Brackish water European perch tolerates significantly higher salinities than freshwater conspecifics due to a physiological specialization. Therefore, brackish water European perch populations may not receive recruitment from freshwater, which raises conservation issues regarding brackish water perch populations due to climate change and fisheries.
Keywords: Brackish water, osmoregulation, plasticity
Abstract
Although considered a stenohaline freshwater species, European perch (Perca fluviatilis) inhabit brackish waters. The present study determined the maximum salinity tolerance and osmoregulatory capability on individuals originating from brackish water and from freshwater populations. The fish were acclimated for 3 weeks to salinities of 0, 10, 12.5, 15, 17.5 and 20 after an initial stepwise increase to the target salinity. The maximum salinity tolerance was determined as the test salinity below which the fish could not acclimate and lost equilibrium. Blood plasma osmolality was measured if the fish had not lost equilibrium after the acclimation period. The maximum salinity tolerance was 17.5 for brackish water European perch and 10 for fresh water European perch. The high salinity tolerance of the brackish water European perch was caused by their ability to both hyper- and hypo-osmoregulate, whereas the freshwater originating fish could only hyper-osmoregulate. The results showed that maximum salinity tolerances and osmoregulatory capabilities depends on the origin habitat salinity. Due to genetic differentiation between European perch populations in brackish and fresh water, the possibility of brackish water European perch being a subspecies of European perch is discussed, yet vital knowledge concerning heritability of salinity tolerance traits is still missing. Regardless of species status, within-species plasticity in the ability to cope with varying salinities have substantial ecological and conservation implications and underlines the need for managing brackish water and freshwater European perch stocks separately.
Introduction
Environmental salinity constitutes a physiological challenge in teleosts due to osmotic water movement and ion diffusion between the environment and the internal milieu of the fish, predominantly occurring over the gills, which are permeable to facilitate respiratory gas exchange, acid-base regulation, and ammonia excretion (Evans et al., 2005). Teleosts must keep their internal osmolalities around 300–400 mOsm kg−1 to maintain homeostasis (Evans et al., 2005), and only tolerate a slight variation in internal osmolality (Lutz, 1972). To counteract osmotic distress, they osmoregulate (Larsen et al., 2014). Osmoregulation in water with salinities below iso-osmotic level (hyper-osmoregulation) consists of producing diluted urine via the renal system and taking up ions through specialized branchial cells (Larsen et al., 2014). Osmoregulation in water above iso-osmotic level (hypo-osmoregulation) is a vastly different physiological process than hyper-osmoregulation, and is obtained by imbibing ambient water, taking up the water through the gastro-intestinal tract, and excreting excess monovalent ions through specialized branchial cells and renal excretion of divalent ions (Larsen et al., 2014).
The vast majority of fish species are physiologically specialized to either hyper- or hypo-osmoregulate, and have limited abilities to do both (Evans, 1984). Therefore, many species have only limited dispersal potential in brackish water of estuaries and coastal areas (Potter et al., 2015). Amongst these is the European perch (Perca fluviatilis), a species widely distributed all over the Eurasian continent where it is common in estuaries such as the Baltic Sea (Thorpe, 1977; Craig, 2000; Couture and Pyle, 2015). There is currently inconsistency in the literature concerning the maximum salinity tolerance and osmoregulatory capability of the species. In Christensen et al. (2017), European perch survived at 420 mOsm kg−1, equivalent to a salinity of 15, whereas in Lutz (1972) and Overton et al. (2008) the fish succumbed at salinities above iso-osmotic conditions (ca. 300 mOsm kg−1, equivalent to a salinity of 10). The discrepancy may derive from intraspecific differences in salinity tolerance and osmoregulatory capability arising from whether the fish originates from varying salinities in brackish water estuaries or stable salinity habitats of freshwater lakes and streams. Population genetic studies have shown substantial differentiation among freshwater and brackish water European perch, where the origin habitat salinity explain around 20 % (Christensen et al., 2016; Nesbø et al., 1999; Skovrind, 2015; Skovrind et al., unpublished data). However, the discrepancy in maximum salinity tolerance could also derive from different methodological approaches between studies, and the hypothesis that origin habitat salinity determines the maximum salinity tolerance and osmoregulatory capabilities, therefore, ought to be tested within the same experimental framework.
European perch is an ecological key species, and socio-economically important for human consumption, for commercial, and for recreational fisheries (Thorpe, 1977; Craig, 2000; Couture and Pyle, 2015), and elucidating any discrepancies in maximum salinity tolerance amongst European perch populations would therefore be valuable knowledge for conservation of the species. The present study determined the maximum salinity tolerance and evaluated the osmoregulatory capability of European perch originating from brackish water and freshwater populations.
