Abstract
Our case series describes two siblings with complex fibrosing lung diseases. The first patient was initially given a diagnosis of sarcoidosis based on imaging and exclusion of alternative diagnoses. A number of years after diagnosis, he had rapid deterioration of his disease and following surgical lung biopsy, his lung fibrosis was re-classified as chronic hypersensitivity pneumonitis (cHP) with a usual interstitial pneumonia pattern. He subsequently underwent successful lung transplantation. The second patient presented with rapidly progressing exertional dyspnoea. His bloods, imaging, bronchoalveolar lavage and histology were discussed at our multidisciplinary team meeting. His histology was most in keeping with subacute on cHP with overlapping imaging features between the two siblings. He was treated accordingly but unfortunately succumbed to his illness shortly after diagnosis. These cases highlight the difficulties differentiating between the various interstitial lung disease (ILD) subtypes and the challenges in management while also increasing awareness of familial ILD.
Keywords: medical management, respiratory medicine, interstitial lung disease, drugs: respiratory system, cardiothoracic surgery
Background
The distinction between interstitial lung disease (ILD) subgroups is important because each disease is managed differently. However, it can be difficult due to the overlap of clinical features. History of exposure to offending antigen in chronic hypersensitivity pneumonitis (cHP), extra-pulmonary manifestations in sarcoidosis, the presence of connective tissue disease and, if feasible, bronchoalveolar lavage (BAL), bronchoscopic biopsy or surgical lung biopsy (SLB) can be helpful.1
These cases also highlight the existence of familial ILD and the limited availability of genomic DNA and precise phenotypic information from historic cases due to the frequent late-onset and terminal nature of ILD.2
Case presentation
Our first patient, a 67-year-old man, was admitted with a 1-week history of dyspnoea, cough and fever. He had a background history of sarcoidosis for which he was on maintenance prednisolone and methotrexate (MTX) and hypertension for which he was taking an ACE inhibitor. He was a lifelong non-smoker and worked as a maintenance manager with no exposure to dust fumes or asbestos. There were no pets or birds at home. He was admitted under his primary respiratory physician and the specialist ILD services were consulted.
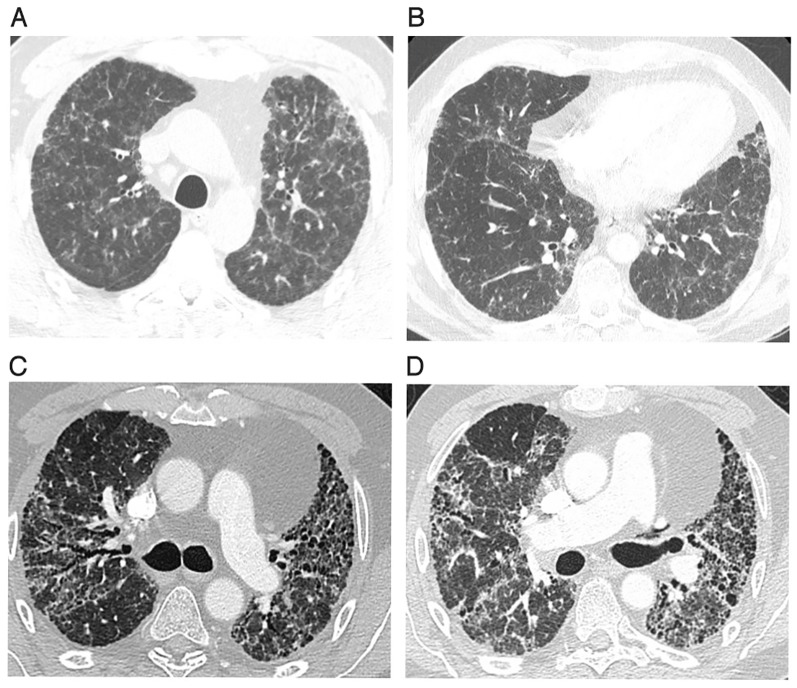
He was diagnosed with sarcoidosis 4 years previously. At that time, he had a chest X-ray (CXR) performed for symptoms suggestive of a lower respiratory tract infection. This showed new increased interstitial markings in a peri-hilar distribution. A CT thorax was pursued. This showed diffuse ground-glass infiltrates throughout both lungs and centrilobular and perifissural nodules in a subpleural distribution. Changes were slightly more predominant in the upper lobes. There was no honeycombing (figure 1A).
