Abstract
Objectives:
Pain assessment is enigmatic. Although clinicians and researchers must rely upon observations to evaluate pain, the personal experience of pain is fundamentally unobservable. This raises the question of how the inherent subjectivity of pain can and should be integrated within assessment. Current models fail to tackle key facets of this problem, such as what essential aspects of pain are overlooked when we only rely on numeric forms of assessment, and what types of assessment need to be prioritized to ensure alignment with our conceptualization of pain as a subjective experience. We present the multimodal assessment model of pain (MAP) as offering practical frameworks for navigating these challenges.
Methods:
This is a narrative review.
Results:
MAP delineates qualitative (words, behaviors) and quantitative (self-reported measures, non–self-reported measures) assessment and regards the qualitative pain narrative as the best available root proxy for inferring pain in others. MAP offers frameworks to better address pain subjectivity by: (1) delineating separate criteria for identifying versus assessing pain. Pain is identified through narrative reports, while comprehensive assessment is used to infer why pain is reported; (2) integrating compassion-based and mechanism-based management by both validating pain reports and assessing underlying processes; (3) conceptualizing comprehensive pain assessment as both multidimensional and multimodal (listening/observing and measuring); and (4) describing how qualitative data help validate and contextualize quantitative pain measures.
Discussion:
MAP is expected to help clinicians validate pain reports as important and legitimate, regardless of other findings, and help our field develop more comprehensive, valid, and compassionate approaches to assessing pain.
Key Words: pain assessment, qualitative methods, mixed-methods, patient communication, narrative medicine, medical ethics, brain imaging, definition of pain, compassionate care, mechanism-based management
Pain is an enigmatic phenomenon that is challenging to treat and study. One challenging attribute is its subjective nature. Pain is defined as a subjective experience,1 which means that it cannot be directly observed by those who are not experiencing it. Yet, clinicians and researchers rely upon observations and measures to assess and infer the pain experienced by other people. This raises the fundamental question of how the inherent subjectivity of pain can and should be addressed and integrated within its assessment.
Current frameworks for guiding pain assessment do not adequately tackle this problem. For instance, biopsychosocial frameworks encourage a multidimensional approach to pain assessment. However, they do not specify the different ways through which this assessment can be conducted. They also do not delineate how different forms of assessment relate to the subjective experience of pain. Different approaches to assessment include observing direct expressions of pain, such as the words and behaviors used by the person in pain. They also include administering quantitative measures of pain, such as self-report questionnaires as well as physiological and psychophysical measures. The overwhelming emphasis in the pain literature is on quantitative methodologies. As a result, pain assessment strategies are typically focused on aspects of pain most readily communicated through numbers, such as pain intensity ratings or pain threshold levels. Although quantitative pain measures are vital to understanding and targeting mechanisms and benchmarking management, they often overlook important attributes of the subjective experience, such as personal context and meaning, which can profoundly shape the experience of pain. Current models do not adequately emphasize what aspects of the pain experience can be uniquely accessed through more qualitative forms of assessment, such as talking, observing, and listening. Previous reports show that patients with pain often do not feel listened to or understood by their health care providers.2–6 These findings highlight the need for assessment models that specifically emphasize how to address subjectivity related to pain.
Current models of pain assessment also fail to provide adequate guidance on what forms of assessment should be prioritized to best align with our conceptualization of pain as a subjective experience. This creates ambiguity in what should be regarded as a root proxy for the pain experience. Specifying an observable, root proxy for pain is an essential step in establishing a conceptual bridge between the nonobservable experience of pain and our assessment methodology. Recent debate on how to best integrate physiological measures of brain activity within pain assessment illustrates the ambiguity in this area. On the one side, there have been calls for using measures of brain activity as objective biomarkers of the pain experience,7–9 whereas on the other, self-report is prioritized as the best root proxy for pain.10,11 Ambiguity in this area raises important challenges for people assessing pain. For instance, when faced with discrepancies across different forms of assessment, clinicians report uncertainty in trying to decide which of their assessment findings should be relied upon as indicators for the nonobservable pain experience.12–16 Although there is preliminary consensus among leaders in brain imaging research that self-reports of pain should be prioritized over physiological measures,17 there is still a lack of clarity in what this means for clinical practice and research. For instance, what do we mean by self-reports of pain? If the intention is to support patient autonomy, should self-report measures be regarded on equal footing with direct narrative reports of pain? Also, from a research perspective, how should objective measures of pain be best anchored to more subjective forms of assessments? Assessment frameworks are needed to help inform decision-making around these questions.
Polarities of opinion in what should be regarded as a root proxy for pain emphasize the potentially competing pillars of what we value in pain assessment. Objective measures of pain are valued, in part, for their usefulness in guiding mechanism-based management.7–9 Self-report is valued, in part, for its ability to support patient autonomy and to provide compassion-based care.10,11,18 Failure to support both of these pillars is associated with important risks. For instance, failure to identify underlying pain mechanisms can result in, at best, a waste of time for the patient and clinician and, at worst, iatrogenesis by providing a rationale for potentially harmful treatments. Similarly, failure to validate pain reports and show compassion can increase patient distress, degrade therapeutic alliance, and undermine hope for improvement.5,16,19,20 Current models of assessment do not provide adequate guidance on how to navigate these competing values. Without guidance in this area, there is increased potential for conflating the validation of a pain experience with the identification of its underlying mechanisms. For instance, pain reports that can be linked to specific mechanisms may be validated as legitimate, while reports without clear links may be dismissed as spurious. Not delineating these aspects of assessment raises the risk that people living with pain continue to feel stigmatized and alienated when certain findings from their assessment are used to invalidate their reports of pain.2–6,20,21 Assessment frameworks are needed to help establish criteria for both legitimizing pain and supporting the principles of mechanism-based management.
