiourethane silane, this material reached stress values that were still statistically lower than the unsilanized filler. Previous studies have demonstrated that unsilanized fillers can be used to relieve part of the polymerization stress, but with detrimental effects to mechanical properties [38], as already mentioned. Therefore, it was expected that the unsilanized filler would lead to lower polymerization stress values compared to silanized fillers, which was indeed the case in relation to both methacrylates and thiol materials, but not in relation to the thiourethane silane. The thiol silane provided stress values intermediary to the methacrylate and thiourethane counterparts, but still statistically higher than the unsilanized filler. This is relevant for two reasons. (1) The thiol silane was included to test the hypothesis that chain-transfer agents tethered to the filler surface are able to delay gelation and reduce stress, but only the latter has been observed — as previously mentioned, the thiol concentration was likely not high enough to elicit the delayed gelation response [40], but was still able to alleviate part of the stress due to expected toughening of the material via the formation of flexible carbon–sulfur bonds [41]. (2) The chain-transfer effect was expected to be greater for the thiourethane silane because of the higher concentration of thiols expected with this material, but that was not observed either. Yet, the reduction in stress was even more significant than with the thiol silane. In this case, not only did the carbon–sulfur bonds likely provided stress relief, but the thio-carbamate bonds present in the thiourethane structure must have contributed to this effect [42]. In addition, other studies have demonstrated active strand behavior with oligomeric surface functionalization [43,44], and this will be further investigated in future studies utilizing stress relaxation experiments in dynamic mechanical analysis. In any event, it is important to highlight that the thiourethanemodified materials reached this lower stress result without compromise to the final conversion and elastic modulus.
In conclusion, silanization with thiourethane significantly reduced the polymerization stress, without compromising conversion and mechanical properties of composites. Since similar effects were previously observed by adding thiourethane to resin matrix, the incorporation of this oligomer to dental composites seems to be a promising strategy to obtain materials with reduced polymerization stress. Further studies associating the incorporation of thiourethane in both filler and resin matrix remain necessary to assess any possible synergistic effect.
Acknowledgments
The authors would like to express their gratitude to Dr. Jeffrey Stansbury and Dr. Parag Shah for conducting the thermogravimetrical analysis at the University of Colorado, Boulder. The authors also acknowledge funding from CAPES (ALFS: 99999.006169/2014–07; AS:200116/2014–2) and NIHNIDCR (CSP: R15 DE0232011; U01 DE023756; K02: DE025280).
R E F E R E N C E S
- [1].Pallesen U, van Dijken JW. A randomized controlled 30 years follow up of three conventional resin composites in Class II restorations. Dent Mater 2015;31:1232–44. [DOI] [PubMed] [Google Scholar]
- [2].Beck F, Lettner S, Graf A, Bitriol B, Dumitrescu N, Bauer P, et al. Survival of direct resin restorations in posterior teeth within a 19-year period (1996–2015): a meta-analysis of prospective studies. Dent Mater 2015;31:958–85. [DOI] [PubMed] [Google Scholar]
- [3].Ástvaldsdóttir Á, Dagerhamn J, van Dijken JW, Naimi-Akbar A, Sandborgh-Englund G, Tranæus S, et al. Longevity of posterior resin composite restorations in adults — a systematic review. J Dent 2015;43:934–54. [DOI] [PubMed] [Google Scholar]
- [4].Opdam NJ, van de Sande FH, Bronkhorst E, Cenci MS, Bottenberg P, Pallesen U, et al. Longevity of posterior composite restorations: a systematic review and meta-analysis. J Dent Res 2014;93:943–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJ. Longevity of posterior composite restorations: not only a matter of materials. Dent Mater 2012;28:87–101. [DOI] [PubMed] [Google Scholar]