Figure 1.
A–D: These images demonstrate the progression of disease over the 4-year period in our first patient. (A) Shows a CT of the chest on first presentation. It demonstrates diffuse bilateral ground-glass opacities with multiple centri-lobular and subpleural nodules. These changes were most prominent in the upper lobes. (B) Shows a chest CT 2 years later showing mild progression of fibrosis changes with persistent pulmonary nodules and ground glass opacification and mild bronchiectasis. (C) Shows chest CT 4 years later (admission described above) showing extensive pulmonary fibrosis with significant traction bronchiectasis and honeycombing. (D) Shows his final CT pulmonary angiogram prior to lung transplantation. This shows low lung volumes, significant bilateral fibrosis, honeycombing and bronchiectasis.
He was referred for a respiratory opinion and initial workup included spirometry, diffusion capacity assessment, laboratory testing and a bronchoscopy. Spirometry revealed FVC 3.41 L (85% predicted) and TLco was 57% predicted. Laboratory testing revealed an elevated serum ACE at 106 U/L (8–65 U/L). The remainder of the laboratory workup was unremarkable including cell counts, renal function, liver function, and autoimmune and vasculitic screens. Bronchoscopic airway inspection was normal and transbronchial biopsies did not reveal any granuloma. BAL was negative for microbiology, culture and sensitivity. Cell counts were not measured. His case was discussed at our multidisciplinary team (MDT). The recommendation was to treat as pulmonary sarcoidosis as the most likely diagnosis. He was started on corticosteroids.
His CXR and CT thorax were repeated during admission for an exacerbation 3 months later. Imaging demonstrated new mediastinal lymphadenopathy and stable interstitial change. Endobronchial ultrasound-guided biopsies were pursued, the results of which were inconclusive with no evidence of granulomata. Following further MDT discussion, a mediastinoscopy was performed, which was also inconclusive. Again, no granulomata were identified.
Despite corticosteroids, his disease progressed with worsening respiratory symptoms and imaging with the development of honeycombing and traction bronchiectasis (figure 1B). MTX therapy was commenced in addition to corticosteroids, and despite dual therapy, he continued to decline, culminating in this admission.
Investigations
Laboratory workup on this admission revealed a normal ACE 45 U/L (8–65 U/L) and an elevated ANA 1:400 (homogenous and nucleolar pattern). ENA, ANCA, RF and anti-CCP were all within normal limits. White cell count (WCC) was marginally elevated at 11.6×109/L with normal WCC differential. The remainder of the laboratory tests were normal. Serum immunoglobulins revealed a reduced IgG at 6.4 g/L (8–15 g/L) and an elevated IgE 196Ku/L (1−100Ku/L). IgA and IgM were normal.
Arterial blood gas showed hypoxemia with PaO27.81 KPA (10–13 KPa). PaCO2 was normal.
CT thorax revealed extensive pulmonary fibrosis affecting all lobes, worst in the upper lobes. The fibrosis had progressed from previous imaging. There was evidence of honeycombing, traction bronchiectasis (figure 1C) and stable mediastinal and hilar lymphadenopathy. There was no air-trapping or mosaic attenuation.
He was unable to complete pulmonary function testing due to dyspnoea and his most recent spirometry (3 months prior to admission) had shown FVC 2.06 L (55% predicted) and DLCO was 31% predicted. On 6-min walk test, he walked 180 m and desaturated to 76% on room air. Transthoracic echocardiogram was performed. It showed normal left ventricular systolic function with normal right ventricular function and no evidence of pulmonary hypertension.
Differential diagnosis
Progression of sarcoidosis.
Sarcoidosis with a superimposed infectious process (bacterial, viral, fungal).
Sarcoidosis with superimposed drug-related lung fibrosis.
Pulmonary hypertension.
cHP.
Pleuroparenchymal fibroelastosis.
Idiopathic pulmonary fibrosis (IPF).
Vasculitis.
