Abstract
Transgenic TgMISIIR-TAg (TAg) mice express the oncogenic virus SV40 in Mullerian epithelial cells. Female TAg mice spontaneously develop epithelial ovarian carcinoma, the most common type of ovarian cancer in women. Female TAg mice are infertile, but the reason has not been determined. We therefore investigated whether female TAg mice undergo puberty, demonstrate follicular development, maintain regular cycles, and ovulate. Ovarian cancers in women commonly develop after menopause. The occupational chemical 4-vinylcyclohexene diepoxide (VCD) accelerates follicle degeneration in the ovaries of rats and mice, causing early ovarian failure. We therefore used VCD dosing of mice to develop an animal model for menopause. The purpose of this study was to characterize reproductive parameters in female TAg mice and to investigate whether the onset of ovarian failure due VCD dosing differed between female TAg and WT C57BL/6 mice. As in WT female mice, TAg female mice underwent puberty (vaginal opening) and developed cyclicity in patterns that were similar between the groups. Vehicle-only TAg mice had fewer ovulations (numbers of corpora lutea) than WT animals. VCD exposure delayed the onset of puberty (day of first estrus) in TAg as compared with WT mice. Morphologic evaluation of ovaries revealed many more degenerating follicles in TAg mice than WT mice, and more VCD-dosed TAg mice were in ovarian failure than VCD-dosed WT mice. These results suggest that despite showing similar onset of sexual maturation, TAg mice have increased follicular degeneration and fewer ovulations than WT. These features may contribute to the inability of female TAg mice to reproduce.
Abbreviations: CL, corpus luteum; TAg, TgMISIIR-Tag transgenic mice; pnd, postnatal day; VCD, 4-vinylcyclohexene diepoxide
Ovarian cancer ranks 5th in cancer deaths among women and is the most common cause of cancer death from gynecologic malignancies in women.13 One reason is that tumor development does not present with recognizable symptoms, and there are no reliable markers for its identification.4 Furthermore, the incidence of ovarian cancer is increased in postmenopausal women.12 The American Cancer Society projects that in the United States in 2018, more than 14,000 women will die from ovarian cancer and more than 22,000 will receive a new diagnosis of ovarian cancer.1
The transgenic TgMISIIR-TAg (TAg) mouse strain is one in which the transforming region of SV40, under the control of the Mullerian inhibitory substance type II receptor gene promoter, is expressed in epithelial cells in the reproductive tract of young female mice.7 The most common form of ovarian cancer in women is of epithelial origin,4 and TAg mice have been generated as a model for spontaneous development of epithelial ovarian cancer. Female TAg mice develop bilateral ovarian carcinoma with 100% penetrance.11 Female TAg mice are infertile, but little has been reported regarding the reproductive characteristics of the female TAg mouse.
C57BL/6 mice are an inbred strain widely used as a background for many genetically altered mice. In C57BL/6 mice, the reproductive cycle is 4 to 5 d long and involves 4 stages: proestrus, estrus (the time of ovulation), metestrus, and diestrus. Vaginal opening precedes the onset of estrus and acquisition of reproductive capacity (puberty), which occurs on or near postnatal day (pnd) 48 in female C57BL/6 mice.10 Those events have not previously been reported for female TAg mice.
The chemical 4-vinylcyclohexene diepoxide (VCD) accelerates the rate of atresia in small follicles in the ovaries of rats and mice.14 VCD requires repeated exposure to be effective and has been used to cause early ovarian failure in mice.9 This chemical has been useful for generating an animal model for menopause. In a previous study, VCD dosing of pnd 4 rats produced small-sized follicle loss similar to that seen in older animals (pnd 28) that were treated with VCD.5 Therefore, VCD exposure is effective in both young and older animals, and it is of interest to investigate ovarian function and to determine whether VCD dosing will cause ovarian failure in TAg mice, which are known to get cancer at a young age. These findings will direct future studies to observe whether VCD dosing might accelerate the spontaneous development of ovarian tumors.
Therefore, the purpose of the current study was to characterize reproductive parameters in female TAg mice and to investigate whether the onset of ovarian failure due to VCD dosing differs between TAg and WT C57BL/6 mice.
Materials and Methods
Materials.
We purchased VCD (catalog no. 94956) and sesame oil (catalog no. S3547) from Sigma Chemical Company (St Louis MO).
Animals.
