ABSTRACT
A distinct taxon of the Drosophila microbiota, Lactobacillus plantarum, is capable of stimulating the generation of reactive oxygen species (ROS) within cells, and inducing epithelial cell proliferation. Here, we show that microbial-induced ROS generation within Drosophila larval stem cell compartments exhibits a distinct spatial distribution. Lactobacilli-induced ROS is strictly excluded from defined midgut compartments that harbor adult midgut progenitor (AMP) cells, forming a functional ‘ROS sheltered zone’ (RSZ). The RSZ is undiscernible in germ-free larvae, but forms following monocolonization with L. plantarum. L. plantarum is a strong activator of the ROS-sensitive CncC/Nrf2 signaling pathway within enterocytes. Enterocyte-specific activation of CncC stimulated the proliferation of AMPs, which demonstrates that pro-proliferative signals are transduced from enterocytes to AMPs. Mechanistically, we show that the cytokine Upd2 is expressed in the gut following L. plantarum colonization in a CncC-dependent fashion, and may function in lactobacilli-induced AMP proliferation and intestinal tissue growth and development.
KEY WORDS: ROS, Nrf2, Stem cell, Non-cell autonomous, Drosophila, JAK/STAT
Highlighted Article: Lactobacilli-induced ROS generation in the Drosophila larval midgut is confined to enterocytes, whereas undifferentiated adult midgut progenitor cells exist in a distinguishable ‘ROS sheltered zone’.
INTRODUCTION
Stem cell growth and differentiation in the metazoan gut are processes that are largely regulated by intrinsic signaling that is initiated via local growth factors and other small molecules. A prominent example are reactive oxygen species (ROS), which can be deliberately generated in diverse physiological contexts by multiple highly conserved enzymes such as the NADPH oxidase (Nox) family. ROS mediate cell signaling via their effects on ‘sensor’ proteins that are typically characterized by oxidant-sensitive cysteine residues that may be rapidly and reversibly modified by ROS (Jones and Neish, 2017). We have reported that specific taxa of lactobacilli, a common component of the insect and mammalian microbiota, have the capacity to stimulate ROS production and consequent epithelial cell proliferation in Drosophila larval epithelial cells by processes that require the catalytic action of Nox enzymes (Jones et al., 2013). These observations were recently corroborated within the Lemaitre group (Iatsenko et al., 2018).
We have also reported that lactobacilli-induced ROS generation in epithelial cells elicits cytoprotection against exogenous insults via the activation of the Nrf2 cell-signaling pathway (Jones et al., 2015). In addition, emerging literature describes roles of the Nrf2 pathway in cell proliferation (Murakami and Motohashi, 2015). Indeed, in Drosophila, CncC (the homolog of Nrf2) was identified as a crucial regulator of intestinal stem cell (ISC) proliferation through the maintenance of cellular redox balance; CncC was reported to be constitutively active in ISCs of the adult midgut, in which it maintains low ROS levels and ISC quiescence (Hochmuth et al., 2011). Because of these reports, we investigated the role of CncC in lactobacilli-induced signaling in the Drosophila larva midgut, which comprises two cell populations: differentiated enterocytes (ECs) and islets of undifferentiated adult midgut progenitors (AMPs) (Takashima et al., 2011).
Herein, we show that lactobacilli-induced generation of ROS in the larval midgut is confined to ECs, and is undetectable in regions that harbor AMPs, which functionally forms a distinguishable ‘ROS sheltered zone’ (RSZ). Interestingly, the RSZ is imperceptible in germ-free larvae, but quickly forms following mono-association with Lactobacillus plantarum, although not with other representatives of fly commensal bacteria. We also show that EC-specific activation of ROS-sensitive CncC/Nrf2 stimulated the proliferation of AMPs in both conventional and germ-free larvae, which shows that CncC signals can be transduced from ECs to AMPs. Gene ontology analysis and qPCR of midgut tissue from L. plantarum-fed larvae identified highly represented transcripts of upd2, the gene product of which has been shown to regulate intestinal homeostasis via JAK/STAT signaling (Hombría et al., 2005). Consistently, reduction of Upd2 abrogated the capacity of L. plantarum to induce epithelial proliferation in the midgut. Our data suggest that lactobacilli-induced AMP proliferation occurs by a non-cell autonomous mechanism that involves Nrf2 pathway signaling.