- [6].van de Sande FH, Opdam NJ, Rodolpho PA, Correa MB, Demarco FF, Cenci MS. Patient risk factors’ influence on survival of posterior composites. J Dent Res 2013;92:78S–83S. [DOI] [PubMed] [Google Scholar]
- [7].Kuper NK, Opdam NJ, Ruben JL, de Soet JJ, Cenci MS, Bronkhorst EM, et al. Gap size and wall lesion development next to composite. J Dent Res 2014;93:108S–13S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Montagner AF, Maske TT, Opdam NJ, de Soet JJ, Cenci MS, Huysmans MC. Failed bonded interfaces submitted to microcosm biofilm caries development. J Dent 2016;52:63–9. [DOI] [PubMed] [Google Scholar]
- [9].Montagner AF, Opdam NJ, Ruben JL, Bronkhorst EM, Cenci MS, Huysmans MC. Behavior of failed bonded interfaces under in vitro cariogenic challenge. Dent Mater 2016;32:668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boaro LC, Fróes-Salgado NR, Gajewski VE, Bicalho AA, Valdivia AD, Soares CJ, et al. Correlation between polymerization stress and interfacial integrity of composites restorations assessed by different in vitro tests. Dent Mater 2014;30:984–92. [DOI] [PubMed] [Google Scholar]
- [11].Ferracane JL, Hilton TJ. Polymerization stress — is it clinically meaningful? Dent Mater 2016;32:1–10. [DOI] [PubMed] [Google Scholar]
- [12].Soares CJ, Faria-E-Silva AL, Rodrigues MP, Vilela ABF, Pfeifer CS, Tantbirojn D, et al. Polymerization shrinkage stress of composite resins and resin cements — what do we need to know? Braz Oral Res 2017;31:e62. [DOI] [PubMed] [Google Scholar]
- [13].Bicalho AA, Valdívia AD, Barreto BC, Tantbirojn D, Versluis A, Soares CJ. Incremental filling technique and composite material — part II: shrinkage and shrinkage stresses. Oper Dent 2014;39:E83–92. [DOI] [PubMed] [Google Scholar]
- [14].Moraes RR, Garcia JW, Barros MD, Lewis SH, Pfeifer CS, Liu J, et al. Control of polymerization shrinkage and stress in nanogel-modified monomer and composite materials. Dent Mater 2011;27:509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maghaireh GA, Taha NA, Alzraikat H. The silorane-based resin composites: a review. Oper Dent 2017;42:E24–34. [DOI] [PubMed] [Google Scholar]
- [16].Lu H, Carioscia JA, Stansbury JW, Bowman CN. Investigations of step-growth thiol-ene polymerizations for novel dental restoratives. Dent Mater 2005;21:1129–36. [DOI] [PubMed] [Google Scholar]
- [17].Park HY, Kloxin CJ, Scott TF, Bowman CN. Stress relaxation by addition-fragmentation chain transfer in highly crosslinked thiol-yne networks. Macromolecules 2010;43:10188–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bacchi A, Pfeifer CS. Rheological and mechanical properties and interfacial stress development of composite cements modified with thio-urethane oligomers. Dent Mater 2016;32:978–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bacchi A, Nelson M, Pfeifer CS. Characterization of methacrylate-based composites containing thio-urethane oligomers. Dent Mater 2016;32:233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bacchi A, Dobson A, Ferracane JL, Consani R, Pfeifer CS. Thio-urethanes improve properties of dual-cured composite cements. J Dent Res 2014;93:1320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bacchi A, Consani RL, Martim GC, Pfeifer CS. Thio-urethane oligomers improve the properties of light-cured resin cements. Dent Mater 2015;31:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Faria-E-Silva AL, Pfeifer CS. Impact of thio-urethane additive and filler type on light-transmission and depth of polymerization of dental composites. Dent Mater 2017;33:1274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Faria-E-Silva AL, Pfeifer CS. Delayed photo-activation and addition of thio-urethane: impact on polymerization kinetics and stress of dual-cured resin cements. J Dent 2017;65:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Edmondson S, Osborne VL, Huck WT. Polymer brushes via surface-initiated polymerizations. Chem Soc Rev 2004;33:14–22. [DOI] [PubMed] [Google Scholar]