Experimental protocols were approved by the IACUC. Female C57BL/6 (WT) breeder mice (age, 8 wk) were purchased from Jackson Laboratory (Bar Harbor, ME). Initially, 6 TAg male mice were obtained from the Fox Chase Cancer Center (Philadelphia, PA) to start the colony; 1 to 3 C57BL/6 female mice were cohoused with each TAg male mouse for mating. Pregnancy of female mice was determined by the presence of a copulatory plug. Pups were born on day 20 or 21 after mating. Pups were evaluated for sex, and tail tips were collected from female mice for genotyping at either The University of Arizona Genetics Core (under the direction of Dr Michael Hammer) or TransnetXY (Cordova, TN). An average of 4 TAg female mice was obtained per litter.
Dosing.
On pnd 7, female mice received sesame oil (vehicle) alone or containing VCD (80 mg/kg) at 2.5 mL/kg body weight through intraperitoneal injection. Daily dosing with sesame oil only (WT, n = 10; TAg, n = 9) continued for 15 d or containing VCD continued for 15 d (WT, n = 9; TAg, n = 6) or 20 d (WT, n = 14; TAg, n = 5). On pnd 56 the animals were killed by CO2 inhalation, and ovaries were collected, sectioned (thickness, 6 µm; every 100 µm), and stained with hematoxylin and eosin for histologic evaluation.
Cycle detection.
Cycle stage in each mouse was determined daily by vaginal cytology, conducted between 0900 and 1000 as previously described.8 For animals with vaginal opening, vaginal cytology was conducted on days 28 through 56 for mice with 15 d of injections and on days 32 through 56 for mice with 20 d of injections. For each animal, the cycle stage was recorded daily as proestrus, estrus, metestrus, or diestrus. Ovarian failure was assigned when animals showed 10 to 15 consecutive days of metestrus or diestrus.15
Histologic preparation.
At the conclusion of the experiments, animals were euthanized by CO2, and reproductive tracts were excised. Reproductive tracts were maintained intact for fixation and embedding, and care was taken to maintain identical orientation for every reproductive system. Reproductive tracks were fixed in 10% buffered formalin and embedded in paraffin for histologic analysis.
Ovarian size.
All ovaries were approximately ellipsoid and were fixed with the long axis parallel to the cut surface. Ovarian size was estimated from stained slides by calculating the surface area (that is, π × 0.5 [vertical axis length {in mm}] × 0.5 [horizontal axis length {in mm}]) in the largest ovarian tissue section. Ovarian size was normalized to body weight by dividing the ovarian area by body weight.
Follicular classification.
Follicles were designated as primordial, primary, growing, and antral as previously described and assessed qualitatively.3
Number of corpora lutea (CL).
As an indicator of ovulation, CL, including those for the current cycle and regressing CL from the previous cycle, were counted on every section of both ovaries from the vehicle-only animals. CL counts rather than follicle counts were used as a reliable measure to confirm ovulation because CL form after every ovulation at the site of ovulation.5
Statistical analyses.
All data were compared by using SPSS statistical software (version 24, IBM, Chicago, IL). Based on results from Shapiro–Wilk tests of normality and Levene tests of homogeneity of variances, all data values were found to be nonparametric. Consequently, Kruskal–Wallis tests were performed to determine whether differences between groups were statistically significant. When applicable, Kruskal–Wallis tests were followed by Mann–Whitney posthoc tests to determine differences between specific groups. For all tests, statistical significance was assigned at P < 0.05, and all data are presented as mean ± SEM.
Results
Time in estrus–proestrus compared with metestrus–diestrus.