RESULTS AND DISCUSSION
Discrete localization of cellular ROS within the Drosophila midgut and hindgut stem cell microenvironment
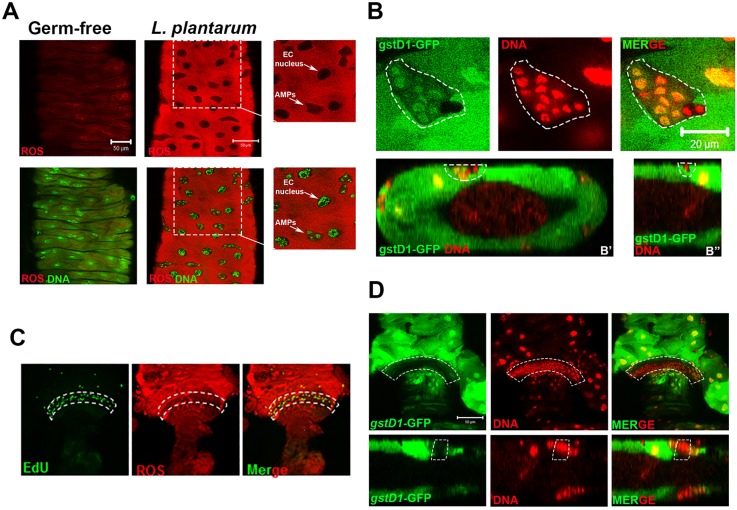
In past work, we have shown that ingestion of L. plantarum by germ-free larvae rapidly induces ROS generation in the Drosophila midgut shortly after ingestion – events that are not elicited by other members of the fly commensal microbiota (Jones et al., 2013). Higher-resolution examination of the microenvironment that surrounds the larval midgut stem cell niche revealed a striking discrete localization of tissue redox alterations. ROS generation was reduced or absent in the larval midgut stem cell niche that harbors AMPs, and in the nuclei of ECs as detected using the ROS-sensitive hydro-Cy3 dye (Fig. 1A). Similar discrete localization was detected using the redox-sensitive gstD1-gfp reporter, which transcriptionally responds to the ROS-sensitive CncC/Nrf2 signaling pathway (Sykiotis and Bohmann, 2008) (Fig. 1B). Z-stack projection analysis of larval midguts revealed that AMPs were situated adjacent to the circular muscle, insulated from the gut lumen and from direct contact with bacteria, which suggests that redox events require bacterial contact (Fig. 1B). In addition, a niche of proliferating ISCs is found situated in the posterior intestine (hindgut), confined to an anterior narrow segment known as the hindgut proliferation zone (HPZ) (Takashima et al., 2008). ROS levels within the HPZ were noticeably lower, as detected by the ROS-sensitive hydro-Cy3 dye (Fig. 1C) and the redox-sensitive gstD1-gfp reporter fly (Fig. 1D). These data indicate that L. plantarum-induced ROS generation and redox-dependent signaling is limited to gut ECs, and that midgut AMPs, and proliferating cells in the HPZ exist in a ‘ROS sheltered zone’ (RSZ).
Fig. 1.
Discrete localization of cellular ROS within the Drosophila midgut and hindgut stem cell microenvironment. (A) ROS generation in the midgut ISC niche of germ-free third instar larvae colonized with L. plantarum for 4 h. Food also contained 100 μM of the ROS-sensitive hydro-Cy3 dye. Magnified images of boxed areas shown on right. (B) Detection of GFP from the ROS-responsive gstD1-gfp element in the midgut ISC niche of germ-free third instar larvae colonized with L. plantarum. (B′) Transverse section and (B″) longitudinal section of the larval midgut. (C) Detection of ROS using the hydro-Cy3 dye, and of EdU-positive cells in the hindgut pylorus of w1118 larvae that were colonized with L. plantarum for 2 h. (D) Detection of GFP using gstD1-gfp in the hindgut pylorus of germ-free third instar larvae that were colonized with L. plantarum 4 h. Bottom panels show longitudinal section of the hindgut pylorus. Region of low GFP is outlined by dashed white lines. Dashed white lines outline the midgut stem cell niche (B) and the hindgut proliferation zone (C,D). Scale bars: 50 μm in A,D; 20 μm in B.
