SUMMARY
In the developing brain immature synapses contain calcium-permeable AMPA glutamate receptors (AMPARs) that are subsequently replaced with GluA2-containing calciumimpermeable AMPARs as synapses stabilize and mature. Here we show that this essential switch in AMPARs and neuronal synapse maturation is regulated by astrocytes. Using biochemical fractionation of astrocyte-secreted proteins and mass spectrometry, we identified astrocyte-secreted chordin like 1 (Chrdl1) is necessary and sufficient to induce mature GluA2containing synapses to form. This function of Chrdl1 is independent of its role as an antagonist of BMPs. Chrdl1 expression is restricted to cortical astrocytes in vivo, peaking at the time of the AMPAR switch. Chrdl1 KO mice display reduced synaptic GluA2 AMPARs, altered kinetics of synaptic events, and enhanced remodeling in an in vivo plasticity assay. Studies have shown that humans with mutations in Chrdl1 display enhanced learning. Thus astrocytes, via the release of Chrdl1, promote GluA2-dependent synapse maturation, and thereby limit synaptic plasticity.
eToc
Blanco-Suarez et al. identify that synapse maturation is not intrinsic to neurons, but is regulated by neighboring astrocytes. They demonstrate that astrocyte-secreted Chrdl1 increases GluA2 AMPA receptor levels at synapses, inducing synapse maturation and inhibiting plasticity.
INTRODUCTION
An important step in the development of the central nervous system (CNS) is the maturation and stabilization of appropriate synaptic connections between neurons, ensuring that correct pathways remain functional throughout life. A major component of excitatory glutamatergic synapse maturation is a switch in the subtype of AMPA glutamate receptor (AMPAR) present at excitatory synapses, from calcium-permeable to GluA2-containing calcium-impermeable channels (Brill and Huguenard, 2008; Kumar et al., 2002). This switch in AMPARs contributes to synapse maturation and stabilization by decreasing the entry of calcium into the postsynaptic cell, limiting plasticity mechanisms that require signaling through calcium-dependent pathways (Henley and Wilkinson, 2016; Jia et al., 1996; Traynelis et al., 2010). The molecular mechanisms that underlie this switch in AMPARs in the context of development remain unknown.
AMPA glutamate receptors (AMPARs) conduct fast synaptic signals at excitatory glutamatergic synapses (Traynelis et al., 2010). There are 4 AMPAR subunits (GluA1–4), which assemble into tetramers (Traynelis et al., 2010). AMPAR composition determines synaptic properties, e.g. the duration of the synaptic response and whether calcium permeates the channel. During cortical development, the switch from calcium-permeable to GluA2-containing calcium-impermeable AMPARs occurs at specific times in each cortical layer, occurring first in layer 4 neurons at P78, then layer 2/3 neurons at P12–14 and finally in layer 5 neurons at P15–16 (Brill and Huguenard, 2008; Kumar et al., 2002). Conflicting results exist about whether neuronal activity is required to promote the subunit switch of AMPARs, with some studies showing it is necessary and others that the switch occurs independently of activity (Liu and Cull-Candy, 2000; Zhu et al., 2000). Whether this switch is intrinsic to neurons, or regulated by other cells, is not known.
Astrocytes are the main class of glial cells in the CNS (Khakh and Sofroniew, 2015). During development astrocytes secrete multiple signals that induce formation or elimination of synapses, and regulate synaptic strength (Clarke and Barres, 2013). We previously showed that astrocyte-secreted glypicans 4 and 6 (Gpc4 and 6) specifically recruit GluA1 calcium-permeable AMPARs to synapses, promoting the formation of immature synapses (Allen et al., 2012). However, Gpc4 and 6 have no effect on GluA2 clustering (Allen et al., 2012). Based on this we hypothesize that astrocytes can regulate the subunit composition of AMPARs at synapses by releasing distinct signals, with Gpc4 and 6 inducing nascent immature GluA1-containing synapses and a second factor promoting synapse maturation by increasing synaptic levels of calcium-impermeable GluA2-containing AMPARs.
In the present study, we identify chordin like-1 (Chrdl1) as the astrocyte-secreted factor responsible for increasing synaptic GluA2 AMPARs. Chrdl1 has been classified as a member of the chordin family of secreted bone morphogenetic protein (BMP) antagonists due to sequence homology (Nakayama et al., 2001; Sakuta et al., 2001). Chrdl1 has three cysteine rich repeats (CR), which are homologous to von Willebrand factor C (vWFC) domains. Chrdl1 can bind multiple BMP ligands, with no detectable binding to other members of the transforming growth factor beta (TGFbeta) super-family including activin and TGFbeta (Nakayama et al., 2001). BMP signaling has been shown to promote synaptogenesis at the Drosophila neuromuscular junction (Ball et al., 2015; Berke et al., 2013; Fuentes-Medel et al., 2012) and in vertebrates (Shen et al., 2004; Xiao et al., 2013). Alternatively, Chrdl1 may act in a BMP-independent manner by interacting with additional ligands via its vWFC domains. There is precedent for this from other astrocyte-secreted synaptogenic factors. For example, Gpc4 was identified as a molecule that binds and sequesters growth factors in the extracellular space via its glycosaminoglycan side chains (Hagihara et al., 2000), analogous to Chrdl1 binding and sequestering BMPs. However, we identified that the mechanism Gpc4 uses to recruit GluA1 AMPARs to synapses is independent of this function, and instead is via Gpc4 signaling through a presynaptic receptor (Farhy-Tselnicker et al., 2017).