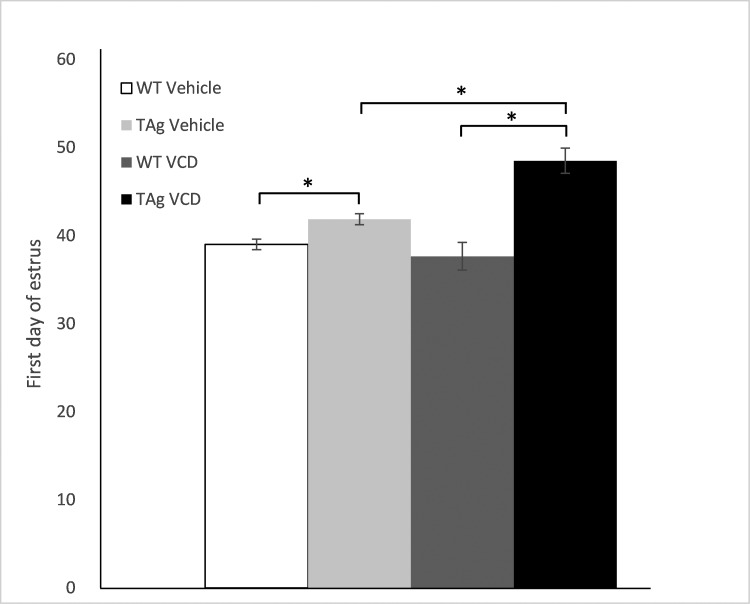
All mice had vaginal opening by day 33. One WT and 2 TAg vehicle-only mice did not display cyclicity at any time point and were considered to be infertile (Table 1). In the remaining animals, the day of first estrus (onset of cyclicity) was different (P < 0.05) between WT and TAg mice in vehicle-only animals (Table 2). Furthermore, VCD dosing delayed (P < 0.05) the day of first estrus in TAg mice (Table 2). The day of first estrus did not differ between VCD-dosed and vehicle-only WT mice (Figure 1, Table 2). The percentages of time in estrus–proestrus compared with metestrus–diestrus were similar in both vehicle-only WT and vehicle-only TAg mice. VCD dosing significantly (P < 0.05) decreased the percentage of time in estrus–proestrus in both WT and TAg mice. In addition, the reduction in percentage time in estrus–proestrus was greater (P < 0.05) in VCD-dosed TAg compared with VCD-dosed WT mice (Figure 2).
Table 1.
Number of animals in each group that were assigned to each reproductive status
| Cycling | Late puberty | Impending ovarian failure | Ovarian failure | |
| WT vehicle (n = 10) | 9 | 0 | 0 | 1 |
| TAg vehicle (n = 9) | 7 | 0 | 0 | 2 |
| WT VCD (n = 23) | 6 | 4 | 9 | 4 |
| TAg VCD (n = 11) | 1 | 3 | 4 | 3 |
Table 2.
Postnatal day of first estrus and percentage of time (mean ± SE) in each cycle phase for each group
| Postnatal day of first estrus | Time (%) in estrus or proestrus | Time (%) in metestrus and diestrus | |
| WT vehicle | 39.0 ± 0.6 | 35.2 ± 4.4 | 64.8 ± 4.4 |
| TAg vehicle | 41.9† ± 0.7 | 31.2 ± 6.4 | 68.7 ± 6.4 |
| WT VCD | 36.2 ± 0.9 | 14.8 ± 1.5a | 85.2 ± 1.5 |
| TAg VCD | 48.5 ± 1.7a,b | 4.8 ± 1.2a,b | 95.2 ± 1.2a,b |
Value significantly (P < 0.05) different from that for vehicle control.
Value significantly (P < 0.05) different from that for WT.
Figure 1.
Postnatal day on which first estrus began. Values are group means ± SE (WT vehicle, n = 10; TAg vehicle, n = 9; WT VCD, n = 23; and TAg VCD, n = 11). *, Values are significantly (P < 0.05) different.
Figure 2.
Percentage of days spent in estrus or proestrus and in metestrus or diestrus (M/D). Values are group means ± SE (WT vehicle, n = 10; TAg vehicle, n = 9; WT VCD, n = 23; and TAg VCD, n = 11). *, Values are significantly (P < 0.05) different.
Cycle length.
Mean cycle duration (that is, number of days from estrus to next estrus) was quite variable between animals and did not differ between vehicle-only WT mice (5.3 ± 0.4 d) and vehicle-only TAg animals (5.4 ± 0.3 d). In addition, dosing with VCD did not affect cycle length in either group (WT VCD, 6.1 ± 0.5 d; TAg VCD, 6.8 ± 1.3 d).
Ovarian size.