Materials and methods
Animal care and experimental protocols followed the guidelines of the Danish Experimental Animal Inspectorate.
Experimental animals
The brackish water European perch were obtained from a harbor site in Køge Bugt (55°31″N, 12°19E) in the western Baltic Sea where the salinity is on average 12 all year round (Skovrind et al., 2013; Christensen et al., unpublished data). The salinity in this area fluctuates considerably between around 0 to just above 20 during periods of sea water intrusion from Kattegat (Christensen et al., unpublished data), and the change in salinity can happen with up to 10 per day. The freshwater European perch were obtained from Lake Esrom (55°58′N, 12°22E), an inland lake with no downstream connection to a brackish water European perch population. The experimental animals were caught in April, May, and June, in 2016, 2017 and 2018, by angling and with cast net and transported to the Marine Biological Section, Elsinore, Denmark (permit number 12-7410-000008) (see Table 1 for details about size and sample size). All fish were kept in freshwater (non-chlorinated Elsinore tap water) at 20°C in 60 L aerated aquaria for 3 weeks before the experiments began, to allow for acclimation to the laboratory facilities, and to alleviate any effect of former temperature acclimation. The lighting scheme was 12 h light, 12 h dark. They were fed sliced herring (Clupea harengus) three times a week. Excess food was siphoned from the tanks and 2/3 of the water exchanged once a week. The water was filtered continuously through biofilters.
Table 1:
Maximum salinity tolerance of European perch (Perca fluviatilis) originating from brackish water (BW) and fresh water (FW).
| Origin | Salinity | Amb Osm | N | BM | SL | Long-term acclimated? |
|---|---|---|---|---|---|---|
| BW | 0 | 52 | 7 | 36.6 ± 3.8 | 13.4 ± 0.4 | Yes |
| 10 | 300 | 5 | 51.6 ± 4.9 | 14.1 ± 0.3 | Yes | |
| 12.5 | 363 | 7 | 10.0 ± 1.5 | 8.5 ± 0.3 | Yes | |
| 15 | 428 | 6 | 11.9 ± 3.8 | 8.4 ± 0.8 | Yes | |
| 17.5 | 498 | 7 | 25.3 ± 10.8 | 10.9 ± 0.9 | Yes | |
| 17.5 | 498 | 7 | 22.0 ± 10.8 | 10.4 ± 1.4 | No (LOE) | |
| 20 | 559 | 10 | 12.3 ± 1.6 | 8.7 ± 0.4 | No (LOE) | |
| FW | 0 | 51 | 9 | 16.6 ± 2.8 | 11.0 ± 0.9 | Yes |
| 10 | 278 | 8 | 16 ± 0.9 | 10.3 ± 0.1 | Yes | |
| 12.5 | 375 | 6 | 36.3 ± 6.5 | 13.0 ± 0.7 | No (LOE) |
Ambient salinity as salinity and osmolality (Amb Osm; mOsm kg−1), sample size (N), body mass (BM; g), standard length (SL; cm), and whether the fish accomplished long term acclimation to the salinity (3 weeks) is given. Loss of equilibrium (LOE) is indicated.
Maximum salinity tolerance
Brackish water and freshwater European perch were acclimated to salinities of 0, 10, 12.5, 15, 17.5 and 20 to determine their maximum salinity tolerance and osmoregulatory abilities. These salinities mimic the naturally occurring salinities in the western Baltic Sea where the brackish water European perch were obtained from. The target salinity was reached by increasing the salinity once a day starting from freshwater in the sequence 0, 10, 12.5, 15, 17.5 and 20, by adding filtered sea water from Kattegat. The fish were monitored twice a day, and euthanized if loss of equilibrium (LOE) occurred. LOE was defined as the point where the fish could not maintain an upright position, did not react to light tapping against the aquarium, and did not respond to being pinched in the tail. After 3 weeks at the target salinity, a blood sample was taken by caudal puncture. The blood sample was centrifuged (Sprout, Heathtrow Scientific, IL, USA) at 2000 g for 3 min, and the plasma osmolality measured in an osmometer (Vapor Pressure Osmometer 5520, Wescor Environmental, Logan, UT, USA), calibrated with the manufacturer’s standards. The fish were fed throughout the acclimation period, yet fasted 3 days prior to blood sampling. The maximum salinity tolerance was defined as the salinity level below which all fish reached LOE within 3 weeks.