Treatment
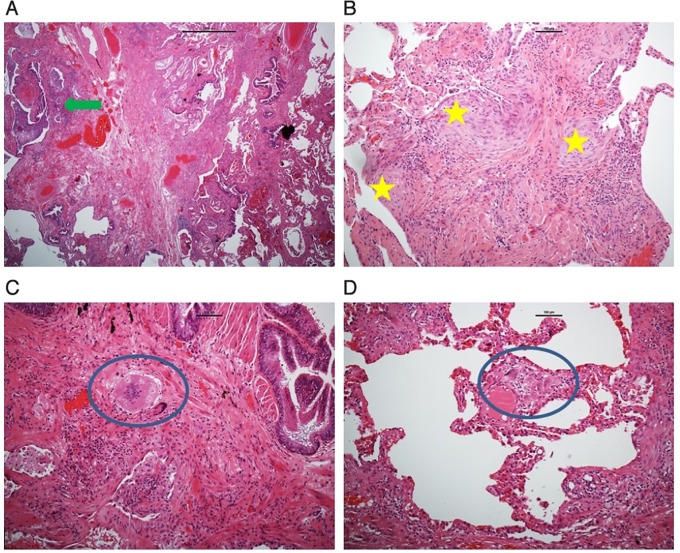
He was treated for superimposed infection with antibiotics and steroids. Unfortunately, his symptoms failed to improve, and following MDT discussion, he underwent video-assisted thoracoscopic SLB. Macroscopically, there was a cobbled appearance to the lung. Microscopically, there was patchy subpleural interstitial fibrosis with spatial and temporal heterogeneity, numerous fibroblastic foci and honeycombing, more diffuse in the upper lobe and minimal in the middle and lower lobes. There were some loose giant cells granulomas in the interstitium, some with asteroid bodies, but not along the lymphatics and not sarcoid-like in appearance. No vasculitis and no malignancy were seen (figure 2).
Figure 2.
A–D: These images demonstrate histopathology images from surgical lung biopsy performed in our first patient. (A,B) Show lung parenchyma with interstitial fibrosis and spatial and temporal heterogeneity with honeycombing (green arrow) and numerous fibroblastic foci (yellow stars). (C,D) Show numerous loose giant cells granulomas (blue circles) which were observed peri-bronchially (C) and in the interstitium (D) (scale bar A=1000 μm, B–D=100 μm).
His history was revisited and again, there was no evident exposure history, no occupational exposures and no family history of ILD.
His case was again discussed at the MDT. The consensus was rapidly progressive cHP (loose giant cell granuloma) with a usual interstitial pneumonia (UIP) pattern (honeycombing, fibroblast foci and patchy subpleural distribution of interstitial fibrosis). Mycophenolate mofetil (MMF) was started and maintenance corticosteroids were weaned. MTX was discontinued. Long-term oxygen therapy was started at 6 L/min. A referral was made to the lung transplantation service. He was discharged home on Day 14 on maintenance MMF 1000 mg twice daily and deltacortril 10 mg.
Outcome and follow-up
He remained stable for 3 months before requiring another admission for increasing dyspnoea and fevers. CT thorax was repeated and while negative for pulmonary embolism, it revealed further progression of fibrosis (figure 1D). He completed a 14-day course of corticosteroids and antibiotics to cover for superimposed infection. Despite this, he remained dependent on 10 L of high flow O2 at rest. The heart and lung transplant unit was contacted, and he was accepted to their care. He subsequently underwent a successful single right-sided lung transplant.
Less than 1 year later, his brother, a 65-year-old man, was admitted with a 3-month history of progressive exertional dyspnoea. He had been told he had ‘mild lung scarring’ in the past. Laboratory testing, spirometry and chest imaging were performed. Bloods revealed normal inflammatory markers and renal function. Serum ACE was mildly elevated at 69 U/L (8–65 U/L), and pANCA was weakly positive. ANA, ENA and MPO and PR3 antibodies were negative. Spirometry showed an FVC of 63% and DLCO was 28% predicted. CT thorax revealed diffuse interstitial changes in both lung fields with no lobar predominance. There was beading along the fissures. There was no air-trapping or mosaic attenuation.
Bronchoscopy with BAL and transbronchial biopsies were pursued in the first instance. Airway inspection was normal. BAL revealed a neutrophil predominance of 26%. Transbronchial biopsy histology showed focal interstitial fibrosis, florid type two pneumocytes, hyperplasia and organising pneumonia in specimens with bronchial mucosa and alveolar parenchyma.