Compartmentalization of redox state within the Drosophila larval midgut is sensitive to colonization with L. plantarum
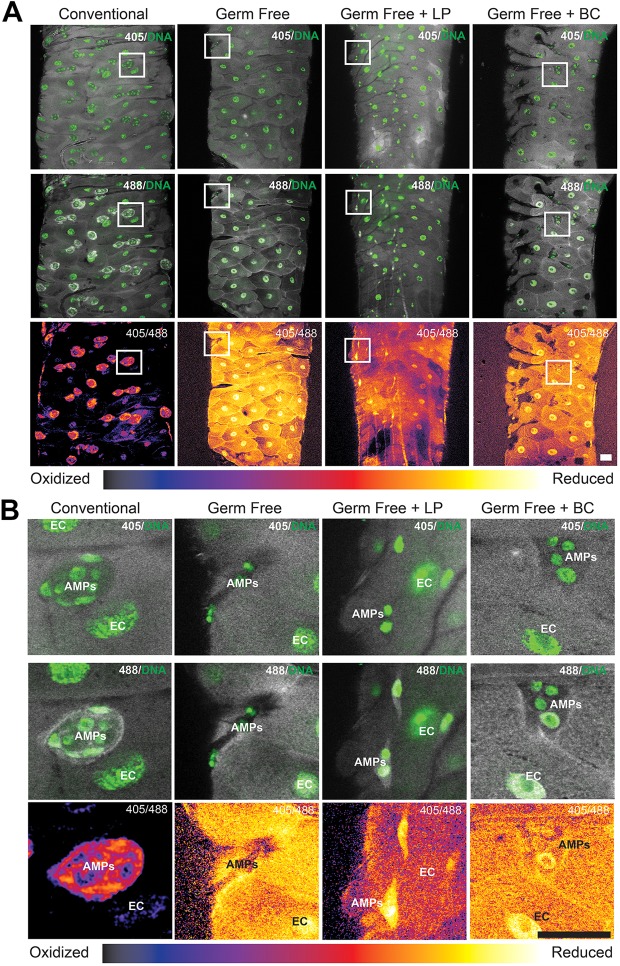
To substantiate the data in Fig. 1, we employed a Drosophila line that harbors redox probes that are sensitive to cytosolic H2O2 (cyto-roGFP2-Orp1) (Albrecht et al., 2011). Consistently, discreet compartmentalization of cell redox state was detected within the midgut of conventionally raised Drosophila larvae with elevated levels of the reduced form of roGFP2-Orp1 (488 nm) within AMP cell niches, which are identified by the tight clustering of small nuclei interspersed between ECs, together forming a redox gradient between the ECs and AMPs (Fig. 2A,B). However, in germ-free larval midguts, no RSZ was detected (Fig. 2A,B). Strikingly, feeding of L. plantarum to germ-free larvae for 4 h, which we have shown to induce ROS generation in ECs (Jones et al., 2013), established a redox gradient between ECs and AMPs, whereas feeding of the non-ROS-generating Bacillus cereus to germ-free larvae for the same duration did not (Fig. 2A,B). Collectively, these data demonstrate that the larval midgut exists in a reducing environment in a germ-free state, whereas AMP niches are maintained in a compartmentalized reducing environment under conventionally colonized conditions, or post-contact with bacteria that induce ROS generation within ECs.
Fig. 2.
Compartmentalization of redox state within the Drosophila larval midgut is sensitive to colonization with L. plantarum. (A) Detection of the ubiquitous expression of the redox-sensitive roGFP from the genotype tub-GAL4 UAS-cyto-roGFP2-Orp1 in the third instar larval midgut, in which reduced roGFP is excited at 405 nm (trend towards yellow) and oxidized roGFP is excited at 488 nm (trend towards blue). Note strong detection of oxidized (488 nm) roGFP surrounding AMP islets in conventionally raised, but not in germ-free larvae. Note reduced state of ECs in germ-free larvae. Colonization of germ-free larvae with L. plantarum (LP), but not with B. cereus (BC) induces the emergence of compartmentalization of redox states between the ECs and AMPs. (B) Magnified views of boxed areas of midgut regions outlined in A. Scale bars: 25 μm.
Expression of CncC/Nrf2 signaling in third instar larval ECs activates AMP proliferation
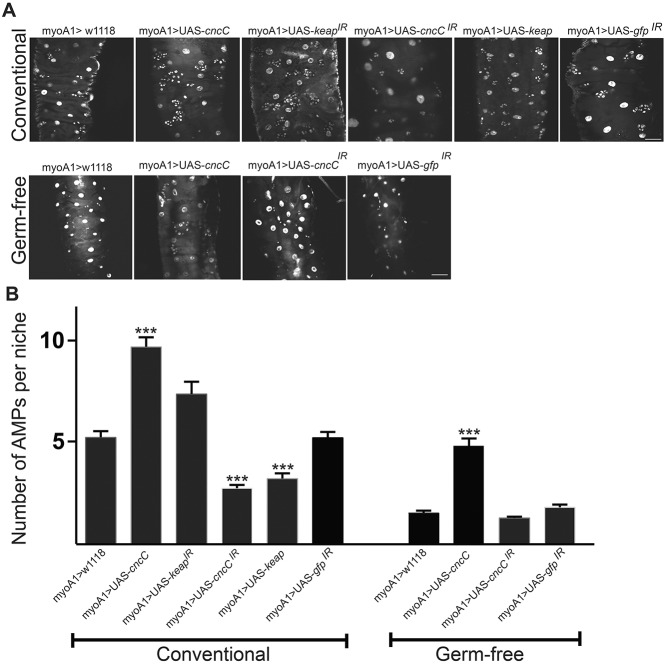
We have reported that L. plantarum-induced ROS generation elicits cytoprotection against pro-oxidative insults via the activation of CncC/Nrf2 signaling (Jones et al., 2015). In the course of these studies, we noticed that constitutive expression of cncC within ECs induces supraphysiologic AMP proliferation in conventionally raised Drosophila larvae (Fig. 3A,B). Consistently, diminishing keap1 transcript levels in ECs, which has the net effect of relieving Keap1-mediated inhibition of CncC nuclear translocation, also results in increased numbers of AMPs in the larval midgut (Fig. 3A,B). Conversely, diminishing cncC transcript levels, or constitutively expressing keap1, significantly decreases the number of AMPs in the larval midgut (Fig. 3A,B), altogether showing that modulating the activity of CncC within ECs of conventionally raised larvae influences the number of AMPs within niches. We also observed that germ-free third instar larvae have significantly fewer AMP cells per niche compared with developmental stage-matched conventionally raised larvae. Strikingly, increased expression of cncC in ECs of germ-free larvae significantly increases AMP numbers within niches in the midgut, having the effect of rescuing AMP numbers to similar levels to those that are detected in wild-type conventionally raised flies (Fig. 3A,B). These data demonstrate that CncC/Nrf2 pathway activity in larval ECs influences cell numbers in the spatially distinct adjacent AMP niche, and suggests that this pathway is a conduit for transferring bacterial signals from the gut lumen to proliferating AMPs.
