Abstract
Programmed cell death-1 (PD-1) inhibitors stimulate immune recognition of tumour cells in cancer patients, but have significant autoimmune side effects including pneumonitis. We report the case of a patient with asthma and mild eosinophilia who developed unusual pulmonary side effect of bronchiectasis, severe eosinophilia (absolute eosinophil count: 3200 c/mm3) and elevated IgE levels (7050 IU/mL; normal: <164 IU/mL) 4 months into therapy with the PD-1 inhibitor pembrolizumab. Aspergillus fumigatus IgG was elevated at 15.60 U/mL (normal: <12.01 U/mL). He responded to therapy with corticosteroids and voriconazole and was able to resume pembrolizumab thereafter with good clinical response.
Keywords: lung cancer (oncology), unwanted effects / adverse reactions
Background
Programmed cell death-1 (PD-1) inhibitors represent a novel treatment option for patients with metastatic lung cancer. These medications show promise when compared with cytotoxic chemotherapy in metastatic disease. However, widespread immune activation that is responsible for the antitumour activity also results in autoimmune phenomena on normal tissues including skin, endocrine glands and lungs.
Case presentation
A 63-year-old man with a history of childhood asthma and 47 packs per year history of tobacco abuse was diagnosed with squamous cell carcinoma of the lung metastatic to his sacrum and liver. The tumour was found to have 50% expression of PD-1. He was offered initial therapy with pembrolizumab 200 mg intravenously for 3 weeks. History included a diagnosis of severe peanut allergy as well as asthma controlled on budesonide/formoterol and as-needed albuterol without prior hospitalisation. He had intermittent idiopathic eosinophilia (3%–10%). Seven days after his first dose of pembrolizumab, he was admitted with shortness of breath. CT chest imaging detailed new multifocal pneumonia and mucus impaction of his right upper lobe. Admission white blood count was 12×109/L with 39% eosinophils (absolute eosinophil count: 3200 c/mm3). Bronchoscopy was performed which found ‘thick mucus’ with negative cultures. He was treated for multilobar pneumonia and completed a course of levofloxacin without steroids.
Three weeks after his first dose of pembrolizumab, he reported continued issues with thick sputum. He had white blood cell count of 10 000 c/mm3 with 10% eosinophils. A second dose of pembrolizumab 200 mg intravenously was administered. Nine days later he was seen for acute worsening of his pelvic pain and ambulatory dysfunction, for which he was prescribed 60 mg of prednisone with instructions to taper over the next 3 weeks. Two weeks later with his prednisone down to 20 mg, he was admitted again for dyspnoea, in which bronchiectasis and pneumonitis was again noted on imaging. Improvement in both primary tumour and metastatic disease was apparent. Eosinophils were undetectable, however the patient had continued on 20 mg of prednisone at the time of admission bloodwork. He was discharged on antibiotics, new home oxygen and was tapered off steroids over the next 2 weeks.
4 weeks later he presented to the hospital with subacute worsening of his dyspnoea, and coughing up hard, branching bits of sputum. He was tachycardic (heart rate: 119 bpm) and tachypneic at rest, with significant worsening with mild exertion. He had diffuse lower lung crackles and end-expiratory wheezing throughout.
Investigations
Chest CT imaging revealed increase in nodular opacities and bronchiectasis (figures 1 and 2). White blood cell count was 12 300 with 11% eosinophils. He was empirically treated for pembrolizumab-induced pneumonitis with 80 mg of prednisone. Sputum grew a single colony of Aspergillus fumigatus. IgE level was 7050 IU/mL (normal: <164 IU/mL). Aspergillus fumigatus IgG was elevated at 15.60 U/mL (normal: <12.01 U/mL). Thyroid Stimulating Hormone (TSH) was found to be 0.009 uIU/mL (normal: 0.45–5.3 uIU/mL) with high-normal free T4 and T3 levels.
Figure 1.
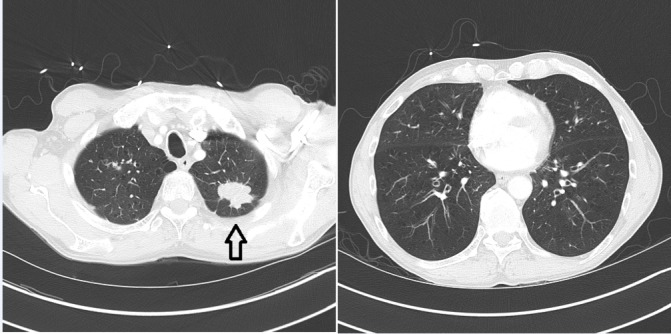
CT chest at time of diagnosis of lung cancer, including images of lower lungs at time of diagnosis; a spiculated 3.6 × 2.8 cm left upper lobe mass is demonstrated (black arrow).
Figure 2.