Here we performed a biochemical screen and identified Chrdl1 as the astrocyte-secreted protein necessary and sufficient to regulate GluA2 synaptic levels and synapse maturation. We find that in vivo expression of Chrdl1 in astrocytes has both temporal and regional heterogeneity, peaking in the cortex at the time of synapse maturation. We demonstrate that Chrdl1 KO mice show enhanced remodeling in response to altered sensory input, showing that Chrdl1 is repressing plasticity in vivo. These findings identify an important role for non-neuronal cells in the sculpting of neural circuits, and have implications for understanding synapse maturation during development and the regulation of synaptic plasticity in the adult.
RESULTS
Astrocytes secrete a factor that is sufficient to increase GluA2 AMPARs at synapses
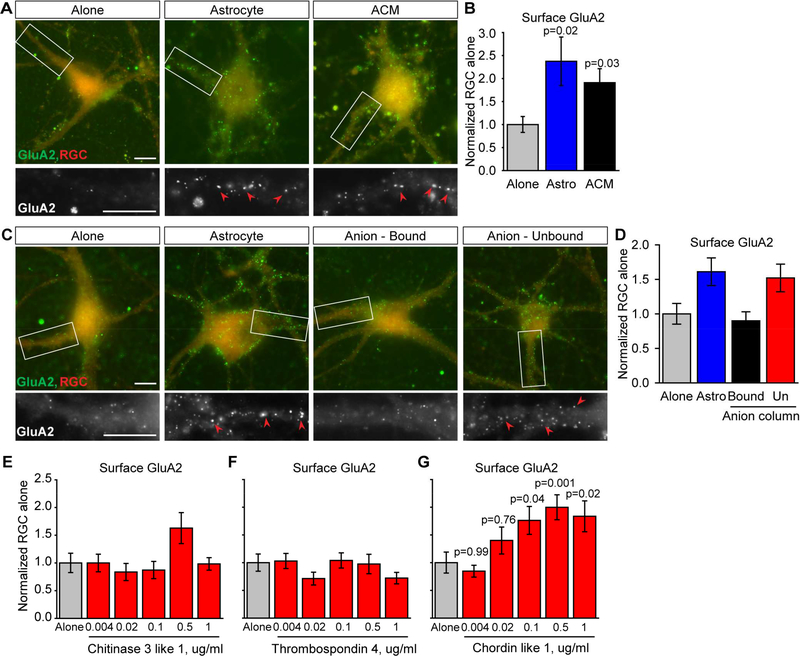
To directly identify the astrocyte-secreted factor that is sufficient to increase synaptic GluA2 AMPARs, we carried out a screen using cultured retinal ganglion cell neurons (RGCs) isolated from the rat retina (Allen et al., 2012; Ullian et al., 2001). RGCs cultured in the absence of other cell types form few synapses and show little synaptic activity. Adding astrocytes or factors secreted by astrocytes (astrocyte conditioned media (ACM)) for 6 days greatly increases RGC synapse number, synaptic activity and surface AMPARs, making this a robust system to identify astrocyte-secreted proteins that regulate synapses (Allen et al., 2012; Ullian et al., 2001). To assay surface levels of GluA2 AMPARs on RGC dendrites we used a live immunostaining assay on non-permeabilized cells, with an antibody specific to the N-terminal region of GluA2. We found that RGCs grown in the presence of astrocytes or ACM for 6 days have a significant ~2-fold increase in surface clusters of GluA2 AMPARs (Figure 1A-B; astrocyte 2.37±0.53-fold, ACM 1.91±0.30-fold, compared to alone), demonstrating that astrocytes secrete a factor that is sufficient to increase levels of GluA2 AMPARs at synapses.
Figure 1. Identification of an astrocyte-secreted factor that is sufficient to increase surface clustering of GluA2 AMPARs.
(A,B) Astrocyte-secreted proteins increase surface clustering of GluA2. (A) Example images of RGC neurons cultured alone, with an astrocyte feeder layer, or astrocyte conditioned media (ACM) for 6 days. Top panel, red labels whole neuron, green surface GluA2. Bottom panel, zoom of dendrite, GluA2 white. Arrowheads mark example GluA2 clusters. (B) Quantification of A, surface GluA2 normalized to RGC alone. Example experiment shown, N=29–30 cells per condition, repeated >3 times with same result. (C,D) Column fractionation of ACM identified the GluA2 clustering activity in the unbound fraction from an anion column. (C) Example images, as in A. (D) Quantification of C, example experiment shown, N=30 cells per condition, repeated >3 times with same result. (E-G) Testing candidate proteins for GluA2 clustering activity. RGCs were treated with multiple doses of purified recombinant proteins for 6 days and assayed for surface clustering of GluA2. Chitinase 3 like 1 (Chi3l1) (E) and Thrombospondin 4 (Thbs4) (F) had no effect; Chordin like 1 (Chrdl1) (G) significantly increased surface clustering of GluA2 at multiple doses. Example experiments shown, N=29–30 cells per condition; Chi3l1 and Thbs4 repeated twice, Chrdl1 3 times, with same result. Scale bar = 10μm. Bar graphs mean±s.e.m.. Statistics by one-way ANOVA, significance as stated on graph compared to alone. See also Table S1.