Fig. 3.
Expression of CncC/Nrf2 signaling in third instar larval ECs activates AMP proliferation. (A) Detection of AMPs using a staining for DNA within the distal midgut of larvae of the indicated genotype that expresses a transgene or element under the control of the EC-specific myoIA-GAL4 driver fly. (B) Quantification of the number of AMPs per niche within the images in A. The number of AMPs per island was counted for ten genetic conditions (five Drosophila intestines per condition; 32-85 islands). A non-parametric Kruskal–Wallis ANOVA (P=0.05) was performed on the data with a post-hoc Dunn's multiple comparison to determine significant differences in mean ranks (P<0.05). Data are mean±s.e.m. ***P<0.001. Scale bars: 25 μm.
L. plantarum upregulates upd2 expression and JAK/STAT signaling in the larval midgut
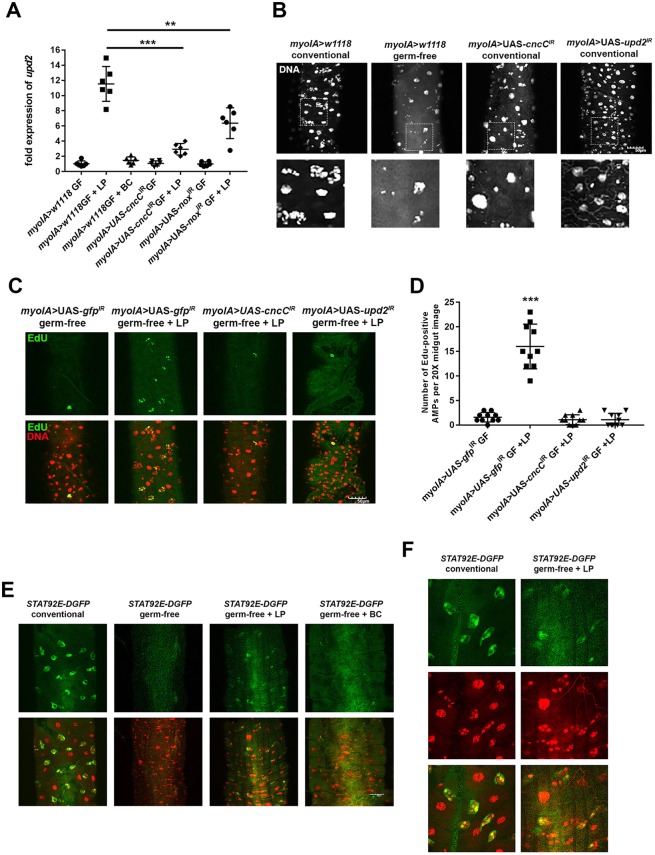
We previously reported on a microarray analysis in which we compared gene expression in the midguts of germ-free third instar larvae with expression in germ-free third instar larvae that had ingested L. plantarum for 4 h (Jones et al., 2015). Gene ontology analysis identified highly represented transcripts of the cytokine upd2 in L. plantarum-fed larvae. Furthermore, because the family of Unpaired (Upd) cytokines has been implicated in paracrine signaling following injury to the Drosophila gut, we hypothesized that upd2 expression responds to bacterial colonization and functions as the cytokine that transduces signals from ECs to AMPs in the context of symbiotic host cell and microbe interactions. Consistently, upd2 expression is rapidly upregulated in the midgut following ingestion of L. plantarum for 4 h in germ-free wild-type larvae, but not following the feeding of larvae with B. cereus, which is a commensal bacterium isolated from the fly that does not activate CncC signaling (Fig. 4A). By contrast, we did not detect any upregulation of the cytokines upd1 and upd3 following colonization with L. plantarum (data not shown). Importantly, EC-targeted depletion of cncC transcript levels (myoIA>UAS-cncCIR) and targeted depletion of dnox (also known as nox) transcript levels (myoIA>UAS-noxIRGF) significantly decreases the capacity of L. plantarum to induce upd2 expression (Fig. 4A). Intriguingly, we show that the effects of EC-specific depletion of Upd2 levels (myoIA>UAS-upd2IR) results in significantly reduced total organismal size of conventionally raised larvae and adults compared with age-matched conventionally raised myoIA>w1118, indicating a function for Upd2 within gut ECs in normal development (Fig. S1). In addition, conventionally raised myoIA>UAS-upd2IR adult flies are of similar body weight to germ-free myoIA>w1118 adult flies (Fig. S1), showing that the organismal phenotype that results from a depletion of Upd2 in ECs elicits similar effects to the complete absence of gut bacteria. Furthermore, we show that constitutive expression of sod1, or catalase, or the inclusion of the antioxidant N-acetylcysteine (NAC) into the larval food, significantly decreases the capacity of L. plantarum to induce upd2 expression (Fig. S2). Indeed, higher amounts of NAC in the larval propagation food completely inhibits pupa from eclosing into adults. These data indicate that modifying the redox state using NAC can impede development in Drosophila (Fig. S2). Analysis of the intestines of age-matched third instar larvae of myoIA>UAS-upd2IR and control lines revealed considerably altered intestinal architecture. EC-specific depletion of Upd2 