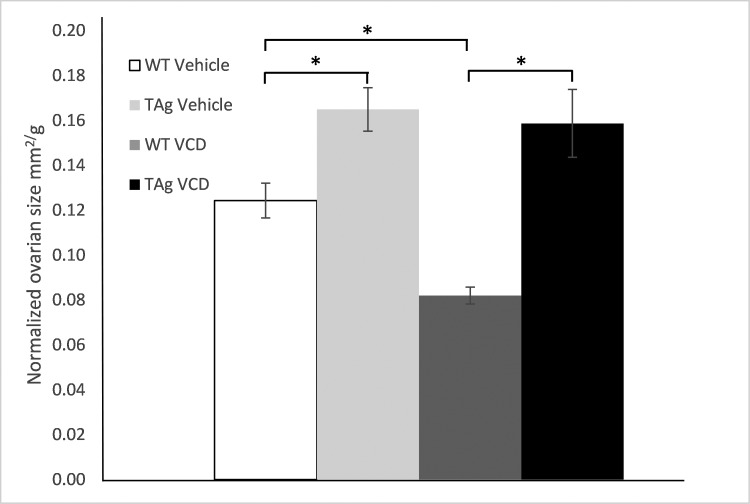
When normalized to body weight, both vehicle-only and VCD-dosed TAg ovaries were significantly larger (P < 0.05) than their WT counterparts (Table 3). VCD led to a significant decrease (P < 0.05) in ovarian size in WT mice but not TAg mice (Figure 3) which had . In addition, TAg mice weighed less (P < 0.05) on average than WT mice (Table 3).
Table 3.
Body weight and absolute ovarian size (mean ± 1 SD)
| Body weight (g) | Absolute ovarian size (mm2) | |
| WT vehicle (n = 9) | 17 ± 1 | 2.16 ± 0.46 |
| TAg vehicle (n = 9) | 16 ± 1 | 2.59† ± 0.44 |
| WT VCD (n = 11) | 19 ± 1a | 1.57 ± 0.19a |
| TAg VCD (n = 5) | 16 ± 2b | 2.60 ± 0.76b |
Value significantly (P < 0.05) different from that for vehicle control.
Value significantly (P < 0.05) different from that for WT.
Figure 3.
Ovarian area. Values are group means ± SE (WT vehicle, n = 9; TAg vehicle, n = 9; WT VCD, n = 11; and TAg VCD, n = 5). *, Values are significantly (P < 0.05) different.
CL number.
As an indicator of ovulation, CL were counted on every section of both ovaries from the vehicle-only mice. The CL count (mean ± SEM) for WT animals was 7.5 ± 1.6 CL, significantly greater (P < 0.05) than the mean CL number for TAg animals, which was 2.3 ± 0.9 CL.
Reproductive status.
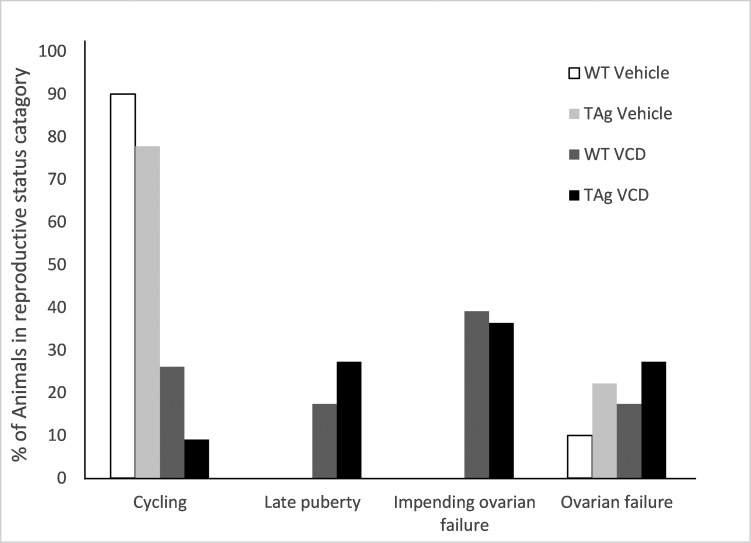
On the basis of cyclicity, the reproductive status of each animal was assigned according to the following categories: late onset of puberty, with puberty defined as the day of first estrus; normal cycling; early irregular cyclicity, indicative of impending ovarian failure; and ovarian failure. During the period in which vaginal cytology was performed, 90% of vehicle-only WT and 78% of vehicle-only TAg mice were cycling. Furthermore, 22% of vehicle-only TAg mice were in ovarian failure. In VCD-dosed mice, 26% of WT mice were cycling normally, compared with only 9% of TAg mice; 17% of WT showed late onset of puberty, compared with 27% of TAg mice; 39% of WT mice and 36% of TAg animals had early irregular cyclicity; and 17% of WT and 27% of TAg mice were in ovarian failure (Table 1, Figure 4).
Figure 4.