This paper introduces the multimodal assessment model of pain (MAP; Table 1 and Fig. 1), a novel framework that aims to address these gaps by: (1) specifying a root proxy for the subjective pain experience; (2) characterizing how different assessment methodologies relate to pain subjectivity; and (3) offering frameworks to further integrate the subjective pain experience within pain research and practice. The first sections of this paper aim to delineate MAP’s nomenclature, postulates, and applications to clinical practice and research. This is followed by a general discussion of how MAP relates to the broader literature and implications for future work.
TABLE 1.
Key Postulates and Applications of the MAP
FIGURE 1.
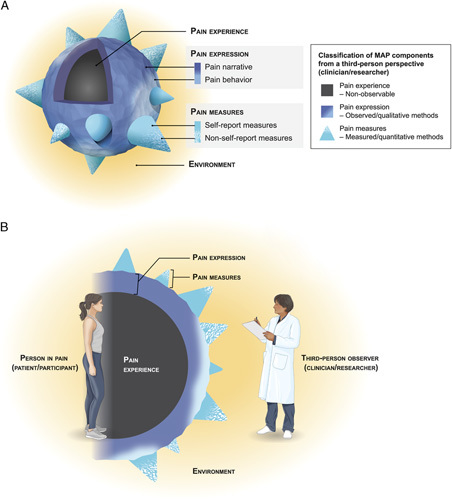
The MAP. A, MAP components are shown from a third-person perspective. This 3-dimensional view emphasizes the nonobservable nature of the pain experience and the relative breadth and scope of the different model components. B, MAP components are depicted in 2-dimensional cross-section and are oriented to both first-person and third-person perspectives. This view emphasizes how pain experience is a function of the whole person, who is influenced by environmental and contextual factors, and how this person relates to different assessment methodologies used in research and practice settings. In both images, the size and shape of MAP components reflect their capacity to address subjectivity related to pain; components with greater breadth and volume have greater potential to address pain-related subjectivity. The textured surface of pain expression represents the idiosyncratic collection of words and behaviors that any particular individual may use to express pain. This is in contrast to the smooth surface of pain measures, which require expressions of pain to be translated into standardized metrics. Cone size represents the relative ability of different pain measures to quantify different aspects of pain expression; measures with relatively larger cones indicate that they address a broader scope of pain expression. Gradients are used to depict the intimate link between the pain narrative and pain behavior, as well as the measures that bridge traditional classification as either self-report or non–self-report (eg, psychophysical measures). MAP indicates multimodal assessment model of pain.
MAP NOMENCLATURE AND POSTULATES
MAP classifies the experience of pain and the observable attributes related to this experience within the following 3 components: pain experience, pain expression, and pain measures. Pain experience is defined as an unpleasant sensory and emotional experience,1 and is understood to be a function of the whole person. Pain expression is the broad collection of qualitative words and behaviors that communicate pain and is divided into 2 subcomponents—pain narrative, representing the words used to describe pain, and pain behavior, representing pain-related nonverbal and para-verbal behaviors. Pain measures are the quantitative tools used to assess pain and are subcategorized into self-report measures and non–self-report measures.
The environment is understood to include everything surrounding the person in pain and influences all MAP components. Examples of environmental factors include the characteristics of the people around the person in pain, such as their visual appearance, demeanor, and relationship to the person reporting pain, as well as the characteristics of the physical environment, such as lighting, physical objects, and familiarity to the person in pain. Consistent with past research, dynamic processes are understood to link MAP components, such as those involving the personal encoding of pain experience into pain expression and pain measures and the observer decoding of pain expression and pain measures.22–26 Both environmental and personal factors are understood to shape these processes.
Considering Pain Assessment From a Third-Person Perspective
MAP delineates first-person and third-person perspectives related to pain (Fig. 1B). The first-person perspective is from the person experiencing pain (eg, patients, participants). The third-person perspective is from those observing this person (eg, clinicians, researchers). MAP is designed for clinicians and researchers and is therefore categorized from a third-person perspective. From this third-person perspective, pain expression and pain measures can be assessed, whereas pain experience cannot be observed.
Delineating the Capacity of Different Methodologies to Assess Subjectivity
Assessment methodologies that target pain expression and pain measures differ in their capacity to accommodate different expressions of the subjective pain experience. Compared with pain measures, pain expression has a greater capacity to assess subjectivity as it can accommodate more variance in the idiosyncratic ways that pain can be communicated. The person (MAP is designed for use among people who are able to provide meaningful self-report; existing frameworks address how to ethically approach pain assessment among those who cannot provide self-report [please see Table 2 for references]) in pain can use a broad, personally derived and unique collection of words and behaviors (eg, analogies, facial expression, postures) to express different aspects and ideas related to the subjective pain experience.23,29,43,50–56 These expressions of pain can be directly observed by clinicians and assessed via qualitative methodologies by researchers.
TABLE 2.
Rationale for Selecting Pain Narrative as the Root Proxy for Identifying the Nonobservable Experience of Pain

Alternatively, clinicians must rely on validated measures, and researchers must use quantitative methodologies, to translate these different expressions of pain into pain measures (eg, numeric ratings, thresholds, brain-activation patterns39,57–60). In theory, this quantification process could at best match the richness and complexity of qualitative expressions of pain. In practice, however, this would involve generating validated measures for every potential combination of words and behaviors (and each of their conveyed personal meanings61) that individuals associate with pain. Accordingly, MAP assumes that only a subset of pain expression is quantified as pain measures. Thus, pain measures have comparatively less potential for accommodating idiosyncratic expressions of pain as they are selected and generated by the assessor and only permit expression via standardized metrics.
Identifying a Proxy for Pain Experience
MAP identifies the qualitative pain narrative as the best available root proxy for pain experience. Establishing an observable, root proxy is intended to serve as a link between the nonobservable pain experience and assessment methodology and can be used to help identify people in pain and to establish correlates of pain. By definition, such a proxy cannot be invalidated by other pain-related measures or expressions. Although there is no perfect proxy for the nonobservable pain experience,10 there are several reasons why pain narrative is best suited for this role.