results in markedly fewer cells within the AMP niche (Fig. 4B), altogether implicating Upd2 activity in ECs as a modulator of gut development. We previously demonstrated that mono-association of germ-free larvae with L. plantarum induces AMP cell proliferation (Jones et al., 2013). To implicate a function for Upd2 in L. plantarum-induced AMP proliferation, we show that mono-association of germ-free larvae with an EC-specific depletion of upd2 or of cncC transcript levels (myoIA>UAS-upd2IR and myoIA>UAS-cncCIR, respectively) completely abrogates the pro-proliferative influence of L. plantarum (Fig. 4C,D). Upd2 has been shown to regulate organismal homeostasis via the activation of JAK/STAT signaling (Rajan and Perrimon, 2012). Signaling involves the translocation of STAT92E into the nucleus to induce target gene expression. To detect and localize STAT signaling, we used the 10XSTAT92E-DGFP reporter fly, which drives the expression of GFP under the Stat92E promoter (Bach et al., 2007). We show that GFP expression in this fly fluoresces in the midgut AMP niche of conventionally raised larvae, but not in germ-free larvae (Fig. 4E). Consistently, feeding of germ-free larvae with L. plantarum induces GFP expression from the 10XSTAT92E-DGFP reporter, whereas feeding with B. cereus does not (Fig. 4E). Higher-magnification images of midguts in Fig. 4E confirm that GFP is expressed within AMPs following L. plantarum feeding (Fig. 4F). Together, these data show that Upd2 and JAK/STAT signaling respond to L. plantarum feeding of larvae, and may function in transferring symbiotic bacteria-induced pro-proliferative messages from ECs to AMPs.
Fig. 4.
L. plantarum upregulates upd2 expression in the larval midgut. (A) Measurement of upd2 transcript levels in third instar larval midguts of the indicated genotypes. Germ-free larvae were mono-associated with either L. plantarum (LP) or B. cereus (BC) for 4 h. All upd2 transcript levels were normalized to levels that were detected in the conventionally raised myoIA>w1118 flies, which serve as control groups with an isogenic genetic background, and were averaged and set to a value of 1. For each data point, five larval intestines were pooled and total RNA was extracted. (B) Third instar larvae posterior midgut tissue from the indicated genotypes. Note fewer AMPs, with many niches only harboring one AMP per niche/island following EC-specific depletion of CncC or Upd2. Bottom panels show magnified views of boxed areas. (C) Detection of EdU-positive cells in the midgut of germ-free Drosophila third instar larvae of the indicated genotypes following mono-association with L. plantarum for 24 h. (D) Quantification of EdU-positive cells in the midgut of phenotypes indicated in C. Note significant increase in the numbers of proliferating AMPs in response to L. plantarum in control, but not in myoIA>UAS-upd2IR or with myoIA>UAS-cncCIR. (E) Detection of GFP-positive cells in the midgut of larvae that harbor a JAK/STAT-responsive 10XSTAT92E-DGFP reporter fly. GFP levels were compared between germ-free larvae and larvae mono-associated with L. plantarum or with B. cereus for 24 h. (F) Higher-magnification images of 10XSTAT92E-DGFP reporter detailed in E, showing colocalization of GFP expression with the smaller nuclei AMPs following L. plantarum feeding. **P<0.01 (nonparametric unpaired t-test); ***P<0.001 (unpaired t-test). n=6 in A; n=10 in D. Quantifications show mean values (middle bars) and s.e.m. (outer bars); individual datapoints plotted. Scale bars: 50 μm.