Percentage of mice in each assigned reproductive phase. Each animal was assigned as cycling, late-onset of first estrus or puberty, impending ovarian failure, or ovarian failure. Values are the percentage of each stage relative to the total of each group (WT vehicle, n = 10; TAg vehicle, n = 9; WT VCD, n = 23; and TAg VCD, n = 11).
Ovarian histology.
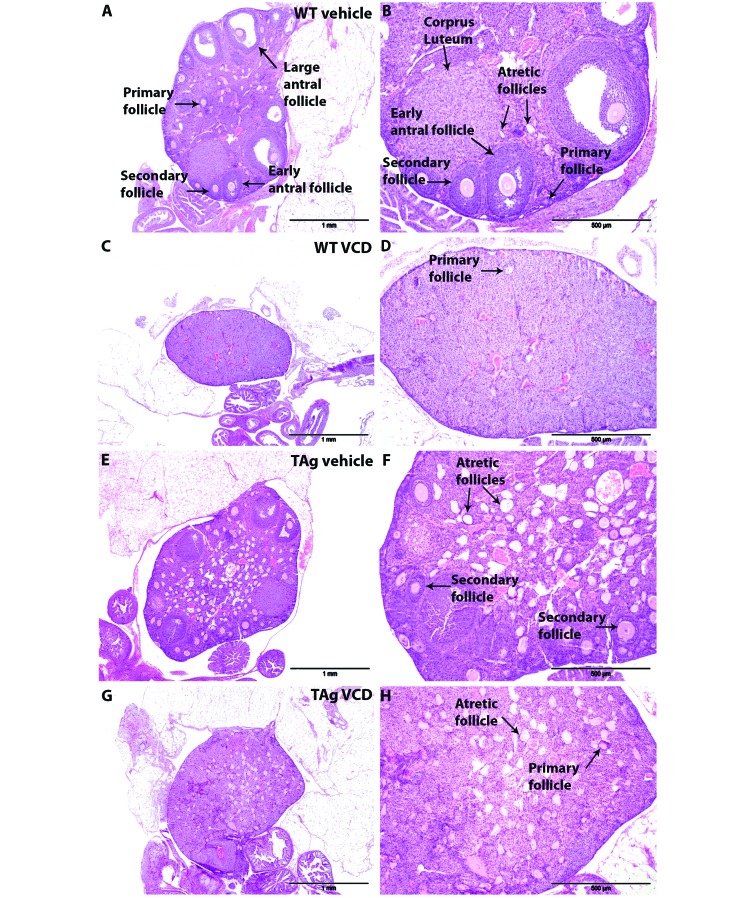
Ovaries from vehicle-only and VCD-dosed WT and TAg mice were collected and prepared for histologic evaluation (Figure 5). Follicles in all stages of development (primary, secondary, early antral, large antral) were observed in all sections from cycling WT and TAg animals (Figure 5 A, B, E, and F). All ovaries contained numerous areas that appeared to be remnants of follicles in which the oocyte had degenerated (that is, atretic follicles), and atretic follicles seemed to be much more numerous in ovaries from vehicle-only and VCD-dosed TAg mice (Figure 5 E, F, G, and H) compared with WT animals (Figure 5 A, B). Ovaries from both vehicle-only and VCD-dosed WT animals contained CL (Figure 5 A, B, E, and F), and ovaries of vehicle-only and VCD-dosed TAg mice (Figure 5 C, D, G, and H) had fewer CL than WT mice (Figure 5 A, B).
Figure 5.
Sections of ovaries from representative vehicle-only and VCD-dosed mice. Ovaries from vehicle-only WT mice have normally developing follicles and corpora lutea; magnification, 4× (A), 10× (B). Ovaries from VCD-dosed WT have few normally developing follicles; magnification, 4× (C), 10× (D). Compared with WT, vehicle-only TAg ovaries have scattered normally developing follicles, fewer corpora lutea, and many atretic follicles; magnification, 4× (E), 10× (F). VCD-dosed TAg ovaries have many atretic follicles and few corpora lutea; magnification, 4× (G), 10× (H). Hematoxylin and eosin stain.
Discussion
Whereas patterns of cyclicity were similar between groups, the day of first estrus was later in vehicle-only TAg mice than in WT mice. In addition, VCD dosing further delayed the onset of puberty in TAg but not WT mice. In vehicle-only TAg had fewer ovulations (as determined by number of CL) than vehicle-only WT animals, suggesting that TAg mice do not cycle as regularly as WT mice. In regard to reproductive status, morphologic evaluation of ovaries revealed much more extensive degeneration of follicles in TAg mice than in WT animals in either treatment group. Furthermore, more VCD-dosed TAg mice than VCD-dosed WT mice were in ovarian failure.