His case was discussed at MDT. In the absence of connective tissue disease and vasculitis, and in the context of his family history, the imaging and histopathology findings were most compatible with a subacute on cHP. He strictly denied any exposures and was a lifelong non-smoker. He was referred for lung transplant assessment, however, he had rapid progression of his disease and died shortly after diagnosis.
Following the death of the second patient, a sister of the two patients mentioned that their father has died over 20 years previously with what had been described as ‘fibrosing alveolitis’.
Discussion
Making a correct diagnosis in ILD allows better estimation of the prognosis and appropriate treatment. This has become more important with the advent of anti-fibrotic in IPF, and the desire for an accurate diagnosis prior to listing for lung transplant. Many ILDs have overlapping clinical and radiological features and getting diagnostic pathology can be difficult.
This case series highlights specifically how it can be difficult to differentiate between sarcoidosis, cHP and IPF. For the purpose of this discussion, we will focus on the diagnosis of cHP, which can be particularly challenging because of the lack of validated diagnostic criteria.3 There is often disagreement among physicians about what actually constitutes cHP even in an MDT setting.4 A group in Canada recently developed a consensus-based approach to cHP diagnosis using the modified Delphi method.5 Experts placed the highest value on exposure identification, air trapping and mosaic attenuation on CT thorax, and poorly formed non-necrotising granulomas on lung biopsy. This consensus is a helpful step in the development of international guidelines but still requires clinical validation.
Morriset et al 5 also place emphasis on careful history-taking for exposures as HP is caused by sensitisation to an inhaled antigen. The reported inciting antigens are numerous and can be classified as either avian, microbial or chemical in nature.3 However, identification of exposure to an offending antigen can be absent in up to 50% of cases of cHP5 such as in both of our cases, where blood testing, radiology and histology are relied on heavily.
Patients with cHP often have a family history of ILD. This suggests that genetics also play an important role in the pathogenesis of cHP.6 There have been studies exploring the role for human leucocyte antigen typing, tumour necrosis factor alpha and tissue inhibitors of metalloproteinases in the development of acute HP. Other groups have tried to identify possible genetic susceptibilities to ILD.6 Despite this, most of the cases of familial cHP described in the literature have been reported in the setting of an, often remote, antigen exposure history.7 8 Okamoto et al 6 found that patients who develop cHP and have a family history of ILD are more likely to manifest a UIP pattern compared with those without a family history. Further research will be needed to determine whether patients with a family history of ILD should undergo earlier screening.
CHP remains a treatment dilemma and due to a lack of randomised controlled trials, treatment decisions are often guided by expert opinion.3 This usually compiles of antigen removal, corticosteroids, and/or cytotoxic drugs.3 Antigen avoidance is a relatively straightforward endeavour, except in the 50% of cases where an inciting antigen is not identifiable.5 Corticosteroid use has been assessed in one randomised placebo-controlled trial in which patients with acute farmer’s lung showed no significant improvement in lung function, compared with placebo, at 12 months.9
Cytotoxic agents such as MMF and azathioprine (AZA) have been assessed in one retrospective analysis by Morisset and colleagues.10 This revealed that treatment with either MMF or AZA was not associated with improved FVC but was associated with a statistically significant improvement in DLCO of 4.2% after 1 year of treatment. However, patients were recruited from academic centres and were only followed up for a median period of 11 months. There is a small series report on the role for rituximab11 12 in treating refractory patients.
Further prospective trials are required to guide treatment plans for this patient cohort. Ultimately lung transplant, like in our case, may still be required for many of these patients.
Patient’s perspective.
My main concern prior to the lung transplant was that I was getting worse. I was really worried about my future as I was getting progressively weaker. Since the transplant I have improved marvellously. I can now walk at my own pace for about twenty-five minutes without stopping. I am getting stronger all the time. I can’t yet keep up with my wife, but she does walk very fast. I was asked repetitively about exposure to things such as birds, feathers, damp and mould, however the doctors did tell me that in a large percentage of cases no definite cause is ever identified. I was puzzled about the cause of this disease. All the doctors have told me that in many cases the cause is not identified.
Learning points.