- [25].Barbey R, Lavanant L, Paripovic D, Schüwer N, Sugnaux C, Tugulu S, et al. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chem Rev 2009;109:5437–527. [DOI] [PubMed] [Google Scholar]
- [26].Chen K, Ma YQ. Interactions between colloidal particles induced by polymer brushes grafted onto the substrate. J Phys Chem B 2005;109:17617–22. [DOI] [PubMed] [Google Scholar]
- [27].Watts DC, Satterthwaite JD. Axial shrinkage-stress depends upon both C-factor and composite mass. Dent Mater 2008;24:1–8. [DOI] [PubMed] [Google Scholar]
- [28].Aydınoğlu A, Yoruç ABH. Effects of silane-modified fillers on properties of dental composite resin. Mater Sci Eng C 2017;79:382–9. [DOI] [PubMed] [Google Scholar]
- [29].Mirabedini A, Mirabedini SM, Babalou AA, Pazokifard S. Synthesis, characterization and enhanced photocatalytic activity of TiO2/SiO2 nanocomposite in an aqueous solution and acrylic-based coatings. Prog Org Coat 2011;72:453–60. [Google Scholar]
- [30].Ye Y, Zeng X, Li H, Chen P, Ye C, Zhao F. Synthesis and characterization of nano-silica/polyacrylate composite emulsions by sol-gel method and in-situ emulsion polymerization. J Macromol Sci A 2011;48:42–6. [Google Scholar]
- [31].Seo JH, Chen LJ, Verkhoturov SV, Schweikert EA, Revzin A. The use of glass substrates with bi-functional silanes for designing micropatterned cell-secreted cytokine immunoassays. Biomaterials 2011;32:5478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Seo JH, Shin DS, Mukundan P, Revzin A. Attachment of hydrogel microstructures and proteins to glass via thiol-terminated silanes. Colloids Surf B Biointerfaces 2012;98:1–6. [DOI] [PubMed] [Google Scholar]
- [33].Rydholm AE, Bowman CN, Anseth KS. Degradable thiol-acrylate photopolymers: polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials 2005;26:4495–506. [DOI] [PubMed] [Google Scholar]
- [34].Lung CYK, Matinlinna JP. Aspects of silane coupling agents and surface conditioning in dentistry: an overview. Dent Mater 2012;28:467–77. [DOI] [PubMed] [Google Scholar]
- [35].Han X, Wu C, Sun S. Photochemical reactions of thiol-terminated self-assembled monolayers (SAMs) for micropatterning of gold nanoparticles and controlled surface functionality. Appl Surf Sci 2012;258:5153–6. [Google Scholar]
- [36].Tan KY, Ramstedt M, Colak B, Huck WT, Gautrot JE. Study of thiol–ene chemistry on polymer brushes and application to surface patterning and protein adsorption. Polym Chem 2016;7:979–90. [Google Scholar]
- [37].Biggs C, Walker M, Gibson MI. “Grafting to” of RAFTed responsive polymers to glass substrates by thiol–ene and critical comparison to thiol–gold coupling. Biomacromolecules 2016;17:2626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Condon JR, Ferracane JL. Reduced polymerization stress through non-bonded nanofiller particles. Biomaterials 2002;23:3807–15. [DOI] [PubMed] [Google Scholar]
- [39].Bacchi A, Yih JA, Platta J, Knight J, Pfeifer CS. Shrinkage/stress reduction and mechanical properties improvement in restorative composites formulated with thio-urethane oligomers. J Mech Behav Biomed Mater 2017;78:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pfeifer CS, Wilson ND, Shelton ZR, Stansbury JW. Delayed gelation through chain-transfer reactions: mechanism for stress reduction in methacrylate networks. Polymer 2011;52:3295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Podgórski M, Becka E, Claudino M, Flores A, Shah PK, Stansbury JW, et al. Ester-free thiol-ene dental restoratives — part A: resin development. Dent Mater 2015;31:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Harrisson S, Liu X, Ollagnier JN, Coutelier O, Marty JD, Destarac M. RAFT polymerization of vinyl esters: synthesis and applications. Polymers 2014;6:1437–88. [Google Scholar]
- [43].Pfistera LA, Stachurskib ZH. Micromechanics of stress relaxation in amorphous glassy PMMA part II: application of the RT model. Polymer 2002;43:7409–17. [Google Scholar]
- [44].Mathiesen D, Vogtmann D, Dupaix RB. Characterization and constitutive modeling of stress-relaxation behavior of poly(methyl methacrylate) (PMMA) across the glass transition temperature. Mech Mater 2014;71:74–84. [Google Scholar]