Here, we demonstrate that proliferation of midgut AMPs is potently stimulated by specific strains of commensal bacteria that are characterized by their ability to stimulate ROS production in epithelial cells. Somewhat paradoxically, stem cells exist in a well-defined lower ROS environment relative to surrounding tissues, which forms a functional region that we designate an RSZ. In the RSZ, AMPs replicate and are not quiescent. We also show elevated AMP proliferation in response to lactobacilli-induced ROS generation in midgut ECs that surround the AMPs, thus demonstrating that AMP are sensitive to altered ROS levels with neighboring cells, and that signaling to AMPs occurs by a non-cell autonomous mechanism. Our data point to a highly compartmentalized distribution of ROS-generating cells within the larval midgut, and support the previous speculation surrounding compartmentalization of ROS levels within tissues (Hansen et al., 2004).
An essential pathway for regulating and/or responding to ROS levels in cells is the Nrf2 pathway (Jones et al., 2012). We recently showed that lactobacilli can induce Nrf2 signaling in ECs, thereby demonstrating a molecular mechanism for lactobacilli-mediated intestinal cytoprotection (Jones et al., 2015). In addition, Nrf2 has been implicated in proliferative control of gut stem cells via regulation of ROS; it has been shown that loss of Nrf2 in ISCs causes accumulation of ROS and accelerates age-related degeneration of the intestinal epithelium (Hochmuth et al., 2011). In the larval Drosophila gut, JAK/STAT signaling is essential for the development of the fly hindgut during late embryogenesis, and during the larval growth (Johansen et al., 2003). In the adult midgut, JAK/STAT signaling induces stem cell proliferation and functions in the differentiation of progenitor cells into EC and enteroendocrine cells (Jiang et al., 2009). Furthermore, JAK/STAT signaling has crucial functions in tissue regeneration following injury by bacterial infection: Upd3 is secreted from ECs to activate JAK/STAT signaling in ISCs, which stimulates their proliferation, with the net effect of rapid regeneration of the damaged midgut epithelium (Buchon et al., 2009). We show here that upd2 is upregulated in the midgut in response to mono-association with L. plantarum by a mechanism that is dependent, at least in part, on dNox and Nrf2 signaling. However, because levels of L. plantarum-induced upd2 expression were only significantly diminished, but not completely annulled, in myoIA>UAS-cncCIR or myoIA>UAS-dnoxIR larvae, it is likely that upd2 expression is regulated by other intrinsic functional elements that regulate tissue homeostasis.
We previously reported that lactobacilli with gut epithelium induce ROS generation, with downstream influences on cell proliferation by mechanisms that require their mucus binding properties (Ardita et al., 2014). Indeed, epithelial ROS that is generated in response to bacteria functions in a signaling role, by which ROS-sensitive cellular enzymes could be influenced by bacterial-induced changes in redox status (Matthews et al., 2018). This hypothesis is supported by reports that reversible oxidative inactivation of a wide range of regulatory enzymes is a recognized mechanism of signal transduction (Ray et al., 2012). Taken together, these data suggest that a eubiotic microbiota that induces the generation of physiological levels of ROS within ECs represents a mechanism whereby microbes influence epithelial development. We emphasize that the role of bacteria in this case would be to promote and optimize homeostasis, a role that is not a fundamental requirement as germ-free animals can develop to adulthood, albeit with abnormal gut architecture.
MATERIALS AND METHODS
Fly strains and bacteria
Flies were maintained on standard media. Crosses were propagated in humidity-controlled incubators at 25°C unless otherwise noted. The gstD-gfp reporter line, and UAS-cncC, UAS-cncCIR, UAS-keap and UAS-keapIR were gifts from Dirk Bohmann (Sykiotis and Bohmann, 2008). MyoIA-GAL4 was a gift from Shigeo Takashima (Takashima et al., 2008). The lines UAS-catalase (24621), UAS-sod1 (24491), UAS-upd2IR (33949) and the 10XSTAT92E-DGFP (26199) reporter were obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537). The Drosophila line cyto-roGFP2-Orp1 was a gift from Tobias Dick (Albrecht et al., 2011). The L. plantarum and B. cereus strains used were isolated from the intestine of w1118 fly stocks in our laboratory as reported in Jones et al. (2013). 16S analysis revealed that the closest taxonomically related bacterium to our isolated strain of L. plantarum is L. plantarum ATCC 14917.