As described in Table 2, when compared with other model components: (1) pain narrative is the most conceptually aligned with the definition of pain; (2) best supports ethical principles when applied to practice; and (3) is commonly regarded as a root source of validity in research and practice. Narrative is understood to be the richest medium for communicating a subjective experience as it permits unique access to personal meaning and is highly sensitive to personal context.27–32 This richness is an essential attribute of a proxy for pain experience as it creates a more robust foundation for establishing other potential correlates of pain. Consistent with the foundational arguments in previous critiques,24,62,63 the qualitative pain narrative is preferred as a proxy over quantitative self-report measures as it provides a more comprehensive medium for expressing subjectivity. Pain narrative is also preferred over pain behavior as it offers greater precision as a medium for communication and avoids important ethical challenges to autonomy that occur when observable behaviors are used to invalidate reported experiences.18,33–35,45–49
THE ROLE OF MAP IN FURTHER INTEGRATING PAIN SUBJECTIVITY WITHIN CLINICAL PRACTICE
Dissociating Assessment Findings From the Validation of the Pain Experience
The historical inclination to only legitimize pain reports linked to pathology can result in treatment being contingent on the identification of “real” pain.14,15,64,65 When pain is a clear symptom of tissue damage (ie, “real” from a clinicopathologic paradigm14), treatment is provided; otherwise pain reports may be dismissed as having purely psychological or socially motivated origins and clinically neglected. Although this approach is not consistent with current evidence or biopsychosocial models of practice, many clinicians still find it challenging to accept pain without a link to pathology and many patients still feel stigmatized by clinicians who dismiss their reports of pain.2–6,12–16,20,21,66,67 The challenge for patients in these clinical encounters is that assessment findings are being used to validate or invalidate their experience of pain. This challenge can persist when applying current mechanism-based management paradigms68–71 to pain reports that cannot be adequately explained.
MAP addresses this challenge by offering clinicians distinct frameworks for identifying and assessing pain. These are centered on 2 fundamental questions—(1) how do I identify “real” pain? and (2) how can I understand the processes that have led my patient to report pain? The first question is directly addressed by identifying pain narrative as the best available proxy for the nonobservable pain experience. Using pain narrative as a clinical proxy for the subjective experience of pain is expected to help clinicians acknowledge and legitimize all patient reports of pain as valid and important experiences, which is an essential element in compassionate and ethical pain management.15,34,72,73
The second question is addressed through a triangulation process that integrates all of MAP’s observed and measured components. Although pain narrative is arguably the best way to identify a “real” pain experience, it is insufficient (when considered in isolation) in the comprehensive assessment of pain.22,24 Comprehensive pain assessment should consider all data that can potentially help explain the factors and processes that have led a patient to report pain.9,10,22,24,40,65,74 Potentially explanatory data are accessed via a range of assessment strategies, including talking, observing, and listening to patients as well as having them complete standardized questionnaires, tasks, and physiological measures. Because each assessment methodology is associated with unique advantages and limitations, there is inherent value in strategically combining them to best explain the processes and factors that have led a patient to report pain.9,10,22,24,40,65 Triangulation is commonly conceptualized as combining multiple data sources to evaluate convergence on a particular domain.31,75,76 The proposed triangulation process involves evaluating how all observed and measured components of MAP converge to explain the processes that have led a patient to report pain. By shedding light on underlying processes, this triangulation strategy is expected to assist clinicians in classifying and diagnosing patients and thereby help implement current mechanism-based models of management.68–71
Integrating Mechanism-based and Compassion-based Management
Mechanism-based pain management has many advantages, but raises the risk of stigmatizing unexplained pain.14 Accepting patients’ pain narratives as accurate representations of their experiences mitigates stigma by ensuring that at a minimum—even when mechanisms are unclear and no viable treatment can be provided—a potential suffering experience is validated and addressed with compassion (This call to validate all reports of pain in the context of pain assessment is not intended to restrict therapeutic interventions that may help patients critically reflect on, and potentially amend, the content and meaning of their pain narratives). MAP is also expected to help clinicians shift their thinking about pain, from a symptom and diagnosis, to a subjective experience. An analogy for this conceptual shift is the difference between feelings of sadness versus a diagnosis of clinical depression. When feelings of sadness are reported to a clinician, he or she may conduct a comprehensive assessment to determine whether these feelings are reflective of a broader clinical depression. However, even if the clinician concludes that the diagnostic criteria for clinical depression are not met, the patient’s report of sadness are still validated as legitimate and important. Similarly, MAP facilitates thinking about pain report as a means of communicating a personal experience (like reports of sadness), rather than something that can be confirmed or rejected through links to pathology, known mechanisms or diagnostic criteria (like clinical depression). This conceptual shift may help clinicians better validate patients’ subjective pain experiences while simultaneously advocating management strategies that are designed to target its root causes. Future research will determine whether MAP can help integrate these principles within clinical training programs to reduce stigma and better empower patients.
Emphasizing That Comprehensive Pain Assessment is Both Multidimensional and Multimodal
Related to this triangulation process, MAP can be used to further expand how comprehensive pain assessment is characterized. Past work has emphasized a multidimensional approach to pain assessment as a means of investigating different pain-related biopsychosocial factors. Guidelines typically advocate assessing these factors through a battery of validated pain measures.44,58,65,77–80 Although this approach has numerous benefits, it does not specifically guide clinicians to qualitatively assess pain expression and thus may overlook important aspects of pain subjectivity. The MAP triangulation process addresses this by emphasizing multimodal assessment, which specifically combines both qualitative and quantitative assessment strategies.