In mice, the first estrus is accompanied by ovulation, the onset of puberty, and the development of reproductive capacity.6 These events are driven by 17β-estradiol (estrogen) which is produced just prior to ovulation in the granulosa cells in large antral follicles.2 The average day of first estrus was similar between vehicle-only WT and TAg mice but was delayed in VCD-treated TAg mice. The reason for this effect might be as follows. Estrogen is produced in follicular granulosa cells to promote ovulation, and VCD accelerates the activation of small preantral follicles in an unregulated manner and can thus accelerate the natural process of cell death (that is, atresia).7 Morphologic evaluation revealed more extensive follicle degeneration in ovaries from TAg as compared with WT mice. Therefore, VCD may have exacerbated atresia, thus delaying both the maturation of granulosa cells and production of sufficient amounts of estrogen to support ovulation. Regardless, TAg mice eventually achieved the first estrus, and cyclicity began.
Cycle length was variable between mice and within one animal. Assessment for vaginal opening began at pnd 7 and was continued through vaginal cytology until pnd 56; the onset of puberty, defined by first estrus, occurred later in that time frame. The likely reason for variable cycle length is that regular rhythms of cyclicity—as occur in more mature mice—have not yet been established. In VCD-dosed animals, onset of cyclicity was delayed (P < 0.05) in TAg compared with WT mice. Why female TAg mice are infertile7 is not clearly understood. In light of our findings regarding cyclicity in TAg mice, the infertility in TAg females cannot be explained by a lack of follicular development.
The ovaries of TAg female mice had follicles at all stages of development, quite similar to WT mice. However, morphologic evaluation revealed that fewer CL were present in ovaries from TAg compared with WT animals. After ovulation, a CL forms in residual follicular cells on the ovary and subsequently produces progesterone for implantation and maintenance of pregnancy.2 Therefore, the reduction in CL counted in TAg ovaries suggests the mice have not ovulated adequate numbers of follicles possibly due to accelerated follicular degeneration.
In WT mice ovarian size (area) was reduced by VCD exposure, consistent with earlier studies showing that VCD causes ovarian atrophy.9 Interestingly, ovarian size was greater in TAg than in WT mice. Whether this phenomenon is related to the tendency of TAg mice to develop ovarian tumors should be the subject of future studies. In addition, unlike the scenario in WT animals, ovarian size was not affected by VCD dosing in TAg mice. Morphologically, VCD accelerated ovarian failure in WT animals, as evidenced by the lack of functional structures (antral follicles and CL) and ovarian atrophy. TAg mice treated with VCD lacked antral follicles and CL, had primary follicles and numerous atretic follicles, but ovarian atrophy was not seen. The delayed responsiveness to VCD in TAg mice supports that small follicle destruction seen in WT mice results in ovarian atrophy, whereas, in TAg mice, loss of functional structures does not result in atrophy. Therefore, the greater ovarian size in TAg as compared with WT mice may be due to an increased stromal compartment that is unresponsive to VCD.
In summary, our study provides the first comparison of the reproductive capacity (estrous cyclicity, puberty onset, and ovarian follicle and CL morphology) of female Tg-MISIIR-TAg transgenic mice with WT mice of the background strain, C57BL/6. The results demonstrate the similarity between those groups, but TAg mice had more follicular atresia than WT mice. Furthermore, TAg animals had fewer CL than WT mice, suggesting a lower level of ovulation in TAg mice that could contribute to their infertility. Future studies will investigate these findings more closely.
Acknowledgments
Support for this study was provided in part by a grant from the National Institutes of Health 1R01CA195723, the UACC Support Grant, P30CA023074, the FCCC Core Grant NCI P30 CA006927, as well as charitable donations (to DCC's laboratory) from the Roberta Dubrow Fund, the Teal Tea Foundation, the Bucks County Board of Associates and the Main Line Board of Associates. The TgMISIIR-TAg mice at Fox Chase are bred, maintained and genotyped with the assistance of the FCCC P30 CA006927-supported Laboratory Animal and Genomic Facilities.