Generation of germ-free Drosophila larvae, and immunostaining and detection of cell proliferation in the intestine of third instar Drosophila larvae
Germ-free Drosophila larvae were generated, and ROS-detected using the hydro Cy3 dye, using methods previously described (Luo et al., 2016). Briefly, Drosophila embryos were harvested and transferred to a cell strainer. In a sterile environment, embryos were thrice washed with sterile PBS, soaked in 50% bleach for 5 min, before rinsing again with sterile PBS. The mesh of the cell strainer was removed to a sterile Petri dish that contained sterilized Drosophila food, and incubated for 24 h at 25°C. After larvae had developed to third instar, they were transferred into another Petri dish that contained 2 ml liquefied sterile Drosophila food that contained a total of 1×106 cfu pure bacterial culture, and a final concentration of 100 μM of hydro-Cy3 ROS-sensitive reagent. After 4 h, the midgut of third instar larvae were dissected and fluorescence detected using IX81 inverted confocal microscopy (Olympus). For the detection of cell proliferation in the midgut of third instar larvae, 5-ethynyl-2′-deoxyuridine (EdU) at a final concentration of 0.4 mM was added to liquefied sterile food, or to liquefied food that contained 1×106 cfu pure bacterial culture. Larvae were incubated in the milieu for 24 h, the midguts were dissected and EdU incorporation was determined according to the manufacturer's protocol (Roche Diagnostics) and Luo et al. (2016). To determine that the germ-free third instar larvae had ingested the bacterial monoculture, larval intestines were dissected into 1 ml sterile PBS and cfu was calculated using the plate count method. We detected 6.4×105 cfu L. plantarum per third instar larval intestine (s.d.=4.2×104, n=5), and 2.64×104 cfu B. cereus per intestine (s.d.=6.2×103, n=5).
Visualization of cellular redox levels in the cyto-roGFP2-Orp1 Drosophila line
Larval midguts were imaged using an Olympus FV1000 confocal microscope equipped with lasers for 405 nm and 488 nm excitation. Intestines were viewed with a 40× objective. The roGFP fluorescence was collected with a 505-525 nm emission band-pass filter for both 488 nm illumination and 405 nm illumination. The ratio of the 405/488 nm laser power and collection parameters (pin hole size, high voltage and gain) were kept constant. Images were imported into Fiji and pixel intensity in the 488 nm-excited channel were divided by pixel intensity of the 405 nm-excited channel. Contrast was adjusted to all ratio images consistently. The ratio was coded by hue on a spectral color scale (Fire LUT) ranging from yellow (fully reduced) to black (fully oxidized).
Quantitative PCR on Drosophila intestines
Five Drosophila larval intestines were dissected and immersed directly in TRIzol before RNA extraction. For each Drosophila line examined, RNA from three independent replicates (each containing five intestines) was extracted. Transcript levels were measured for each replicate in duplicate by qRT-PCR. Transcript levels of upd2 were amplified using primers Upd2-RT-F 5′-cgtcatcgtcatcctcatcatc-3′, and Upd2-RT-R 5′-gctactctcgctgagactcata-3′. Upd2 transcript levels were normalized against rp49 transcript levels that were measured using Rp49-F, 5′-tacaggcccaagatcgtgaa-3′ and Rp49-R, 5′-tctccttgcgcttcttgga-3′. In our experiments, we normalized transcript enrichment to those levels detected in conventionally raised wild-type controls.
Supplementary Material
Acknowledgements
The Bloomington Drosophila Stock Center is supported by the National Institutes of Health (P40OD018537).
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.R.R., A.S.N., R.M.J.; Methodology: A.R.R., L.L., R.M.J.; Formal analysis: A.R.R.; Investigation: A.R.R., L.L.; Resources: L.L.; Data curation: L.L.; Writing - original draft: R.M.J.; Writing - review & editing: A.R.R., A.S.N.; Supervision: R.M.J.
