Abstract
Mirror therapy is a simple, inexpensive, and patient-oriented method that has been shown to reduce phantom sensations and pain caused by amputation and improve range of motion, speed, and accuracy of arm movement and function. Extracorporeal shock wave therapy (ESWT) is a new, reversible, and noninvasive method for the treatment of spasticity after stroke. To investigate the therapeutic effect of the combination of mirror and extracorporeal shock wave therapy on upper limb spasticity in poststroke patients. We randomly assigned 120 patients into four groups: A, B, C, and D. All groups received conventional rehabilitation training for 30 min per day, five times a week, for 4 weeks. Moreover, participants in groups A, B, and C also added mirror therapy, ESWT, and a combination of mirror and ESWT, respectively, for 20 min per day. Motor recovery and spasticity were measured using Fugl–Meyer assessment and modified Ashworth scale. The differences in the Fugl–Meyer assessment and modified Ashworth scale scores in group C were significantly greater than those of group D at all observed time points after treatment and were significantly greater than those of groups A and B (P<0.05), but no significant differences were observed between groups A and B until 12 months. Upper extremity spasticity was improved by combined mirror and ESWT.
Keywords: extracorporeal shock wave therapy, limb spasticity, mirror therapy, stroke
Introduction
Stroke affects 15 million people worldwide annually, among which five million die and another five million are left permanently disabled, making it a major cause of morbidity and mortality (Chapman and Bogle, 2014; Lackland et al., 2014). In addition, stroke survivors experience impaired motor function of the upper limb, which commonly leads to functional limitations and disabilities and affects their daily life (Alt Murphy et al., 2011). Various symptoms of central nervous system damage after stroke may occur, in which muscle spasticity, caused by the upper motor neuron lesion, is a common complication and the most common clinical challenge (Dymarek et al., 2016).
Spasticity, according to Lance (1980), is a typical component of upper motor neuron syndrome characterized by a velocity-dependent increase in tonic stretch reflexes with exaggerated tendon jerks resulting in hyperexcitability of the stretch reflex. Globally, 12 million people have spasticity of the upper or lower limb (Watkins et al., 2002; de Weerd et al., 2011). It occurs in 19 and 39% at 3 and 12 months after stroke, respectively (Yelnik et al., 2010). Because of long-term complications such as chronic pain, joints deformities, heterotrophic ossifications, bones demineralization, or muscles contractures with their atrophy, spasticity seriously affects important functions of daily living, causing discomfort, stiffness, and limitations in physical activities and daily life, and leads to increased medical bills (Francisco and McGuire, 2012; Ward, 2012). Therefore, control and treatment of spasticity in the rehabilitation of upper extremity motor function after stroke have become increasingly important.
Mirror therapy (MT) is a simple, inexpensive, and patient-oriented method (Altschuler and Hu, 2008). Since Ramachandran et al. (1995) first introduced the use of MT, it has been shown to reduce phantom sensations and pain caused by amputation and improve range of motion, speed, and accuracy of arm movement and function (Zeng et al., 2018). In addition, it has been suggested as a promising rehabilitation approach for recovery of motor function of the upper limb in poststroke patients.
Meanwhile, recent studies have shown that extracorporeal shock wave therapy (ESWT) is a new, reversible, and noninvasive method for the treatment of spasticity after stroke (Moon et al., 2013; Santamato et al., 2014; Li et al., 2016). These studies applied ESWT to treat upper or lower limb spasticity and suggested that ESWT is effective in treating spasticity and improving some parameters without causing muscle weakness or unpleasant effects in patients with stroke, cerebral palsy, and multiple sclerosis.
MT and ESWT are both promising and effective methods for motor recovery of upper limb spasticity in poststroke patients. We hypothesized that MT in combination with ESWT could lead to greater improvement of spasticity after stroke. Therefore, in the present study, we combined MT and ESWT as a new therapy (ETMS+MT) to verify our hypothesis and investigate its feasibility and the possible effects on upper limb spasticity in poststroke patients.
Patients and methods
Study design
A randomized controlled trial design was used in this present study, and the patients were divided into four groups (group A, n=30; group B, n=30, group C, n=30; group D, n=30) by random allocation software. Patients in group D received conventional rehabilitation therapy for 30 min per day, five times a week, for 4 weeks. The conventional program consisted of exercise therapy, occupational therapy, and neurodevelopmental facilitation techniques. In addition to the conventional rehabilitation program, patients in groups A, B, and C added MT, ESWT, and MT+ESWT training, respectively, for 20 min per day, five times a week, for 4 weeks (Fig. 1). The procedures were administered by an occupational therapist who was not involved in the assessment of the patients.
Fig. 1.