Biochemical screen to identify the astrocyte-secreted GluA2 clustering factor
To determine which factor being secreted by astrocytes is sufficient to increase surface GluA2 clusters on RGC neurons, we fractionated factors present in ACM based on charge using an anion exchange chromatography column. Column fractions were assayed for the ability to increase surface GluA2 on RGC dendrites, and this identified the unbound fraction from an anion exchange column as sufficient (Figure 1C-D; astrocyte 1.61±0.20-fold, bound 0.90±0.13fold, unbound 1.52±0.20-fold, compared to alone). We used mass spectrometry analysis to identify the proteins present in the anion exchange column unbound fraction, generating a list of fifty candidates (Table S1). To narrow down proteins for testing, we compared the most abundant extracellular proteins detected in the positive fraction with published mRNA expression data from purified brain cells (Cahoy et al., 2008). We focused on proteins that 1) are enriched in astrocytes and absent from neurons, as we hypothesized that this will be a factor specifically made by astrocytes in vivo, and 2) peak in expression in astrocytes at P14, the time when GluA2 is being recruited to synapses (Brill and Huguenard, 2008). This narrowed down the list to three candidates: chitinase 3 like 1 (Chi3l1), chordin like 1 (Chrdl1) and thrombospondin 4 (Thbs4). To determine if any of these proteins is sufficient by itself to increase surface clustering of GluA2, we treated RGCs with purified protein for each candidate at multiple doses (4ng-1μg/ml) for 6 days. Chi3l1 and Thbs4 did not cause a significant increase in surface clustering of GluA2 at any dose tested (Figure 1E-F). Chrdl1 was sufficient to significantly increase surface clusters of GluA2 by ~2-fold at multiple doses (0.1–1μg/ml; Figure 1G), identifying Chrdl1 as a factor secreted by astrocytes that regulates surface clustering of GluA2 AMPARs.
Chrdl1 is secreted by astrocytes and is necessary and sufficient to increase GluA2 clustering, and formation of active synapses
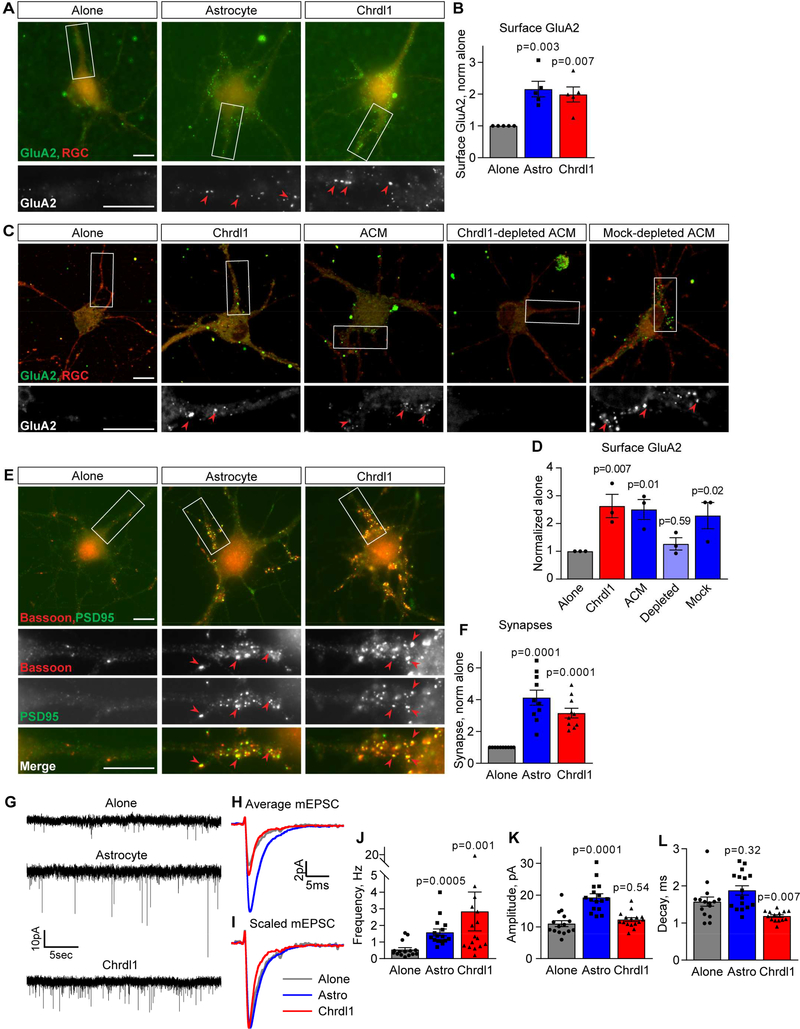
Having identified Chrdl1 via mass spectrometry analysis, we next validated that astrocytes in vitro are secreting Chrdl1 protein. Western blotting of ACM detected Chrdl1 protein, whereas RGC conditioned media did not contain Chrdl1, showing astrocytes and not neurons are secreting Chrdl1 protein in vitro (Figure S1A-B). We then determined the extent of Chrdl1 effects on multiple aspects of synapse formation and function in vitro by treating RGC neurons with purified Chrdl1 protein at 1μg/ml for 6 days (a level of protein similar to that being secreted by astrocytes into ACM in vitro, Fig S1C), the treatment used in all subsequent experiments unless noted. Chrdl1 increased surface clustering of GluA2 to the same level as astrocytes, suggesting that Chrdl1 is a major contributor to this astrocyte function (Figure 2A-B; astrocyte 2.15±0.24-fold, Chrdl1 1.99±0.24-fold, compared to alone). To address this directly we used immunodepletion to remove Chrdl1 from ACM, and compared the ability of Chrdl1-depleted ACM to mock-depleted ACM to increase surface GluA2 (Figure S1D). This demonstrated that Chrdl1-depleted ACM no longer increased surface GluA2 compared to the untreated condition, whereas mock-depleted ACM induced a significant ~2-fold increase in surface GluA2 (Figure 2C-D; Chrdl1 2.63±0.42-fold, ACM 2.51±0.36-fold, Chrdl1-depleted ACM 1.27±0.22-fold, mock-depleted ACM 2.29±0.47-fold, compared to alone).