Data analyses
The blood plasma osmolality values at each treatment were tested for normality with Shapiro–Wilk’s tests, and for variance homogeneity within each of the populations with Levene’s tests. The blood plasma osmolalities at the different salinities was compared within each population with one-way ANOVAs, followed by Tukey’s post hoc test if normally distributed and the variances homogeneous, and with one-way ANOVAs with Welch correction, followed by Games-Howell post hoc test, if normally distributed and the variances heterogeneous. All statistics were computed in SPSS statistics 25 (IBM, Armonk, NY, USA), and the significance level set to an α value of 0.05.
Results
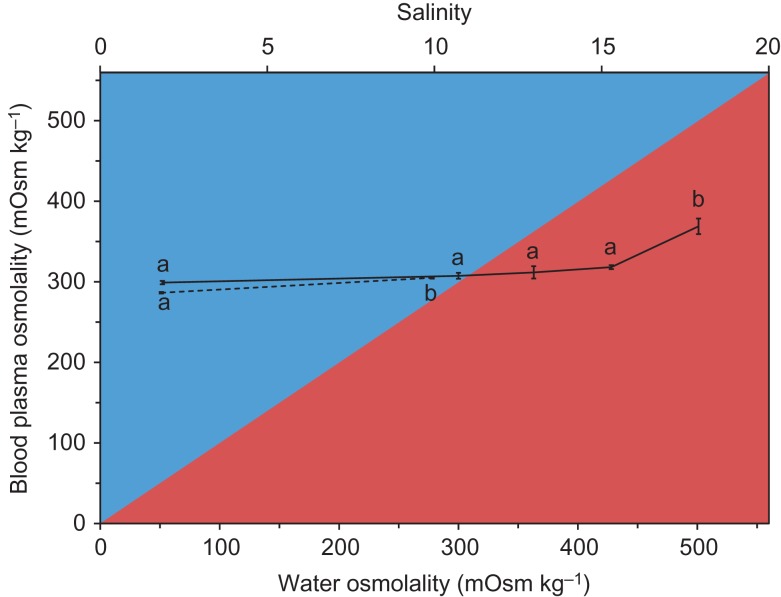
The maximum salinity tolerance of the brackish water European perch was 17.5, yet half of individuals reached LOE before 3 weeks at this salinity (Table 1). The brackish water European perch attempted acclimated to a salinity of 20 reached LOE on a median time of 6 days. The maximum salinity tolerance of the freshwater European perch was 10, and the fish reached LOE on a median time of 10 days at a salinity of 12.5. The blood plasma osmolality ranged from 286 to 369 mOsm kg−1, and increased significantly approaching the maximum salinity tolerance for both the brackish water European perch and the freshwater European perch [one-way ANOVA with Welch correction, F(4, 12.692) = 15.39, P < 0.001, and one-way ANOVA, F(1,15) = 5.931, P = 0.028, respectively] (Fig. 1). The brackish water European perch were able to both hyper- and hypo-osmoregulate, whereas the freshwater European perch were only able to hyper-osmoregulate.
Figure 1:
Osmoregulatory capabilities of European perch (Perca fluviatilis). Blood plasma osmolality is shown in relation to ambient water osmolality. The black line represents fish of brackish water origin, the dashed line represents fish of freshwater origin. Data is shown as the average ± SE. Different letters are assigned to significantly different groups within each population (Games-Howell tests used for the brackish water European perch, Tukey’s test used for the freshwater European perch). For details about sample sizes, please consult Table 1. The blue area is where the fish hyper-osmoregulate and the red area is where the fish hypo-osmoregulate (osmoregulation in water with an osmolality lower and higher than the internal osmolality of the fish, respectively).
Discussion
Maximum salinity tolerance and osmoregulatory capability
The brackish water European perch had a maximum salinity tolerance of 17.5, which was substantially higher than the maximum salinity tolerance of the freshwater European perch (10). Intriguingly, the brackish water European perch had the ability to both hyper- and hypo-osmoregulate, in contrast to the freshwater European perch, which could only hyper-osmoregulate. Other freshwater European perch populations have also been shown unable to hypo-osmoregulate (Lutz, 1972; Overton et al., 2008), which must therefore be considered normal amongst freshwater European perch. Hyper- and hypo-osmoregulation in teleosts are two fundamentally different physiological processes (Larsen et al., 2014), and the ability of the brackish water European perch to do both must thus be associated with physiological specialization to life in brackish water. This, in turn, increases the maximum salinity tolerance and thus enhances the species distribution potential in brackish water.