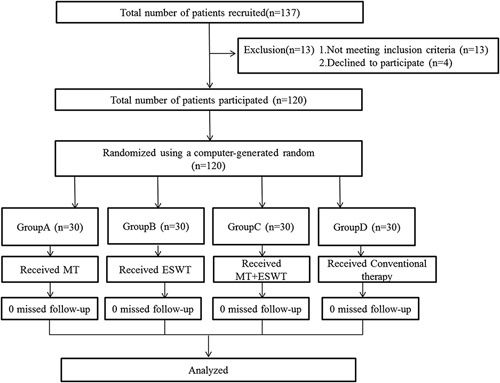
Flow diagram of the study design. ESWT, extracorporeal shock wave therapy; MT, mirror therapy.
Participants
One hundred and thirty-seven poststroke inpatients were recruited for this study from the Department of Oncology, the Second Affiliated Hospital of Wenzhou Medical University, China, from January 2015 to December 2017. Participants with disease duration more than 6 months, with modified Ashworth scale (MAS) score more than 1 and less than 4 for the upper limb flexor tension, with no cognitive problems, and who can understand and follow simple verbal instructions were recruited. One hundred and twenty participants were eligible for the study. The study was approved by the Ethics Committee of the Second Affiliated Hospital of Wenzhou Medical University. All participants provided informed consent according to the Declaration of Helsinki before study enrollment.
Interventions
Patients in group A sat on a stool in front of a table with a 30-cm2 mirror. The affected hand was placed behind the mirror so that it could not be seen, and the unaffected hand was placed in the reflecting side of the mirror. Patients were asked to move their wrist while simultaneously observing the reflection of the unaffected hand. In group B, 2000 shots with a pressure of 2.0–3.0 bar and frequency of 8 Hz were used diffusely for the intrinsic muscles and flexor digitorum tendon of the hand by an ultrasound pointer guide (Terason, t3000; Teratech, Burlington, Massachusetts, USA). The procedure was within tolerable pain limits. Patients in group C performed MT and received ESWT in parallel on the wrist extensor of the affected side.
Outcome measures
All patients in the four groups were examined by the same outcome assessors, who were unaware of treatment procedure. Evaluations were performed before the interventions and 1, 3, 6, and 12 months after the last interventions.
Fugl–Meyer assessment of upper extremity motor recovery
The Fugl–Meyer assessment (FMA), which has impressive test–retest and inter-rater reliability and construct validity, quantitatively measures motor recovery after stroke (Sanford et al., 1993; Gladstone et al., 2002). The score for the motor skill assessment included 66 and 34 points for the affected upper and lower limbs, respectively. The motor skills of the upper limb were assessed in our study. A three-point ordinal scale (0, cannot perform; 1, perform partially; 2, perform completely) was adopted.
Modified Ashworth scale for spasticity assessment
Spasticity is clinically evaluated by the MAS, which is a six-point rating scale with scores ranging from 0 to 4, where 0 indicates no increase in muscle tone and four indicates that the affected limb is rigid during flexion or extension (Gregson et al., 1999). The measurement, which has good inter-rater and intra-rater reliability, is performed by calculating the degree and point of resistance when a muscle is manually stretched.
Statistical analysis
Data in this study were analyzed by SPSS 18.0 (IBM, Armonk, New York, USA). Independent t-test, χ2-test, and Mann–Whitney U-test were used as homogeneity tests for demographic and medical characteristics. Wilcoxon’s matched-pairs signed ranks test was used to compare results obtained before and after intervention. One-way analysis of variance followed by Bonferroni post-hoc tests was used to compare the differences among the four groups. Statistical significance was accepted for P values less than 0.05 in all tests.
Results
Recruitment and sample size
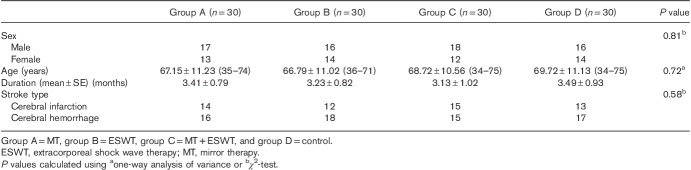
Patients were recruited between January 2016 and December 2017. In total, 120 patients agreed to participate in the study and were assigned to group A (n=30), group B (n=30), group C (n=30) or group D (n=30) (Fig. 1). All the participants completed the full protocol. The 12-month follow-up questionnaire was completed by all of the patients. No adverse effects or complications were observed after the interventions in any of the four groups. There were no significant differences in the demographic and baseline clinical characteristics of the participants in any of the four groups (Table 1).
Table 1.