There are considerable clinical, radiological and histological similarities between pulmonary sarcoidosis, chronic hypersensitivity pneumonitis (cHP) and idiopathic pulmonary fibrosis in particular.
A thorough history is important when considering HP as a diagnosis, however there is no identifiable exposure history in more than 50% of cases of HP.
Patients with interstitial lung diseases including cHP should be asked about family history as they may be genetically predisposed.
Footnotes
Patient consent for publication: Obtained.
Contributors: Category 1: identification of case for write-up: MK, TJM. Planning for data collection and write-up: MOC, TJM. Acquisition of patient consent: TJM, MK. Acquisition of patient information to write-up case report: MOC, AF, MK, TJM. Interpretation and reporting of histopathological slides: AF. Interpretation and reporting of radiological images: MK, TJM. Analysis and interpretation of literature relating to case: MOC. Category 2: drafting the case report: MOC. Critical revision of the case report manuscript: MK, TJM. Category 3: approval of the version of the manuscript to be submitted for publication: MOC, MK, AF, TJM. Correction of manuscript submission: MOC, TJM.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jeong YJ, Lee KS, Chung MP, et al. Chronic hypersensitivity pneumonitis and pulmonary sarcoidosis: differentiation from usual interstitial pneumonia using high-resolution computed tomography. Semin Ultrasound CT MR 2014;35:47–58. 10.1053/j.sult.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 2. Garcia CK, Raghu G. Inherited interstitial lung disease. Clin Chest Med 2004;25:xi 10.1016/j.ccm.2004.10.001 [DOI] [PubMed] [Google Scholar]
- 3. Salisbury ML, Myers JL, Belloli EA, et al. Diagnosis and Treatment of Fibrotic Hypersensitivity Pneumonia. Where We Stand and Where We Need to Go. Am J Respir Crit Care Med 2017;196:690–9. 10.1164/rccm.201608-1675PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walsh SLF, Wells AU, Desai SR, et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respir Med 2016;4:557–65. 10.1016/S2213-2600(16)30033-9 [DOI] [PubMed] [Google Scholar]
- 5. Morisset J, Johannson KA, Jones KD, et al. Identification of Diagnostic Criteria for Chronic Hypersensitivity Pneumonitis: An International Modified Delphi Survey. Am J Respir Crit Care Med 2017. 10.1164/rccm.201710-1986OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Okamoto T, Miyazaki Y, Tomita M, et al. A Familial History of Pulmonary Fibrosis in Patients with Chronic Hypersensitivity Pneumonitis. Respiration 2013;85:384–90. 10.1159/000338123 [DOI] [PubMed] [Google Scholar]
- 7. Johnson CL, Bernstein IL, Gallagher JS, et al. Familial hypersensitivity pneumonitis induced by Bacillus subtilis. Am Rev Respir Dis 1980;122:339–48. 10.1164/arrd.1980.122.2.339 [DOI] [PubMed] [Google Scholar]
- 8. Kokuto H, Matsuda S, Tsuji S, et al. A case of parents and child who simultaneously suffered from summer-type hypersensitivity pneumonitis. Nihon Naika Gakkai Zasshi 2016;105:534–9. 10.2169/naika.105.534 [DOI] [PubMed] [Google Scholar]
- 9. Kokkarinen JI, Tukiainen HO, Terho EO. Effect of corticosteroid treatment on the recovery of pulmonary function in farmer’s lung. Am Rev Respir Dis 1992;145:3–5. 10.1164/ajrccm/145.1.3 [DOI] [PubMed] [Google Scholar]
- 10. Morisset J, Johannson KA, Vittinghoff E, et al. Use of Mycophenolate Mofetil or Azathioprine for the Management of Chronic Hypersensitivity Pneumonitis. Chest 2017;151:619–25. 10.1016/j.chest.2016.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lota HK, Keir GJ, Hansell DM, et al. Novel use of rituximab in hypersensitivity pneumonitis refractory to conventional treatment. Thorax 2013;68:780–1. 10.1136/thoraxjnl-2013-203265 [DOI] [PubMed] [Google Scholar]
- 12. Keir GJ, Maher TM, Ming D, et al. Rituximab in severe, treatment-refractory interstitial lung disease. Respirology 2014;19:353–9. 10.1111/resp.12214 [DOI] [PubMed] [Google Scholar]