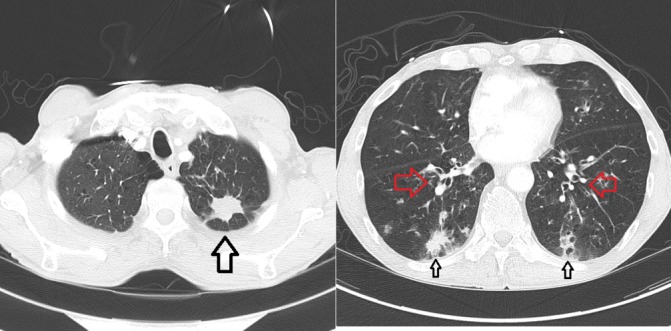
CT chest at time of diagnosis of allergic bronchopulmonary aspergillosis, 4 months into treatment; primary lung lesion in left upper lobe now 2.8 cm in length is decreased in size (large black arrow); new peribronchial thickening is noted (red arrows) and peripheral infiltrates (small black arrows).
Treatment
In light of his persistent eosinophilia, steroid-dependent lung disease with Aspergillus antibodies, and markedly elevated IgE levels, he was treated for allergic bronchopulmonary aspergillosis with voriconazole 200 mg two times per day for 3 months and a tapering dose of steroid over 6 weeks. He was also treated for mild thyroiditis with methimazole.
Outcome and follow-up
After 3 months and without steroids for 4 weeks, his eosinophil count was 3.6%, he was off supplemental oxygen and his IgE level was 3410 U/L. He was restarted on pembrolizumab which he has thus far tolerated well. Repeat CT chest imaging revealed significant improvement in airspace opacities noted on earlier imaging, as well as a significant reduction in size of both the primary mass and his liver metastases. Repeat thyroid levels off methimazole were within normal limits.
Discussion
Immune checkpoint inhibitors are powerful new tools for oncologists. They effectively recruit the patient’s own immune system which had developed tolerance of cancer cells, stimulating them to respond to previously down-regulated tumour antigens.1 Autoimmune side effects of this immune stimulation are now more frequently reported. Endocrine and dermatological autoimmune side effects are most commonly reported, occurring in 33% and 25%, respectively.2 PD-1 inhibition blocks both PD-L1 and PD-L2, which have opposing roles in modulating T-cell functions in allergic disorders involving airway hyper-responsiveness.3 Pneumonitis is reported in randomised trials in approximately 4% of patients, but higher in PD-1 inhibitors than PD-L1 inhibitors, with onset between 9 days and 19 months.4 Those with prior interstitial lung disease, prior asthma and radiation treatment to the lung appear to be at increased risk. Commonly reported patterns on imaging include ground glass infiltrative pattern (37%), an interstitial pattern (22%) and a cryptogenic organising pneumonia pattern (19%).4 Bronchiectasis and obstructive lung pathology has not previously been described.
Allergic bronchopulmonary aspergillosis (ABPA) is believed to be due to sensitisation to Aspergillus that results in robust hypersensitivity response, which may occur in up to 2.5% of patients with asthma. ABPA is now believed to be driven by a florid response to the fungus by type 2 T helper cells (Th2), resulting in marked mucus production, hyper-responsiveness and bronchiectasis, leading to frequent asthma exacerbations with mucus plugging, eosinophilia, bronchiectasis on imaging and elevated IgE levels.5 6 Our patient carried a diagnosis of asthma with low levels of eosinophilia before his lung cancer diagnosis. He had not however had any prior lung-related hospitalisations or exacerbations requiring systemic steroids before his lung cancer diagnosis. After stimulation of his immune system by pembrolizumab, he had significant pulmonary deterioration that included bronchiectasis, significant increase in his eosinophilia and serum and sputum evidence of Aspergillus as well as a markedly increased IgE level, consistent with ABPA, and this appears to be temporally associated with use of pembrolizumab. His bronchiectasis and IgE level as well as his eosinophilia improved with a course of antifungals and corticosteroids.
Learning points.
Patients receiving treatment with programmed cell death-1 (PD-1) inhibitors are at risk for autoimmune effects that include pneumonitis in 4% of patients; obstructive lung disease has not previously been described.
Allergic bronchopulmonary aspergillosis is a robust hypersensitivity response to Aspergillus in asthmatic patient that can cause bronchiectasis, eosinophilia and elevated total IgE.
In patients with underlying asthma with eosinophilia, immune stimulation via PD-1 inhibition may bring out vigorous hypersensitivity responses that include those that meet criteria for allergic bronchopulmonary aspergillosis.
Footnotes
Contributors: AAD and RK both had access to all of the data, contributed significantly to the manuscript and the final editing of the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Khoja L, Day D, Wei-Wu Chen T, et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377–85. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 2. Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One 2016;11:e0160221 10.1371/journal.pone.0160221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh AK, Stock P, Akbari O. Role of PD-L1 and PD-L2 in allergic diseases and asthma. Allergy 2011;66:155–62. 10.1111/j.1398-9995.2010.02458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–17. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Muldoon EG, Strek ME, Patterson KC. Allergic and noninvasive infectious pulmonary aspergillosis syndromes. Clin Chest Med 2017;38:521–34. 10.1016/j.ccm.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 6. Tracy MC, Okorie CUA, Foley EA, et al. Allergic Bronchopulmonary Aspergillosis. J Fungi 2016;2:17 10.3390/jof2020017 [DOI] [PMC free article] [PubMed] [Google Scholar]