General characteristics of participants
Changes in the Fugl–Meyer assessment and modified Ashworth scale of the four group
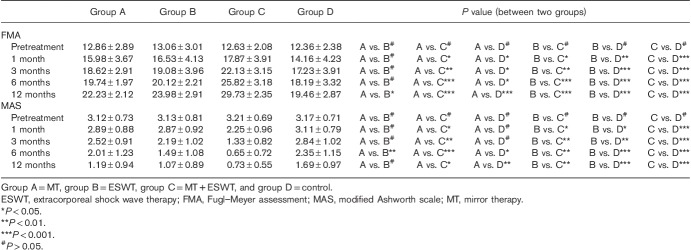
Table 2 presents the comparisons of upper extremity motor performance and spasticity before and after treatment in the four groups. The FMA scores of group C were significantly greater than that of group D at all observed time points after treatment and were significantly greater than groups A and B especially at 6 and 12 months, but no significant differences were observed between groups A and B until the 12 months. Meanwhile, the post-treatment MAS scores were statistically lower in group C than in group D at all observed time points after treatment and were significantly greater than in groups A and B especially at 6 and 12 months. Moreover, the differences in MAS scores between groups A and B reached significance at 6 months.
Table 2.
Changes in the Fugl–Meyer assessment and modified Ashworth scale
Discussion
Our present study results showed that MT combined with ESWT produced greater improvement in upper extremity motor performance and significant reduction in spasticity, and the effects lasted at least 12 months compared with those of MT alone or ESWT alone. This is the first randomized, single-blind study to investigate the feasibility and possible effects of MT combined with ESWT for the treatment of upper limb spasticity in poststroke patients.
Since Altschuler et al. (1999) first reported that MT can be possibly used in rehabilitating the motor function of the affected arms of poststroke patients, more studies found that MT may improve the motor function of the upper extremity in patients with stroke (Mirela Cristina et al., 2015; Colomer et al., 2016; Gurbuz et al., 2016). Moreover, the activation of the primary motor cortex (M1) or mirror neurons has been proposed as the possible mechanism of MT (Garry et al., 2005). However, previous studies reported that MT has invalid effect on upper limb spasticity after stroke (Xu et al., 2017). Various clinical experiment studies have demonstrated that ESWT could be used efficiently in the treatment of musculoskeletal disorders such as chronic tendinopathies, calcific tendinitis of the shoulder, lateral epicondylitis, and plantar fasciitis (Gerdesmeyer et al., 2003). In addition, recent studies have applied ESWT to patients with stroke with upper or lower limb spasticity and showed that ESWT is effective in treating spasticity and improving some parameters (Dymarek et al., 2016). Therefore, in our present study, we hypothesized that MT, which induces motor performance recovery, combined with ESWT could lead to greater improvement of spasticity after stroke. Our results showed that compared with the MT or ESWT group, the MT+ESWT group had significantly improved upper extremity motor performance and reduced spasticity, and the effects lasted 12 months based on FMA and MAS scores, whereas the MT+ESWT groups achieved improvement until 6 and 12 months. More importantly, these findings have not been reported until now.
This study has a few limitations. First, the small number of participants may affect the generalizability of the study findings. Second, we used FMA and MAS to measure motor improvement, but did not evaluate the Brunnstrom stages of motor recovery. Thus, further studies, are needed to evaluate the long-term therapeutic benefits of MT-ESWT on the motor recovery of upper limb spasticity after stroke.
Conclusion
The use of MT+ESWT might be beneficial in the recovery of upper limb spasticity in poststroke patients and could be a promising and effective method for clinical therapy.
Acknowledgements
Zhejiang Province, medical and health science and technology projects (no. 2018PY033).
Conflicts of interest
There are no conflicts of interest.