Funding
R.M.J. is supported in part by the National Institutes of Health grant R01DK098391. A.S.N. is supported, in part, by the National Institutes of Health grants R01DK071604 and RO1AI064462. A.R.R. is funded by the T32DK007771-06 training grant. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.171520.supplemental
References
- Albrecht S. C., Barata A. G., Grosshans J., Teleman A. A. and Dick T. P. (2011). In vivo mapping of hydrogen peroxide and oxidized glutathione reveals chemical and regional specificity of redox homeostasis. Cell Metab. 14, 819-829. 10.1016/j.cmet.2011.10.010 [DOI] [PubMed] [Google Scholar]
- Ardita C. S., Mercante J. W., Kwon Y. M., Luo L., Crawford M. E., Powell D. N., Jones R. M. and Neish A. S. (2014). Epithelial adhesion mediated by Pilin SpaC is required for Lactobacillus rhamnosus GG-induced cellular responses. Appl. Environ. Microbiol. 80, 5068-5077. 10.1128/AEM.01039-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach E. A., Ekas L. A., Ayala-Camargo A., Flaherty M. S., Lee H., Perrimon N. and Baeg G.-H. (2007). GFP reporters detect the activation of the Drosophila JAK/STAT pathway in vivo. Gene Expr. Patterns 7, 323-331. 10.1016/j.modgep.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Buchon N., Broderick N. A., Poidevin M., Pradervand S. and Lemaitre B. (2009). Drosophila intestinal response to bacterial infection: activation of host defense and stem cell proliferation. Cell Host Microbe 5, 200-211. 10.1016/j.chom.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Hansen J. M., Watson W. H. and Jones D. P. (2004). Compartmentation of Nrf-2 redox control: regulation of cytoplasmic activation by glutathione and DNA binding by thioredoxin-1. Toxicol. Sci. 82, 308-317. 10.1093/toxsci/kfh231 [DOI] [PubMed] [Google Scholar]
- Hochmuth C. E., Biteau B., Bohmann D. and Jasper H. (2011). Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8, 188-199. 10.1016/j.stem.2010.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hombría J. C.-G., Brown S., Häder S. and Zeidler M. P. (2005). Characterisation of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 288, 420-433. 10.1016/j.ydbio.2005.09.040 [DOI] [PubMed] [Google Scholar]
- Iatsenko I., Boquete J. P. and Lemaitre B. (2018). Microbiota-derived lactate activates production of reactive oxygen species by the intestinal NADPH oxidase nox and shortens drosophila lifespan. Immunity 49, 929-942.e5. 10.1016/j.immuni.2018.09.017 [DOI] [PubMed] [Google Scholar]
- Jiang H., Patel P. H., Kohlmaier A., Grenley M. O., Mcewen D. G. and Edgar B. A. (2009). Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell 137, 1343-1355. 10.1016/j.cell.2009.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen K. A., Iwaki D. D. and Lengyel J. A. (2003). Localized JAK/STAT signaling is required for oriented cell rearrangement in a tubular epithelium. Development 130, 135-145. 10.1242/dev.00202 [DOI] [PubMed] [Google Scholar]
- Jones R. M. and Neish A. S. (2017). Redox signaling mediated by the gut microbiota. Free Radic. Biol. Med. 105, 41-47. 10.1016/j.freeradbiomed.2016.10.495 [DOI] [PubMed] [Google Scholar]
- Jones R. M., Mercante J. W. and Neish A. S. (2012). Reactive oxygen production induced by the gut microbiota: pharmacotherapeutic implications. Curr. Med. Chem. 19, 1519-1529. 10.2174/092986712799828283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. M., Luo L., Ardita C. S., Richardson A. N., Kwon Y. M., Mercante J. W., Alam A., Gates C. L., Wu H., Swanson P. A. et al. (2013). Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediated generation of reactive oxygen species. EMBO J. 32, 3017-3028. 10.1038/emboj.2013.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. M., Desai C., Darby T. M., Luo L., Wolfarth A. A., Scharer C. D., Ardita C. S., Reedy A. R., Keebaugh E. S. and Neish A. S. (2015). Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 12, 1217-1225. 10.1016/j.celrep.2015.07.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Reedy A. R. and Jones R. M. (2016). Detecting reactive oxygen species generation and stem cell proliferation in the Drosophila intestine. Methods Mol. Biol. 1422, 103-113. 10.1007/978-1-4939-3603-8_10 [DOI] [PubMed] [Google Scholar]
- Matthews J. D., Reedy A. R., Wu H., Hinrichs B. H., Darby T. M., Addis C., Robinson B. S., Go Y.-M., Jones D. P., Jones R. M. et al. (2018). Proteomic analysis of microbial induced redox-dependent intestinal signaling. Redox. Biol. 20, 526-532. 10.1016/j.redox.2018.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S. and Motohashi H. (2015). Roles of Nrf2 in cell proliferation and differentiation. Free Radic. Biol. Med. 88, 168-178. 10.1016/j.freeradbiomed.2015.06.030 [DOI] [PubMed] [Google Scholar]
- Rajan A. and Perrimon N. (2012). Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell 151, 123-137. 10.1016/j.cell.2012.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. D., Huang B.-W. and Tsuji Y. (2012). Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 24, 981-990. 10.1016/j.cellsig.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykiotis G. P. and Bohmann D. (2008). Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76-85. 10.1016/j.devcel.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S., Mkrtchyan M., Younossi-Hartenstein A., Merriam J. R. and Hartenstein V. (2008). The behaviour of Drosophila adult hindgut stem cells is controlled by Wnt and Hh signalling. Nature 454, 651-655. 10.1038/nature07156 [DOI] [PubMed] [Google Scholar]
- Takashima S., Younossi-Hartenstein A., Ortiz P. A. and Hartenstein V. (2011). A novel tissue in an established model system: the Drosophila pupal midgut. Dev. Genes Evol. 221, 69-81. 10.1007/s00427-011-0360-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.