Figure 2. Chrdl1 is sufficient to induce formation of mature synapses in vitro.
(A,B) Chrdl1 increases surface GluA2 to the same level as astrocytes. (A) Example images of RGC neurons cultured alone, with an astrocyte feeder layer, or 1μg/ml Chrdl1 protein for 6 days. Top panel, red labels whole neuron, green surface GluA2. Bottom panel, zoom of dendrite, GluA2 white. Arrowheads mark example GluA2 clusters. (B) Quantification of A, surface GluA2 normalized to RGC alone. N=5 experiments, each experiment 30 cells/condition. (C,D) Depletion of Chrdl1 from ACM abolishes the ability of ACM to increase surface GluA2. (C) Example images as in A of RGC neurons cultured alone, with 1μg/ml Chrdl1 protein, 50μg/ml ACM, Chrdl1-depleted ACM or mock-depleted ACM for 6 days. (D) Quantification of C, surface GluA2 normalized to RGC alone. N=3 experiments, each experiment 30 cells/condition. (E,F) Chrdl1 is sufficient to induce structural synapse formation. (E) Example images of RGC neurons cultured as in A, immunostained for presynaptic bassoon (red) and postsynaptic PSD95 (green). Lower panels zoom of dendrite, arrowheads mark example synapses (colocalized bassoon and PSD95). (F) Quantification of E, synapse number normalized to RGC alone. N=10 experiments, each experiment 30 cells/condition. (G-L) Chrdl1 is sufficient to induce functional synapses and increase synaptic activity. (G) Example mEPSC recordings from RGC neurons treated as in A. (H,I) Average mEPSC from all recordings aligned to rise-time, raw (H), scaled to peak (I). (J-L) Quantification of mEPSCs: Chrdl1 increases mEPSC frequency (J), amplitude is unchanged (K), decay time is decreased (L). N=15–16 cells per condition from 3 experiments. Scale bar = 10μm. Bar graphs mean±s.e.m., with individual data points marked (cells J,K,L; experiments B,D,F). Statistics by one-way ANOVA, significance as stated on graph compared to alone. See also Figure S1.
To determine if Chrdl1 is sufficient to increase synapse formation, we used immunocytochemistry to label pre and postsynaptic markers (with Bassoon and PSD95, respectively), scoring colocalization of synaptic markers as a synapse (Ippolito and Eroglu, 2010). Chrdl1 was sufficient to increase synapse number by ~3-fold, a similar effect to that induced by astrocytes (Figure 2E-F; astrocyte 4.13±0.47-fold, Chrdl1 3.15±0.30-fold, compared to alone).
Given an increase in synapses containing GluA2 AMPARs, we next asked if these synapses are functionally active. We used whole-cell patch clamping to record individual excitatory postsynaptic currents (mEPSCs) from RGCs, in the presence of tetrodotoxin to block action potentials (Figure 2G-I). Chrdl1 induced a significant increase in the frequency of mEPSCs detected, to the same level as astrocytes (Figure 2J, S1E; alone 0.55±0.12Hz, astrocyte 1.58±0.21Hz, Chrdl1 2.84±1.17Hz). Chrdl1 did not induce a significant change in mEPSC amplitude compared to RGCs alone (Figure 2K, S1F; alone 11.03±0.93pA, astrocyte 19.25±1.16pA, Chrdl1 12.28±0.61pA), whereas mEPSC decay time was significantly decreased by Chrdl1 treatment (Figure 2L; alone 1.58±0.12ms, astrocyte 1.88±0.13ms, Chrdl1 1.19±0.13ms). Taken together, these data demonstrate that astrocyte-secreted Chrdl1 is necessary and sufficient to induce the formation of mature functional excitatory synapses containing GluA2 AMPARs, differentiating it from astrocyte-secreted Gpc4 which induces formation of immature nascent synapses containing GluA1 (Allen et al., 2012; Farhy-Tselnicker et al., 2017).
Chrdl1 regulates GluA2 AMPARs in neurons in a BMP-independent manner
Chrdl1 was identified and classified as a member of the chordin family of secreted BMP antagonists due to sequence homology (Coffinier et al., 2001; Nakayama et al., 2001; Sakuta et al., 2001; Ueki et al., 2003). BMP signaling is involved in synaptogenesis (Ball et al., 2015; Berke et al., 2013; Fuentes-Medel et al., 2012; Kalinovsky et al., 2011; Xiao et al., 2013) and dendritic outgrowth (Beck et al., 2001; Esquenazi et al., 2002; Withers et al., 2000) through both SMAD-dependent (transcriptional activation of target genes) and SMAD-independent mechanisms (Lee‐Hoeflich et al., 2004; Liu and Niswander, 2005). Therefore a possible mechanism for how astrocyte-secreted Chrdl1 regulates GluA2 AMPARs is by antagonizing ongoing BMP signaling in neurons.