Tagging studies alongside physio-chemical measurements have shown that brackish water European perch in the western Baltic Sea experience salinities around 12 throughout the year, ranging from 0 to above 20 (Olsen, 2002; Skovrind et al., 2013; Christensen et al., unpublished data). These populations conduct winter migrations into lower reaches of streams (Olsen, 2002; Skovrind et al., 2013; Christensen et al., unpublished data), presumably to save energy on osmoregulation at low temperatures (Christensen et al., 2017). Spawning also occurs in brackish water with successful hatching (Christensen et al., 2016; Skovrind et al., 2013) at salinities higher than the tolerance salinities of eggs and fry in other European perch populations (Klinkhardt and Winkler, 1989; Tibblin et al., 2012). Exposure to high environmental salinities throughout the whole life cycle of European perch in the western Baltic Sea likely adds a substantial selection pressure for higher salinity tolerance in brackish water European perch populations.
Is brackish water European perch an independent species?
Varying salinity tolerances and osmoregulatory capability can be determining factors when assessing management units of closely related fish populations, even to the point of classifying differentiating populations as subspecies or sister species. For instance, in whitefish (Coregonus spp.), a population endemic to the Wadden Sea has been classified as North Sea houting (Coregonus oxyrhynchus), an independent species, in part due to its anadromous lifestyle and higher salinity tolerance compared to European whitefish (Coregonus lavaretus) (Hansen et al., 1999). In pupfish (Cyprinodon variegatus), a population endemic to central Florida is designated a subspecies, the Lake Eustis pupfish (Cyprinodon variegatus variegatus), on behalf of its distinct osmoregulatory capability, and debate is ongoing as to whether it should be regarded an independent species (Brix and Grosell, 2013).
Nesbø et al. (1999) demonstrated genetic differentiation among stationary and anadromous European perch populations in the northern Baltic region. Furthermore, Skovrind (2015) showed significant genetic differentiation between European perch from fresh water and brackish water in the western Baltic region, using full genome representative sequencing on six freshwater, and six brackish water populations (N = 190). The two European perch populations of the present study are from the same study sites as the ones used in Skovrind (2015), and it is likely that the population structure and genetic differentiation is associated with differences in maximum salinity tolerance and osmoregulatory capability between brackish water and fresh water European perch populations. Together with the genetic differentiation, these physiological differences in relation to origin habitat salinity could indicate an emerging speciation between brackish water and freshwater European perch, as it is argued for North Sea houting and Lake Eustis pupfish (Hansen et al., 1999; Brix and Grosell, 2013). However, it remains untested to what extend salinity tolerance and osmoregulatory capability is an inheritable characteristic, which must be clear before a separate species status may apply.
Conservation perspectives
Regardless of species status, the results of the present study are valuable information for ecologists and conservation biologists. Locally, environmental salinity in estuaries and coastal areas is currently susceptible to changes due to altering patterns in river-runoff and evaporation associate with climate change (Harley et al., 2006; Vuorinen et al., 2015). Furthermore, substantial recreational and commercial fisheries for European perch take place in the Baltic Sea (Craig, 2000; Thorpe, 1977; Christensen et al., unpublished data). To conserve the species, and mediate ecological effects of climate change in these areas, the increased salinity tolerance and osmoregulatory capability of brackish water European perch needs to be recognized, as it is unlikely that a depleted stock will receive successful recruitment from nearby freshwater stocks.
It remains unknown whether the varying salinity tolerance and osmoregulatory capability applies to other species of freshwater fishes in estuaries and coastal regions (Potter et al., 2015), or is unique to European perch in the western Baltic Sea. Further exploration into this matter could be target for future studies.
Acknowledgements
The authors would like to express their gratitude to Andreas Ruth, Hedrikur Bergsson, Julius Nielsen, Kevin L. Schauer, Mikkel Skovrind, Morten Bo Søndergaard Svendsen, Sachia Jo Traving and Sofie Jacobsen for their help with catching European perch and fish holding, and to DFK and other office inhabitants for moral support.
Funding
E.A.F. Christensen was supported by the Augustinus Foundation, Christian and Ottilia Brorson’s travel grant, Elisabeth and Knud Petersens’s Foundation, strategic internal Ph.D. funding of Department of Biology, University of Copenhagen, and Knud Højgaard’s Foundation. M.G. is Maytag Professor of Ichthyology and was supported by NSF (IOS 1146695).