References
- 1.American Cancer Society. [Internet]. 2018. Key statistics for ovarian cancer. [Cited 05 January 2018]. Available at: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html
- 2.Bahr JM, Milich KM. 2014. Ovarian physiology, p 3–13. In: Hoyer PB, editor. Ovarian toxicology 2nd ed. Boca Raton (FL): Taylor and Francis Group. [Google Scholar]
- 3.Borman SM, VanDePol BJ, Kao S, Thompson KE, Sipes IG, Hoyer PB. 1999. A single dose of the ovotoxicant 4-vinylcyclohexene diepoxide is protective in rat primary ovarian follicles. Toxicol Appl Pharmacol 158:244–252. 10.1006/taap.1999.8702. [DOI] [PubMed] [Google Scholar]
- 4.Connolly DC, Bao R, Nikitin AY, Stephens KC, Poole TW, Hua X, Harris SS, Vanderhyden BC, Hamilton TC. 2003. Female mice chimeric for expression of the simian virus 40 TAg under control of the MISIIR promoter develop epithelial ovarian cancer. Cancer Res 63:1389–1397. [PubMed] [Google Scholar]
- 5.Devine PJ, Sipes IG, Skinner MK, Hoyer PB. 2002. Characterization of a rat in vitro culture system to study the ovarian toxicant 4-vinylcyclohexene diepoxide. Toxicol Appl Pharmacol 184:107–115. 10.1006/taap.2002.9502. [DOI] [PubMed] [Google Scholar]
- 6.Gaytan F, Morales C, Leon S, Heras V, Barroso A, Avendano MS, Vazquez MJ, Castellano JM, Roa J, Tena-Sempere M. 2017. Development and validation of a method for precise dating of female puberty in laboratory rodents: the puberty ovarian maturation score (Pub-Score). Sci Rep 7:1–11. 10.1038/srep46381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hensley H, Quinn B, Wolf RL, Williams SJ, Williams C, Hamilton TC, Connolly DC. 2007. Magnetic resonance imaging dor detection and determination of tumor volume in a genetically engineered mouse model of ovarian cancer. Cancer Biol Ther 6:1717–1725. 10.4161/cbt.6.11.4830. [DOI] [PubMed] [Google Scholar]
- 8.Lohff JC, Christian PJ, Marion SL, Arrandale A, Hoyer PB. 2005. Characterization of cyclicity and hormonal profile with impending ovarian failure in a novel chemical-induced mouse model of perimenopause. Comp Med 55:523–527. [PubMed] [Google Scholar]
- 9.Mayer LP, Devine PJ, Dyer CA, Hoyer PB. 2004. The follicle-deplete mouse ovary produces androgen. Biol Reprod 71:130–138. 10.1095/biolreprod.103.016113. [DOI] [PubMed] [Google Scholar]
- 10.Pinter O, Beda Z, Csaba Z, Gerendoi I. 2007. Differences in the onset of puberty in selected inbred mouse strains. Joint Meeting of British Endocrine Societies, 9th European Congress of Endocrinology, Budapest, Hungary, 28 Apr–02 May 2017. Endocrine abstracts 14:617. [Google Scholar]
- 11.Quinn BA, Xiao F, Bickel L, Martin L, Hua X, Klein-Szanto A, Connolly DC. 2010. Development of a syngeneic mouse model of epithelial ovarian cancer. J Ovarian Res 3:1–17. 10.1186/1757-2215-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schüler S, Ponnath M, Engel J, Ortmann O. 2013. Ovarian epithelial tumors and reproductive factors: a systemic review. Arch Gynecol Obstet 287:1187–1204. [DOI] [PubMed] [Google Scholar]
- 13.Siegel RL, Miller KD, Jemal A. 2017. Cancer statistics, 2017. CA Cancer J Clin 67:7–30. 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 14.Springer LN, McAsey ME, Flaws JA, Tilly JL, Sipes IG, Hoyer PB. 1996. Involvement of apoptosis in 4-vinylcyclohexene diepoxide-induced ovotoxicity in rats. Toxicol Appl Pharmacol 139:394–401. 10.1006/taap.1996.0180. [DOI] [PubMed] [Google Scholar]
- 15.Wright LE, Christian PJ, Rivera Z, Van Alstine WG, Funk JL, Bouxsein ML, Hoyer PB. 2008. Comparison of skeletal effects of ovariectomy versus chemically induced ovarian failure in mice. J Bone Miner Res 23:1296–1303. 10.1359/jbmr.080309. [DOI] [PMC free article] [PubMed] [Google Scholar]