It should be noted that multimodal assessment, in and of itself, is not novel within current clinical practice. Indeed, even the most biomedically oriented pain assessment would typically include qualitative (eg, what brings you in today?) and quantitative (eg, please rate your pain on this 0 to 10 scale) components. The novel contribution of MAP in this regard is that it provides a framework for prioritizing and integrating these different forms of assessment. This may be particularly useful in helping clinicians avoid narrowly focusing on quantitative measures. For instance, when confronted with a patient that rates his pain intensity as 12 on a 10 point scale, some clinicians may be inclined to focus on the fact that, from a quantitative perspective, this is not a valid response. This focus may in turn lead to increased skepticism about the patient’s report of pain and undermine the therapeutic alliance with the patient. In contrast, by emphasizing the central importance of narrative, MAP may help shift the clinician’s focus to what the patient is trying to communicate, asking for instance, how this rating fits with the patient’s broader narrative. This may in turn facilitate greater understanding and collaboration with the patient. By specifically emphasizing multimodal assessment, MAP may increase the clinical importance of talking, observing, and listening within pain assessment29,56,62,81 and further encourage treatment strategies that focus on qualitative narrative for therapeutic benefit.82–89
THE ROLE OF MAP IN FURTHER INTEGRATING PAIN SUBJECTIVITY WITHIN RESEARCH
Using Qualitative Pain Expressions to Identify and Contextualize Pain Measures
Recent work recommends using personal reports of pain to differentiate measures of pain from measures of nociception.10,17,40 Figure 1 may help implement these recommendations by serving as a visual framework for differentiating these measures. Measures with direct or indirect (indirect links with pain narrative may be established via links to self-report pain measures and pain behavior) links to pain narrative can be classified as pain measures, while other measures may not. This framework may help researchers evaluate potential links between newly developed measures and pain subjectivity and thus help inform the integration of physiological biomarkers within pain assessment.9–11,17,40
Recent work highlights the limited ability of different pain measures to adequately represent the experiences of people living with pain. For instance, pain intensity ratings have been criticized for being too narrow to adequately capture a meaningful cross-section of pain expression.24,62,90–94 This can lead to an overly narrow focus on the sensory aspects of pain and contribute to an overreliance on pharmacological interventions as a means of lowering intensity ratings.94 MAP can serve as a high-level heuristic for framing these concerns by addressing the potential alignment between the relative scopes of pain measures versus pain expression. Figure 1 helps to visualize this relationship through the relative surface area of pain expression that is covered by a given pain measure (eg, measures that quantify additional aspects of pain expression cover a proportionally larger surface area, see note in Fig. 1 for details). This may guide further mixed-method research that anchors different pain measures within qualitative pain expression and that evaluates their potential advantages/disadvantages in representing the subjective experiences of people living with pain.43,95–99
Promoting Qualitative Pain Research
Qualitative methodologies have historically been poorly integrated within leading pain journals, despite their unique ability to access pain subjectivity.29,31,75,76 MAP’s central emphasis on qualitative methodologies highlights their importance for pain research and provides a framework for further integrating them within the pain literature.
DISCUSSION
MAP aligns with and builds on a recent consensus statement by a presidential task force of the International Association for the Study of Pain (IASP) that addresses recent debate on whether brain imaging data should be prioritized over subjective reports of pain.17 Consistent with MAP, the IASP task force recommends that self-reports of pain should be regarded as the sole criteria for identifying the pain experience and that the use of neurophysiological data related to pain should be limited to understanding underlying mechanisms and personalizing treatment. MAP extends these conclusions in 3 ways. First, MAP adds specificity by further delineating between qualitative and quantitative forms of self-report data and by providing an argument for why the qualitative pain narrative should be regarded as the best root proxy for the pain experience. Second, MAP helps contextualize brain imaging data with other qualitative and quantitative behavioral and physiological data by encouraging multimodal approaches to assessment. Third, MAP provides frameworks for how this consensus recommendation might be applied within both clinical and research contexts. Within the context of clinical practice, this involves dissociating multimodal assessment findings from the validation of the pain experience. Within a research context, this involves linking the value of neuroimaging data as a measure of pain to the breadth and depth with which it maps onto different qualitative expressions of pain.
One concern with these conclusions that has been previously raised in the literature, is that relying on self-report as a proxy for the pain experience will result in validating potentially dishonest reports of pain.24,100 This concern has fueled ongoing debate regarding how to define pain. Williams and Craig, for instance, have proposed to amend the current definition of pain, in part, to limit its prioritization of self-report data and thereby provide a clearer mechanism for identifying and challenging pain reports that are judged to be dubious.101–103 MAP aims to provide a fresh lens to consider these concerns and to lay the foundation for clinical management frameworks that will help address them.
MAP brings a novel perspective to this debate by being one of the first models to adopt a third-person perspective on pain assessment. One advantage of this perspective is that it aligns with the third-person, empirical view that is most commonly used to study pain and thus enables us to more clearly delineate, and work within, the limits of our scientific methodologies. From this perspective, the puzzle of how to identify dishonest reports of pain cannot be solved. This is consistent with a recent review of behavioral assessment strategies which indicates that there are no objective proxies that may accurately identify whether a reported pain experience is or is not valid.104 It is also consistent with the conclusions of the IASP task force who indicate that brain imaging data cannot function as a “lie-detector” to validate or invalidate a self-reported pain experience.17 Thus, there are no methodologies that currently exist, or that are expected to be developed, that can be used to objectively verify subjective reports of pain. In light of these methodological limitations, MAP aims to shift the focus of this debate from its unsolvable attributes to more actionable management strategies. It does this by not putting the legitimacy of pain reports into question, but instead, focusing on understanding why the person is reporting pain. Once the reasons for reporting pain can be classified, appropriate treatment can be offered. This shift in focus raises 2 important questions for future research—(1) how to classify patients whose reports of pain have no apparent alignment with other assessment findings? and (2) what to do about their management? The development of such frameworks is no small feat and may end up challenging traditional ways of conceptualizing and classifying pain. However, MAP helps clarify some solid first principles from which to approach this work, namely that it does not make empirical or ethical sense to invalidate subjective reports of pain.