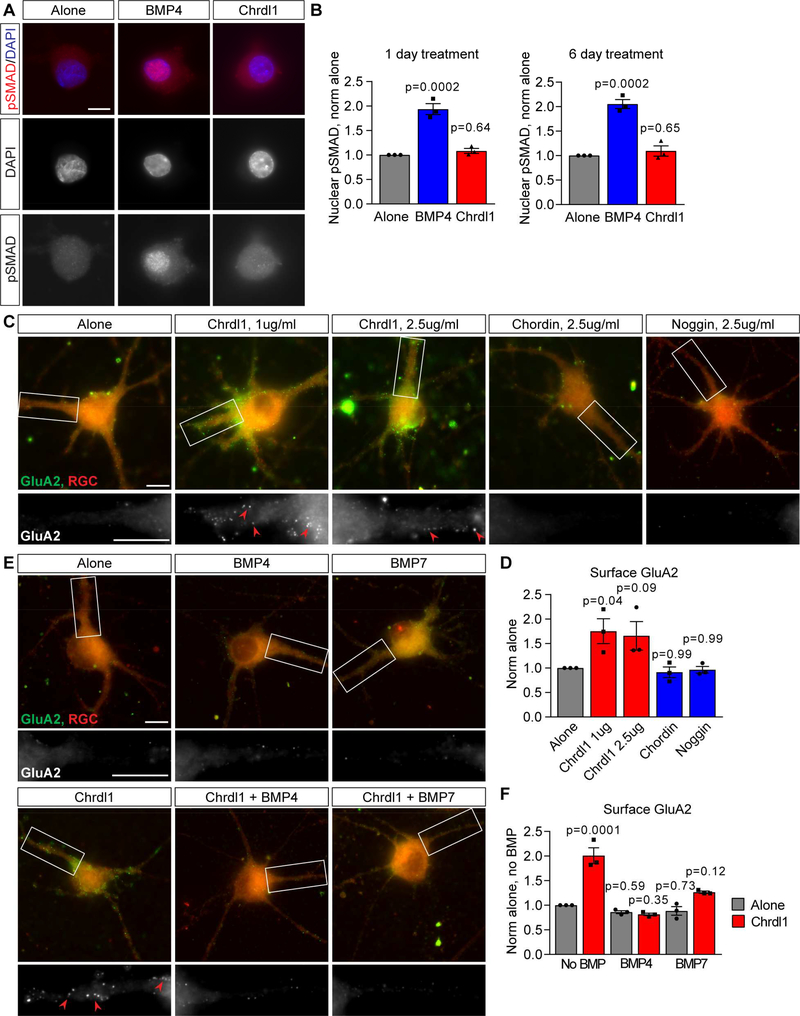
We first asked if there is any evidence of Chrdl1 regulating canonical BMP signaling in neurons by controlling the recruitment of phosphoSMAD (pSMAD) to the nucleus, a necessary step for pSMAD to regulate transcription of BMP target genes (Liu and Niswander, 2005). We found no difference in the level of nuclear pSMAD in RGCs grown alone or treated with Chrdl1 for 1 or 6 days, whereas treatment with BMP4 as a positive control caused a robust nuclear recruitment of pSMAD (Figure 3A-B; 1 day treatment: BMP4 1.94±0.11-fold, Chrdl1 1.08±0.05-fold, compared to alone; 6 day treatment: BMP4 2.05±0.09-fold, Chrdl1 1.09±0.10-fold, compared to alone). We then used an unbiased approach to ask if Chrdl1 regulates expression of BMP target genes, by performing RNA sequencing of RGCs treated with Chrdl1 compared to untreated neurons. We found that Chrdl1 treatment of RGCs did not alter the expression of genes related to BMP signaling (Table S2), or genes involved in excitatory synaptogenesis and AMPAR trafficking (Table S3). The inability of Chrdl1 to alter the translocation of pSMAD to the nucleus or regulate expression of BMP target genes indicates Chrdl1 is likely acting independently of the canonical BMP signaling cascade.
Figure 3. Chrdl1-induced GluA2 clustering is independent of BMP signaling.