References
- Brix KV, Grosell M (2013) Evaluation of pre- and post-zygotic mating barriers, hybrid fitness and phylogenetic relationship between Cyprinodon variegatus variegatus and Cyprinodon variegatus hubbsi (Cyprinodontiformes, Teleostei). J Evol Biol 26: 854–866. [DOI] [PubMed] [Google Scholar]
- Christensen EAF, Skovrind M, Olsen MT, Carl H, Gravlund P, Møller PR (2016) Hatching success in brackish water of Perca fluviatilis eggs obtained from the western Baltic Sea. Cybium 40: 133–138. [Google Scholar]
- Christensen EAF, Svendsen M, Steffensen JF (2017) Plasma osmolality and oxygen consumption of perch Perca fluviatilis in response to different salinities and temperatures. J Fish Biol 90: 819–833. [DOI] [PubMed] [Google Scholar]
- Couture P, Pyle G (2015) Biology of Perch. CRC Press, Boca Raton. [Google Scholar]
- Craig JF. (2000) Percid Fishes—Systematics, Ecology and Exploitation. Blackwell Sceience Ltd., Dunscore. [Google Scholar]
- Evans DH. (1984) The roles of gill permeability and transport mechanisms in euryhalinity In Hoar WS, Randall DJ, eds. Fish Physiology, Vol XIB Academic Press, Orlando, pp 239–283. [Google Scholar]
- Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol Rev 85: 97–177. [DOI] [PubMed] [Google Scholar]
- Hansen MM, Mensberg K-LD, Berg S (1999) Postglacial recolonization patterns and genetic relationships among whitefish (Coregonus sp.) populations in Denmark, inferred from mitochondrial DNA and microsatellite markers. Mol Ecol 8: 239–252. [Google Scholar]
- Harley CDG, Hughes AR, Hultgren KM, Miner BG, Sorte CJB, Thornber CS, Rodriguez LF, Tomanek L, Williams SL (2006) The impacts of climate change in coastal marine systems. Ecol Lett 9: 228–241. [DOI] [PubMed] [Google Scholar]
- Klinkhardt M, Winkler HM (1989) Einfluß der Salinität auf die Befruchtungs- und Entwicklungsfähigkeit der Eier von vier Süßwasserfischarten Plötz (Rutilus rutilus), Barsch (Perca fluviatilis), Kaulbarsch (Gymnocephalus cernua) und Zander (Stizostedion lucioperca). Wiss Zeitschrift Univ Rostock 38: 23–30. [Google Scholar]
- Larsen EH, Deaton LE, Onken H, O’Donnell M, Grosell M, Dantzler WH, Weihrauch D (2014) Osmoregulation and excretion. Compr Physiol 4: 405–573. [DOI] [PubMed] [Google Scholar]
- Lutz PL. (1972) Ionic and body compartment responses to increasing salinity in the perch Perca fluviatilis. Comp Biochem Physiol 42A: 711–717. [DOI] [PubMed] [Google Scholar]
- Nesbø CL, Fossheim T, Vøllestad LA, Jakobsen KS (1999) Genetic divergence and phylogeographic relationships among European perch (Perca fluviatilis) populations reflect glacial refugia and postglacial colonization. Mol Ecol 8: 1387–1404. [DOI] [PubMed] [Google Scholar]
- Olsen JS. (2002) Vækst, migration og reproduction hos en dansk population af brakcandsaborre (Perca fluviatilis L.). Master’s thesis. University of Copenhagen.
- Overton JL, Bayley M, Paulsen H, Wang T (2008) Salinity tolerance of cultured Eurasian perch, Perca fluviatilis L.: effects on growth and on survival as a function of temperature. Aquaculture 277: 282–286. [Google Scholar]
- Potter IC, Tweedley JR, Elliott M, Whitfield AK (2015) The ways in which fish use estuaries: a refinement and expansion of the guild approach. Fish Fish 16: 230–239. [Google Scholar]
- Skovrind M. (2015) Population structure of stenohaline anadromous fishes. Master’s thesis. University of Copenhagen.
- Tibblin P, Koch-Schmidt P, Larsson P, Stenroth P (2012) Effects of salinity on growth and mortality of migratory and resident forms of Eurasian perch in the Baltic Sea. Ecol Freshw Fish 21: 200–206. [Google Scholar]
- Thorpe J. (1977) Synopsis of Biological Data on the Perch Perca fluviatilis Linnaeus, 1758 and Perca Flavescens Mitchill, 1814. Food and agriculture organization of the united nations, Rome. [Google Scholar]
- Vuorinen I, Hänninen J, Rajasilta M, Laine P, Eklund J, Montesino-Pouzols F, Corona F, Junker K, Meier HEM, Dippner JW (2015) Scenario simulations of future salinity and ecological consequences in the Baltic Sea and adjacent North Sea areas-implications for environmental monitoring. Ecol Indic 50: 196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]