Although we have outlined several of the potential advantages of applying MAP to research and practice, it is important to also point out its potential limitations. For instance, from a clinical perspective it could be argued that an increased focus on pain narratives may be overly burdensome for the time constraints of daily practice and, if implemented, may inadvertently encourage patients to fixate on their pain. Expanding the qualitative aspects of pain assessment does raise the risk of increased time spent on assessment. However, this can potentially be mitigated by research that helps identify the best approaches for navigating qualitative pain assessment—there is surprisingly little research in this area—such as which questions should be asked and guidelines on how to frame them. This may help make the qualitative aspects of pain assessment more efficient. Clinical training programs will also likely be needed to translate these findings into practice and to help ensure that an increased focus on pain during the initial assessment does not derail evidence-based approaches to treatment that focus on improving function and quality of life. However, even if a MAP-based approach to clinical assessment may be more labor intensive, it is possible that this initial investment of resources will be offset by improved patient outcomes and result in a net savings of resources and costs across the duration of treatment.
From a research perspective, there may also be important challenges in bridging the cultural gulf between quantitative and qualitative methodologies. For instance, quantitative pain researchers may have difficulty interpreting theoretical jargon used in qualitative methodologies and may perceive qualitative findings to have limited relevance due to their inherent subjectivity and limited generalizability. An essential step in facilitating this process will be identifying key areas of pain research that are both uniquely suited to qualitative methodologies and that hold strong potential for advancing the broader field. One area that is primed to benefit from additional qualitative research is pain-related suffering. Suffering is given a central place within foundational models of pain,105–108 yet remains empirically underdeveloped within the pain literature. Moreover, the limited research on suffering within the pain literature has primarily focused on numeric ratings of its intensity.106 Although easily quantified, intensity ratings provide no information about the nature, meaning or source of suffering and thus may be of limited value in directing patient care. Qualitative and mixed methodologies, on the other hand, can be used to more fully characterize the construct of pain-related suffering and to develop tools to help clinicians better recognize and assess this experience. For instance, these methodologies could be used to develop brief qualitative interview guides that help primary-care clinicians have a conversation with their patients about pain-related suffering and probe the perceived cause, impact, and preferred course of action. Additional qualitative research on pain-related suffering may eventually provide primary-care clinicians with an evidence-base to develop alternate models of care that are not exclusively directed at the sensory aspects of pain15,109—a potentially important contribution in navigating the ongoing challenges related to opioid use in pain management.94
CONCLUSIONS
More than a half-century ago, McCaffery37 highlighted the importance of validating patient narratives about pain when she wrote, the now classic maxim, that “pain is what the person says it is and exists whenever he or she says it does.” Since then, there have been considerable technological advancements that have introduced important new methodologies for assessing pain. In recent years, our field has been challenged to discern how to best integrate these developments with the fundamental values of supporting people in pain. MAP aims to provide practical frameworks to help clinicians and researchers navigate these challenges. It does this by first highlighting that the most subjective aspects of the pain experience are not readily quantified, and then by providing a conceptual bridge to link qualitative methodologies, which target these subjective aspects, to our most objective measures of pain. MAP also offers pragmatic frameworks for better integrating this full range of multimodal assessment strategies within both research and practice. The overarching goal of MAP is to help develop more comprehensive, valid and compassionate approaches to pain assessment that can be used to reduce the widespread and deep feelings of invalidation and suffering that are all too commonly reported by people living in pain.
ACKNOWLEDGEMENTS
The authors thank Jean YiChun Lin for her work in illustrating Figures 1 A and 1B and Maria Romanova for her assistance in illustrating Figure 1B. The authors thank Megan Bradley, Ian Ward, Jennifer Haythornthwaite and Mick Sullivan for their conceptual and editorial input and the anonymous reviewers who helped strengthen this manuscript.
Footnotes
Supported by funds received from the Canadian Institutes of Health Research and the Fond de recherche du Quebec Santé. The authors declare no conflict of interest.