(A,B) Chrdl1 does not regulate recruitment of pSMAD to the nucleus in RGC neurons. (A) Example images of RGCs treated with 1μg/ml Chrdl1 or 250ng/ml BMP4 for 1 day, and immunostained for pSMAD (red) and DAPI to mark the nucleus (blue). BMP4 is a positive control condition, and is sufficient to increase nuclear pSMAD. (B) Quantification of A, nuclear pSMAD level normalized to alone. N=3 experiments, each experiment 30 cells/condition, both 1 day and 6 day treatment. (C,D) Other secreted BMP antagonists do not increase surface clustering of GluA2 in RGCs. (C) Example images of RGC neurons treated with Chrdl1, chordin or noggin for 6 days, concentration as marked. Top panel, red labels neuron, green surface GluA2. Bottom panel, zoom of dendrite, GluA2 white. Arrowheads mark example GluA2 clusters. (D) Quantification of C, surface GluA2 compared to RGC neurons alone (untreated). N=3 experiments, each experiment 30 cells/condition. (E,F) Excess BMP ligands block the ability of Chrdl1 to increase surface GluA2 clustering in RGCs. (E) Example images of RGC neurons treated with 1μg/ml Chrdl1, 250ng/ml BMP4, 250ng/ml BMP7, or Chrdl1 and either BMP for 6 days. Top panel, red labels neuron, green surface GluA2. Bottom panel, zoom of dendrite, GluA2 white. Arrowheads mark example GluA2 clusters. (F) Quantification of E, surface GluA2 normalized to RGCs alone (no BMP). N=3 experiments, each experiment 30 cells/condition. Scale bar = 10μm. Bar graphs mean±s.e.m., with individual data points representing experiments. Statistics by one-way ANOVA, significance as stated on graph compared to alone. See also Figure S1, Table S2, S3.
The RNA sequencing analysis showed that RGCs express mRNA for multiple BMP family members, including BMP2,4,6,7, making inhibiton of their action through a non-canonical BMP pathway a possible mechanism (Table S2). If Chrdl1 is regulating non-canonical BMP signaling in RGC neurons, then we predict that other secreted antagonists of BMP signaling should have the same effect as Chrdl1 in regulating GluA2 AMPARs, by preventing BMP-receptor interaction. We tested two secreted BMP antagonists that bind the same BMPs as Chrdl1: chordin (binds BMP2,4,7) and noggin (binds BMP2,4,5,6,7) (Bragdon et al., 2011). We first validated that the BMP antagonists were functional, by demonstrating they were sufficient to decrease nuclear recruitment of pSMAD in response to BMP4 in HEK cells (Figure S1G-H). Treating RGC neurons for 6 days with either chordin or noggin did not cause an increase in surface GluA2 clusters on RGC dendrites compared to untreated RGCs, whereas Chrdl1 applied in the same experiment did (Figure 3C-D; Chrdl1 1μg/ml 1.75±0.25-fold, Chrdl1 2.5μg/ml 1.66±0.29-fold, chordin 2.5μg/ml 0.91±0.11-fold, noggin 2.5μg/ml 0.96±0.07-fold, compared to alone). The inability of chordin or noggin to phenocopy the effect of Chrdl1 indicates BMP signaling is not involved in Chrdl1-mediated GluA2 recruitment.
We finally asked if adding excess BMP ligand to saturate the CR repeats/BMP binding sites on Chrdl1 would inhibit the ability of Chrdl1 to increase surface levels of GluA2. Chrdl1 shows a high affinity for BMP4 and lower affinity for BMP7 (Nakayama et al., 2001). High levels of BMP4 (250ng/ml) blocked the ability of Chrdl1 to increase surface clustering of GluA2, and BMP7 partially blocked the effect, showing that the CR repeats/BMP binding sites are necessary for Chrdl1 to increase surface GluA2 (Figure 3E-F; Chrdl1 2.01±0.16-fold, Chrdl1+BMP4 0.81±0.03-fold, Chrdl1+BMP7 1.26±0.02-fold, compared to alone). Additionally, if ongoing BMP signaling were regulating surface GluA2 in RGC neurons, then we predict that treatment with BMPs themselves would decrease surface levels of GluA2. However, treatment with either
BMP4 or BMP7 in the absence of Chrdl1 did not significantly alter surface levels of GluA2 on RGCs compared to the untreated condition (Figure 3E-F; BMP4 0.86±0.03-fold, BMP7 0.88±0.09-fold, compared to alone), showing that BMPs are not regulating synaptic levels of GluA2 AMPARs in RGCs. Taken together these results strongly suggest that the mechanism Chrdl1 uses to increase GluA2 AMPARs at synapses is independent of BMP signaling, and is instead through a novel pathway regulated by the CR repeats in Chrdl1.