References
- Alt Murphy M, Persson H, Danielsson A, Broeren J, Lundgren-Nilsson A, Sunnerhagen K. (2011). SALGOT – Stroke Arm Longitudinal study at the University of Gothenburg, prospective cohort study protocol. BMC Neurol 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschuler EL, Hu J. (2008). Mirror therapy in a patient with a fractured wrist and no active wrist extension. Scand J Plast Reconstr Surg Hand Surg 42:110–111. [DOI] [PubMed] [Google Scholar]
- Altschuler EL, Wisdom SB, Stone L, Foster C, Galasko D, Llewellyn DM, et al. (1999). Rehabilitation of hemiparesis after stroke with a mirror. Lancet 353:2035–2036. [DOI] [PubMed] [Google Scholar]
- Chapman B, Bogle V. (2014). Adherence to medication and self-management in stroke patients. Br J Nurs 23:158–166. [DOI] [PubMed] [Google Scholar]
- Colomer C, NOé E, Llorens R. (2016). Mirror therapy in chronic stroke survivors with severely impaired upper limb function: a randomized controlled trial. Eur J Phys Rehabil Med 52:271–278. [PubMed] [Google Scholar]
- De Weerd L, Rutgers WA, Groenier KH, van der Meer K. (2011). Perceived wellbeing of patients one year post stroke in general practice – recommendations for quality aftercare. BMC Neurol 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymarek R, Ptaszkowski K, Slupska L, Halski T, Taradaj J, Rosinczuk J. (2016). Effects of extracorporeal shock wave on upper and lower limb spasticity in post-stroke patients: a narrative review. Top Stroke Rehabil 23:293–303. [DOI] [PubMed] [Google Scholar]
- Francisco GE, McGuire JR. (2012). Poststroke spasticity management. Stroke 43:3132–3136. [DOI] [PubMed] [Google Scholar]
- Garry MI, Loftus A, Summers JJ. (2005). Mirror, mirror on the wall: viewing a mirror reflection of unilateral hand movements facilitates ipsilateral M1 excitability. Exp Brain Res 163:118–122. [DOI] [PubMed] [Google Scholar]
- Gerdesmeyer L, Wagenpfeil S, Haake M, Maier M, Loew M, Wortler K, et al. (2003). Extracorporeal shock wave therapy for the treatment of chronic calcifying tendonitis of the rotator cuff: a randomized controlled trial. JAMA 290:2573–2580. [DOI] [PubMed] [Google Scholar]
- Gladstone DJ, Danells CJ, Black SE. (2002). The Fugl–Meyer assessment of motor recovery after stroke: a critical review of its measurement properties. Neurorehabil Neural Repair 16:232–240. [DOI] [PubMed] [Google Scholar]
- Gregson JM, Leathley M, Moore AP, Sharma AK, Smith TL, Watkins CL. (1999). Reliability of the Tone Assessment Scale and the modified Ashworth scale as clinical tools for assessing poststroke spasticity. Arch Phys Med Rehabil 80:1013–1016. [DOI] [PubMed] [Google Scholar]
- Gurbuz N, Afsar SI, Ayas S, Cosar SN. (2016). Effect of mirror therapy on upper extremity motor function in stroke patients: a randomized controlled trial. J Phys Ther Sci 28:2501–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackland D, Roccella E, Deutsch A, Fornage M, George M, Howard G, et al. (2014). Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 45:315–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW. (1980). The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology 30:1303–1313. [DOI] [PubMed] [Google Scholar]
- Li TY, Chang CY, Chou YC, Chen LC, Chu HY, Chiang SL, et al. (2016). Effect of radial shock wave therapy on spasticity of the upper limb in patients with chronic stroke: a prospective, randomized, single blind, controlled trial. Medicine 95:e3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirela Cristina L, Matei D, Ignat B, Popescu CD. (2015). Mirror therapy enhances upper extremity motor recovery in stroke patients. Acta Neurol Belg 115:597–603. [DOI] [PubMed] [Google Scholar]
- Moon SW, Kim JH, Jung MJ, Son S, Lee JH, Shin H, et al. (2013). The effect of extracorporeal shock wave therapy on lower limb spasticity in subacute stroke patients. Ann Rehabil Med 37:461–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran VS, Rogers-Ramachandran D, Cobb S. (1995). Touching the phantom limb. Nature 377:489–490. [DOI] [PubMed] [Google Scholar]
- Sanford J, Moreland J, Swanson LR, Stratford PW, Gowland C. (1993). Reliability of the Fugl–Meyer assessment for testing motor performance in patients following stroke. Phys Ther 73:447–454. [DOI] [PubMed] [Google Scholar]
- Santamato A, Micello MF, Panza F, Fortunato F, Logroscino G, Picelli A, et al. (2014). Extracorporeal shock wave therapy for the treatment of poststroke plantar-flexor muscles spasticity: a prospective open-label study. Top Stroke Rehabil 21 (Suppl 1):S17–S24. [DOI] [PubMed] [Google Scholar]
- Ward AB. (2012). A literature review of the pathophysiology and onset of post-stroke spasticity. Eur J Neurol 19:21–27. [DOI] [PubMed] [Google Scholar]
- Watkins CL, Leathley MJ, Gregson JM, Moore AP, Smith TL, Sharma AK. (2002). Prevalence of spasticity post stroke. Clin Rehabil 16:515–522. [DOI] [PubMed] [Google Scholar]
- Xu Q, Guo F, Salem HMA, Chen H, Huang X. (2017). Effects of mirror therapy combined with neuromuscular electrical stimulation on motor recovery of lower limbs and walking ability of patients with stroke: a randomized controlled study. Clin Rehabil 31:1583–1591. [DOI] [PubMed] [Google Scholar]
- Yelnik AP, Simon O, Parratte B, Gracies JM. (2010). How to clinically assess and treat muscle overactivity in spastic paresis. J Rehabil Med 42:801–807. [DOI] [PubMed] [Google Scholar]
- Zeng W, Guo Y, Wu G, Liu X, Fang Q. (2018). Mirror therapy for motor function of the upper extremity in patients with stroke: a meta-analysis. J Rehabil Med 50:8–15. [DOI] [PubMed] [Google Scholar]