REFERENCES
- 1.International Association for Study of Pain (IASP). International Association for Study of Pain Task Force on Taxonomy Classification of Chronic Pain. Seattle: IASP Press; 1994. [Google Scholar]
- 2.De Ruddere L, Craig KD. Understanding stigma and chronic pain: a-state-of-the-art review. Pain. 2016;157:1607–1610. [DOI] [PubMed] [Google Scholar]
- 3.Holloway I, Sofaer-Bennett B, Walker J. The stigmatisation of people with chronic back pain. Disabil Rehabil. 2007;29:1456–1464. [DOI] [PubMed] [Google Scholar]
- 4.Slade SC, Molloy E, Keating JL. Stigma experienced by people with nonspecific chronic low back pain: a qualitative study. Pain Med. 2009;10:143–154. [DOI] [PubMed] [Google Scholar]
- 5.Toye F, Seers K, Allcock N, et al. Patients’ experiences of chronic non-malignant musculoskeletal pain: a qualitative systematic review. Br J Gen Pract. 2013;63:e829–e841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner A, Malterud K. It is hard work behaving as a credible patient: encounters between women with chronic pain and their doctors. Soc Sci Med. 2003;57:1409–1419. [DOI] [PubMed] [Google Scholar]
- 7.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 1: the need, reality, challenges, and solutions. Discov Med. 2011;11:197–207. [PubMed] [Google Scholar]
- 8.Borsook D, Becerra L, Hargreaves R. Biomarkers for chronic pain and analgesia. Part 2: how, where, and what to look for using functional imaging. Discov Med. 2011;11:209–219. [PubMed] [Google Scholar]
- 9.Mackey SC. Central neuroimaging of pain. J Pain. 2013;14:328–331. [DOI] [PubMed] [Google Scholar]
- 10.Robinson ME, Staud R, Price DD. Pain measurement and brain activity: will neuroimages replace pain ratings? J Pain. 2013;14:323–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan MD, Cahana A, Derbyshire S, et al. What does it mean to call chronic pain a brain disease? J Pain. 2013;14:317–322. [DOI] [PubMed] [Google Scholar]
- 12.Ferrell BR, Novy D, Sullivan MD, et al. Ethical dilemmas in pain management. J Pain. 2001;2:171–180. [DOI] [PubMed] [Google Scholar]
- 13.Hahn SR. Physical symptoms and physician-experienced difficulty in the physician-patient relationship. Ann Intern Med. 2001;134(pt 2):897–904. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan M. The problem of pain in the clinicopathological method. Clin J Pain. 1998;14:197–201. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan M, Ferrell B. Ethical challenges in the management of chronic nonmalignant pain: negotiating through the cloud of doubt. J Pain. 2005;6:2–9. [DOI] [PubMed] [Google Scholar]
- 16.Toye F, Seers K, Barker KL. Meta-ethnography to understand healthcare professionals’ experience of treating adults with chronic non-malignant pain. BMJ Open. 2017;7:e018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis KD, Flor H, Greely HT, et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat Rev Neurol. 2017;13:624–638. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan M. The new subjective medicine: taking the patient’s point of view on health care and health. Soc Sci Med. 2003;56:1595–1604. [DOI] [PubMed] [Google Scholar]
- 19.Greville-Harris M, Dieppe P. Bad is more powerful than good: the nocebo response in medical consultations. Am J Med. 2015;128:126–129. [DOI] [PubMed] [Google Scholar]
- 20.Toye F, Seers K, Hannink E, et al. A mega-ethnography of eleven qualitative evidence syntheses exploring the experience of living with chronic non-malignant pain. BMC Med Res Methodol. 2017;17:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen RHN, Turner RM, Rydell SA, et al. Perceived stereotyping and seeking care for chronic vulvar pain. Pain Med. 2013;14:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hadjistavropoulos T, Craig KD. A theoretical framework for understanding self-report and observational measures of pain: a communications model. Behav Res Ther. 2002;40:551–570. [DOI] [PubMed] [Google Scholar]
- 23.Prkachin KM, Craig KD. Expressing pain: the communication and interpretation of facial pain signals. J Nonverbal Behav. 1995;19:191–205. [Google Scholar]
- 24.Schiavenato M, Craig KD. Pain assessment as a social transaction: beyond the “gold standard”. Clin J Pain. 2010;26:667–676. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan M. Exaggerated pain behavior: by what standard? Clin J Pain. 2004;20:433–439. [DOI] [PubMed] [Google Scholar]
- 26.Tait RC, Chibnall JT, Kalauokalani D. Provider judgments of patients in pain: seeking symptom certainty. Pain Med. 2009;10:11–34. [DOI] [PubMed] [Google Scholar]
- 27.Carpenter C, Suto M. Qualitative Research for Occupational and Physical Therapists: A Practical Guide. Oxford, UK: Blackwell Publishing Ltd; 2008. [Google Scholar]
- 28.Denzin NK, Lincoln YS. The Sage Handbook of Qualitative Research. Thousand Oaks: Sage; 2011. [Google Scholar]
- 29.Morse JM. Using qualitative methods to access the pain experience. Br J Pain. 2015;9:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polkinghorne DE. Language and meaning: data collection in qualitative research. J Couns Psychol. 2005;52:137–145. [Google Scholar]
- 31.Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ. 1995;311:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Upshur RE.Morse JM, Swanson JM, Kuzel AJ. The status of qualitative research as evidence. The Nature of Qualitative Evidence. Thousand Oaks, CA: Sage; 2001:5–26. [Google Scholar]
- 33.Entwistle VA, Carter SM, Cribb A, et al. Supporting patient autonomy: the importance of clinician-patient relationships. J Gen Intern Med. 2010;25:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peter E, Watt-Watson J. Unrelieved pain: an ethical and epistemological analysis of distrust in patients. Can J Nurs Res. 2002;34:65–80. [PubMed] [Google Scholar]
- 35.Quinlan-Colwell A. Making an ethical plan for treating patients in pain. Nurse Pract. 2013;38:17–21. [DOI] [PubMed] [Google Scholar]
- 36.International Association for the Study of Pain (IASP). International Association for the Study of Pain’s Declaration of Montreal. 2010. Available at: www.iasp-pain.org/Advocacy/Content.aspx?ItemNumber=1821. Accessed October 11, 2017.