Chrdl1 is enriched in astrocytes in vivo and shows heterogeneous spatiotemporal expression
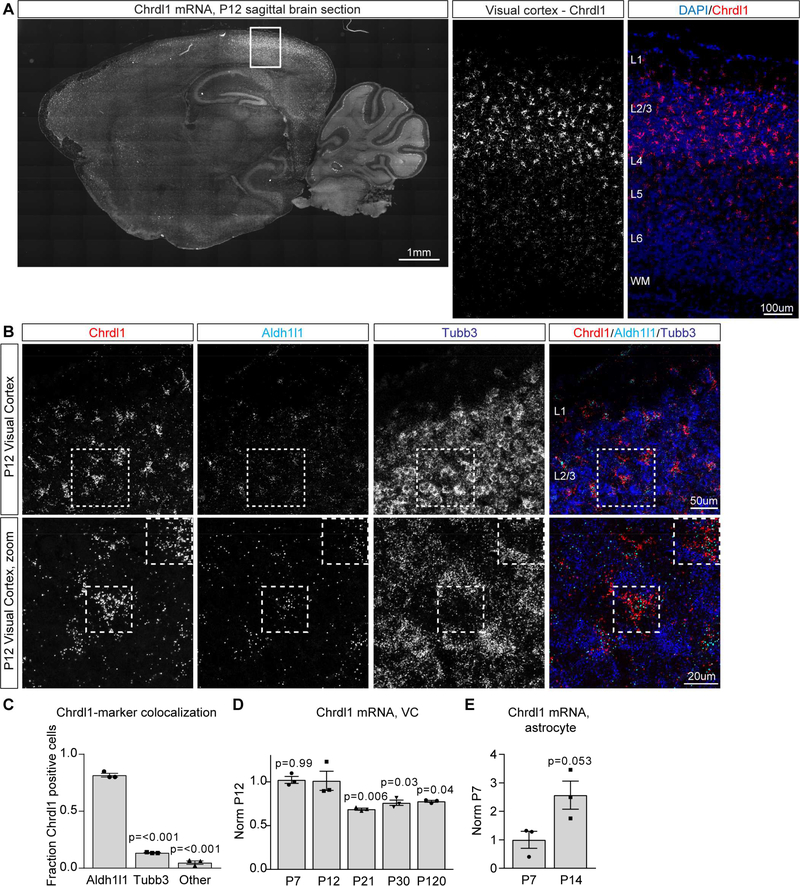
Having demonstrated Chrdl1 is an astrocyte-secreted factor that regulates synaptic GluA2 AMPARs and synapse maturation in vitro, we next examined the role of Chrdl1 in the developing brain. We analyzed the regional and temporal expression of Chrdl1 mRNA using in situ hybridization in brain sections from mice at multiple ages – P7 (initiation of synaptogenesis), P12 (initiation of synapse maturation), P30 (stable synapses) and P120 (adult). Expression of Chrdl1 was detected at all ages, and showed a heterogeneous pattern, being restricted to the cortex, striatum and cerebellum (of note, cerebellar Chrdl1 expression is in Purkinje neurons, not astrocytes) (Figure 4A, S2A-C,S4C). Interestingly, even within the cortex, Chrdl1 expression is heterogeneous, being enriched in upper cortical layers compared to deep layers (Figure 4A, S2A-C).
Figure 4. Chrdl1 is expressed by astrocytes in the visual cortex and peaks in expression at P14 at the time of the AMPAR switch.
(A) Fluorescent in situ hybridization (FISH) for Chrdl1 mRNA at P12, sagittal brain section, Chrdl1 mRNA white (left); zoom in of visual cortex (right), Chrdl1 mRNA red, DAPI to mark nuclei in blue. Representative image of the entire sagittal section is a mosaic of 126 overlapping stitched tiles. Representative images of the visual cortex are cropped from maximum orthogonal projections of z-stacks of mosaics of 14 overlapping stitched tiles. (B) FISH for Chrdl1 (red), Aldh1l1 to mark astrocytes (cyan) and Tubb3 to mark neurons (blue), in the visual cortex at P12. Bottom row, zoom of boxed area in top row. Squares outline example astrocytes expressing Chrdl1. (C) Quantification of overlap between Chrdl1 and Aldh1l1 or Tubb3 at P12, normalized to the total number of DAPI nuclei colocalized with Chrdl1 mRNA. N=3 mice. Statistics by one-way ANOVA compared to Chrdl1+Aldh1l1 condition, significance as marked on graph. (D) qRT-PCR for Chrdl1 mRNA in the visual cortex at multiple developmental timepoints, N=3 mice each age. Statistics by oneway ANOVA, significance as stated on graph compared to P12. (E) qRT-PCR for Chrdl1 from astrocyte mRNA from the visual cortex at P7 and P14, N=3 mice each age. Statistics by t-test, significance as stated on graph. Bar graphs mean±s.e.m., with individual data points per mouse. See also Figure S2,S3.
To determine if astrocytes are the major source of Chrdl1 in the cortex in vivo, we used fluorescent in situ hybridization (FISH) to detect Chrdl1 mRNA, along with an astrocyte marker (Aldh1l1 or GLAST) and a neuronal marker (Tubb3). Strong overlap between Chrdl1 and the astrocyte marker (Aldh1l1 or GLAST) was detected at all ages in the upper layers of the visual cortex, with minimal overlap between Chrdl1 and Tubb3 (Figure 4B, S3A-C). At P12 82% of the Chrdl1 mRNA overlaps with Aldh1l1 mRNA, showing in vivo Chrdl1 mRNA is enriched in astrocytes (Figure 4C; Chrdl1 overlap with: Aldh1l1 81.6±1.6%, Tubb3 13.5±0.2%, other 4.9±1.5%). Further evidence for astrocyte-enriched expression of Chrdl1 comes from published RNA sequencing data, which shows that Chrdl1 is enriched in astrocytes compared to other cell types in the developing cortex (Figure S4D) (Zhang et al., 2014). To determine if the level of Chrdl1 varies across development, we used qRT-PCR to quantify Chrdl1 mRNA level in the whole visual cortex (P7, P12, P21, P30, P120), and specifically in astrocytes in the visual cortex (P7, P14) at multiple postnatal timepoints. We found that Chrdl1 is expressed at every timepoint examined (Figure 4D), and peaks in expression at P12–14, with astrocyte-specific expression of Chrdl1 increasing by 2.57±0.50-fold between P7 and P14 (Figure 4E). This matches the time when the switch to GluA2 AMPARs and synapse maturation is occurring in the upper cortical layers where Chrdl1 expression is highest (Brill and Huguenard, 2008). Therefore, Chrdl1 is expressed by astrocytes in the cortex and peaking at the right time to be regulating the AMPAR switch and synapse maturation in vivo.