- 37.McCaffery M. Nursing Practice Theories Related to Cognition, Bodily Pain and Man-environment Interactions. Los Angeles: University of California; 1968. [Google Scholar]
- 38.Sullivan MD, Derbyshire SW. Is there a purely biological core to pain experience? Pain. 2015;156:2119–2120. [DOI] [PubMed] [Google Scholar]
- 39.Wager TD, Atlas LY, Lindquist MA, et al. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis KD. Is chronic pain a disease? Evaluating pain and nociception through self-report and neuroimaging. J Pain. 2013;14:332–333. [DOI] [PubMed] [Google Scholar]
- 41.Main CJ. Pain assessment in context: a state of the science review of the McGill Pain Questionnaire 40 years on. Pain. 2016;157:1387–1399. [DOI] [PubMed] [Google Scholar]
- 42.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. [DOI] [PubMed] [Google Scholar]
- 43.Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34:50–59. [DOI] [PubMed] [Google Scholar]
- 44.Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain. 2008;137:276–285. [DOI] [PubMed] [Google Scholar]
- 45.Booker SQ, Haedtke C. Assessing pain in nonverbal older adults. Nursing. 2016;46:66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hadjistavropoulos T, Herr K, Prkachin KM, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13:1216–1227. [DOI] [PubMed] [Google Scholar]
- 47.Herr K, Coyne PJ, McCaffery M, et al. Pain assessment in the patient unable to self-report: position statement with clinical practice recommendations. Pain Manag Nurs. 2011;12:230–250. [DOI] [PubMed] [Google Scholar]
- 48.Hadjistavropoulos T, Herr K, Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(suppl):S1–S43. [DOI] [PubMed] [Google Scholar]
- 49.Reid MC, Eccleston C, Pillemer K. Management of chronic pain in older adults. BMJ. 2015;350:h532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agnew DC, Merskey H. Words of chronic pain. Pain. 1976;2:73–81. [DOI] [PubMed] [Google Scholar]
- 51.Bergh I, Jakobsson E, Sjöström B, et al. Ways of talking about experiences of pain among older patients following orthopaedic surgery. J Adv Nurs. 2005;52:351–359; discussion 360–351. [DOI] [PubMed] [Google Scholar]
- 52.Boyd DB, Merskey H. A note on the description of pain and its causes. Pain. 1978;5:1–3. [DOI] [PubMed] [Google Scholar]
- 53.Clarke A, Anthony G, Gray D, et al. “I feel so stupid because I can’t give a proper answer…” How older adults describe chronic pain: a qualitative study. BMC Geriatr. 2012;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diller A. Cross-cultural pain semantics. Pain. 1980;9:9–26. [DOI] [PubMed] [Google Scholar]
- 55.Fordyce WE. Behavioral Methods for Chronic Pain and Illness. Saint Louis: The C. V. Mosby Company; 1976. [Google Scholar]
- 56.Schott GD. Communicating the experience of pain: the role of analogy. Pain. 2004;108:209–212. [DOI] [PubMed] [Google Scholar]
- 57.Bačkonja MM, Attal N, Baron R, et al. Value of quantitative sensory testing in neurological and pain disorders: NeuPSIG consensus. Pain. 2013;154:1807–1819. [DOI] [PubMed] [Google Scholar]
- 58.Dworkin RH, Turk DC, Farrar JT, et al. IMMPACT. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- 59.Prkachin KM. Assessing pain by facial expression: facial expression as nexus. Pain Res Manage. 2009;14:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rolke R, Magerl W, Campbell KA, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. [DOI] [PubMed] [Google Scholar]
- 61.Wideman TH, Hudon A, Bostick G. The added value of qualitative methodologies for studying emotional disclosure about pain. J Pain. 2018;19:1366. [DOI] [PubMed] [Google Scholar]
- 62.Ballantyne JC, Sullivan MD. Intensity of chronic pain—the wrong metric? N Engl J Med. 2015;373:2098–2099. [DOI] [PubMed] [Google Scholar]
- 63.Craig KD. Social communication model of pain. Pain. 2015;156:1198–1199. [DOI] [PubMed] [Google Scholar]
- 64.Purcell TB. The somatic patient. Emerg Med Clin North Am. 1991;9:137–159. [PubMed] [Google Scholar]
- 65.Turk DC, Okifuji A. Assessment of patients’ reporting of pain: an integrated perspective. Lancet. 1999;353:1784–1788. [DOI] [PubMed] [Google Scholar]
- 66.Dewar A, White M, Posade ST, et al. Using nominal group technique to assess chronic pain, patients’ perceived challenges and needs in a community health region. Health Expect. 2003;6:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Synnott A, O’Keeffe M, Bunzli S, et al. Physiotherapists may stigmatise or feel unprepared to treat people with low back pain and psychosocial factors that influence recovery: a systematic review. J Physiother. 2015;61:68–76. [DOI] [PubMed] [Google Scholar]
- 68.Cruz-Almeida Y, Fillingim RB. Can quantitative sensory testing move us closer to mechanism-based pain management? Pain Med. 2014;15:61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Finnerup NB, Jensen TS. Mechanisms of disease: mechanism-based classification of neuropathic pain—a critical analysis. Nat Clin Pract Neurol. 2006;2:107–115. [DOI] [PubMed] [Google Scholar]
- 70.Woolf CJ, Bennett GJ, Doherty M, et al. Towards a mechanism-based classification of pain? Pain. 1998;77:227–229. [DOI] [PubMed] [Google Scholar]
- 71.Woolf CJ, Max MB. Mechanism-based pain diagnosis: issues for analgesic drug development. Anesthesiology. 2001;95:241–249. [DOI] [PubMed] [Google Scholar]
- 72.Edmond SN, Keefe FJ. Validating pain communication: current state of the science. Pain. 2015;156:215–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferrell B. Ethical perspectives on pain and suffering. Pain Manag Nurs. 2005;6:83–90. [DOI] [PubMed] [Google Scholar]
- 74.Fillingim RB, Loeser JD, Baron R, et al. Assessment of chronic pain: domains, methods, and mechanisms. J Pain. 2016;17(suppl):T10–T20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mays N, Pope C. Rigour and qualitative research. BMJ. 1995;311:109–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mays N, Pope C. Qualitative research in health care. Assessing quality in qualitative research. BMJ. 2000;320:50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–121. [DOI] [PubMed] [Google Scholar]
- 78.Turk DC, Dworkin RH. What should be the core outcomes in chronic pain clinical trials? Arthritis Res Ther. 2004;6:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–345. [DOI] [PubMed] [Google Scholar]
- 80.Turk DC, Dworkin RH, Burke LB, et al. Developing patient-reported outcome measures for pain clinical trials: IMMPACT recommendations. Pain. 2006;125:208–215. [DOI] [PubMed] [Google Scholar]
- 81.Loeser JD. The education of pain physicians. Pain Med. 2015;16:225–229. [DOI] [PubMed] [Google Scholar]
- 82.Alperstein D, Sharpe L. The efficacy of motivational interviewing in adults with chronic pain: a meta-analysis and systematic review. J Pain. 2016;17:393–403. [DOI] [PubMed] [Google Scholar]
- 83.Carr DB, Loeser JD, Morris DB. Narrative, Pain, and Suffering. Seattle, WA: IASP Press; 2005. [Google Scholar]
- 84.Cepeda MS, Chapman CR, Miranda N, et al. Emotional disclosure through patient narrative may improve pain and well-being: results of a randomized controlled trial in patients with cancer pain. J Pain Symptom Manage. 2008;35:623–631. [DOI] [PubMed] [Google Scholar]
- 85.Charon R. The patient-physician relationship. Narrative medicine: a model for empathy, reflection, profession, and trust. JAMA. 2001;286:1897–1902. [DOI] [PubMed] [Google Scholar]
- 86.Christiansen S, Oettingen G, Dahme B, et al. A short goal-pursuit intervention to improve physical capacity: a randomized clinical trial in chronic back pain patients. Pain. 2010;149:444–452. [DOI] [PubMed] [Google Scholar]
- 87.McCracken LM, Davies M, Scott W, et al. Can a psychologically based treatment help people to live with chronic pain when they are seeking a procedure to reduce it? Pain Med. 2015;16:451–459. [DOI] [PubMed] [Google Scholar]
- 88.McCracken LM, Morley S. The psychological flexibility model: a basis for integration and progress in psychological approaches to chronic pain management. J Pain. 2014;15:221–234. [DOI] [PubMed] [Google Scholar]
- 89.Yu L, Norton S, McCracken LM. Change in “Self-as-Context” (“Perspective-Taking”) occurs in Acceptance and Commitment Therapy for people with chronic pain and is associated with improved functioning. J Pain. 2017;18:664–672. [DOI] [PubMed] [Google Scholar]
- 90.Bačkonja MM, Farrar JT. Are pain ratings irrelevant? Pain Med. 2015;16:1247–1250. [DOI] [PubMed] [Google Scholar]
- 91.Birnie KA, McGrath PJ, Chambers CT. When does pain matter? Acknowledging the subjectivity of clinical significance. Pain. 2012;153:2311–2314. [DOI] [PubMed] [Google Scholar]
- 92.Krebs EE, Carey TS, Weinberger M. Accuracy of the pain numeric rating scale as a screening test in primary care. J Gen Intern Med. 2007;22:1453–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pasero C, Quinlan-Colwell A, Rae D, et al. American Society for Pain Management Nursing Position statement: prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs. 2016;17:291–292. [DOI] [PubMed] [Google Scholar]
- 94.Sullivan MD, Ballantyne JC. Must we reduce pain intensity to treat chronic pain? Pain. 2016;157:65–69. [DOI] [PubMed] [Google Scholar]
- 95.Bunzli S, Smith A, Schütze R, et al. Making sense of low back pain and pain-related fear. J Orthop Sports Phys Ther. 2017;47:1–27. [DOI] [PubMed] [Google Scholar]
- 96.Bunzli S, Smith A, Schütze R, et al. Beliefs underlying pain-related fear and how they evolve: a qualitative investigation in people with chronic back pain and high pain-related fear. BMJ Open. 2015;5:e008847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bunzli S, Smith A, Watkins R, et al. What do people who score highly on the Tampa Scale of Kinesiophobia really believe? A mixed methods investigation in people with chronic non specific low back pain. Clin J Pain. 2015;31:621–632. [DOI] [PubMed] [Google Scholar]
- 98.Dudgeon BJ, Ehde DM, Cardenas DD, et al. Describing pain with physical disability: narrative interviews and the McGill Pain Questionnaire. Arch Phys Med Rehabil. 2005;86:109–115. [DOI] [PubMed] [Google Scholar]
- 99.Schütze R, Rees C, Slater H, et al. “I call it stinkin’ thinkin”: a qualitative analysis of metacognition in people with chronic low back pain and elevated catastrophizing. Br J Health Psychol. 2017;22:463–480. [DOI] [PubMed] [Google Scholar]
- 100.Craig KD, Versloot J, Goubert L, et al. Perceiving pain in others: automatic and controlled mechanisms. J Pain. 2010;11:101–108. [DOI] [PubMed] [Google Scholar]
- 101.Craig KD, de C Williams AC. Reply. Pain. 2018;159:996–997. [DOI] [PubMed] [Google Scholar]
- 102.Wideman TH, Hudon A, Walton DM. Questions raised by the proposed definition of pain: what characterizes the experience of pain and how is subjectivity validated? Pain. 2018;159:995–996. [DOI] [PubMed] [Google Scholar]
- 103.Williams AC, Craig KD. Updating the definition of pain. Pain. 2016;157:2420–2423. [DOI] [PubMed] [Google Scholar]
- 104.Tuck NL, Johnson MH, Bean DJ. You’d better believe it: the conceptual and practical challenges of assessing malingering in patients with chronic pain. J Pain. 2018. Doi: 10.1016/j.jpain.2018.07.002. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 105.Cassel EJ. The nature of suffering and the goals of medicine. N Engl J Med. 1982;306:639–645. [DOI] [PubMed] [Google Scholar]
- 106.Fishbain DA, Lewis JE, Gao J. The pain-suffering association, a review. Pain Med. 2015;16:1057–1072. [DOI] [PubMed] [Google Scholar]
- 107.Loeser JD. Pain and suffering. Clin J Pain. 2000;16(suppl):S2–S6. [DOI] [PubMed] [Google Scholar]
- 108.Waddell G. A new clinical model for the treatment of low-back pain. Spine. 1987;12:632–644. [DOI] [PubMed] [Google Scholar]
- 109.Esquibel AY, Borkan J. Doctors and patients in pain: conflict and collaboration in opioid prescription in primary care. Pain. 2014;155:2575–2582. [DOI] [PubMed] [Google Scholar]