Chrdl1 absence does not disrupt neuron or astrocyte generation in the visual cortex
To determine the functional importance of Chrdl1 to synapse maturation in vivo, we developed a Chrdl1 knock out (KO) mouse (see Methods). KO of Chrdl1 was confirmed by in situ hybridization and qRT-PCR, with both techniques demonstrating a loss of Chrdl1 mRNA in the KO (Figure S4A-B). Although we demonstrated that Chrdl1 does not regulate GluA2 AMPARs by inhibiting BMP signaling in vitro (Figure 3), we first asked whether other BMP-dependent developmental processes are altered in Chrdl1 KO mice. Inhibition of BMP signaling promotes neurogenesis, and it has been proposed that Chrdl1 promotes a neurogenic fate by inhibiting BMP4 in the adult dentate gyrus (Ueki et al., 2003). We therefore quantified cortical thickness, neuron number and astrocyte number in the P14 visual cortex. We found no difference in any of these developmental parameters in the Chrdl1 KO mice compared to littermate wild type (WT) controls, showing no gross alteration in cell fate has occurred (Figure S5A-D). We further performed RNA sequencing analysis of the P14 visual cortex from Chrdl1 KO and WT mice to determine if genes related to BMP signaling, astrocyte development or synapse formation were altered. No significant differences in gene expression were detected, except for a significant decrease in Chrdl1 in the KO (Tables S2-S4), demonstrating that similar to our in vitro results, Chrdl1 does not strongly regulate neuronal gene expression in vivo.
Chrdl1 KO mice have decreased levels of GluA2 AMPARs at cortical synapses
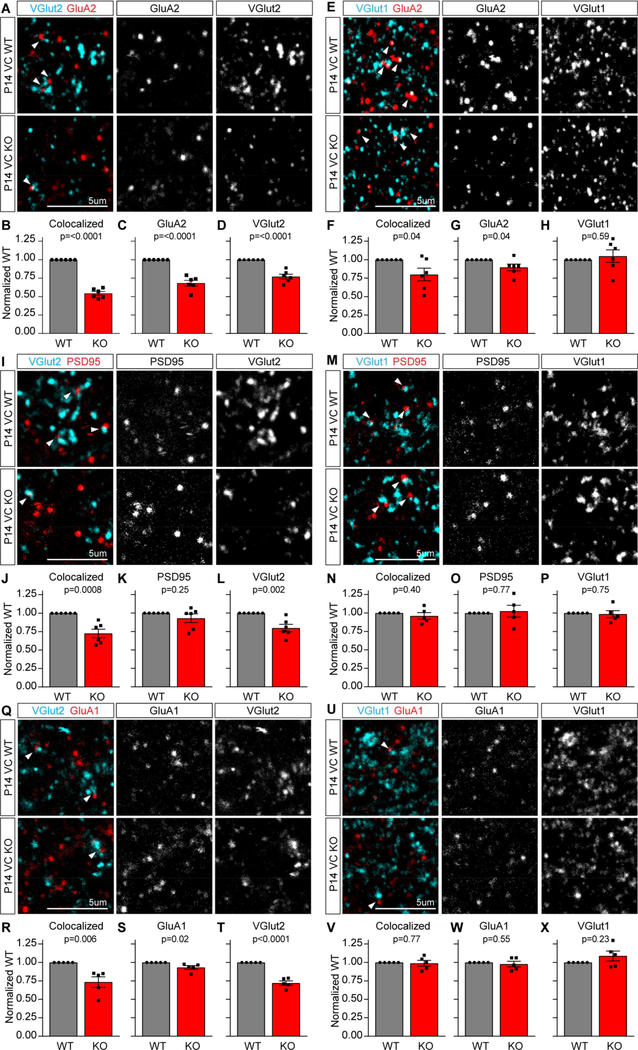
We next asked if features of synapse maturation are altered in the Chrdl1 KO mouse, focusing on synapses formed in the upper layers of the visual cortex at P14, a region where Chrdl1 expression is high and at a time when the switch to GluA2 AMPARs is occurring (Figure 4) (Brill and Huguenard, 2008). To determine if there is an alteration in GluA2 AMPARs present at synapses, we used immunohistochemistry to label GluA2 along with presynaptic markers in P14 visual cortex brain sections, and analyzed the upper cortical layers (Figure 5A,E; Figure S6F). We first asked if the level of GluA2 at thalamocortical synapses is altered in Chrdl1 KO mice, as these synapses predominantly form in upper cortical layers, by analyzing colocalization of presynaptic vesicular glutamate transporter 2 (VGlut2) with GluA2. This showed a significant ~50% decrease in the amount of GluA2 colocalized with VGlut2 in the Chrdl1 KO (Figure 5B; 0.54±0.03-fold of WT), with smaller but significant decreases in the clustering of each marker alone (Figure 5C-D; GluA2 0.68±0.04-fold of WT, VGlut2 0.77±0.03-fold of WT). We then asked if the level of GluA2 at intracortical synapses is also altered, by analyzing colocalization of presynaptic vesicular glutamate transporter 1 (VGlut1) with GluA2. We found a significant ~20% decrease in the colocalization of VGlut1 with GluA2 in the Chrdl1 KO (Figure 5F; 0.80±0.09-fold of WT), with a small but significant decrease in GluA2 (Figure 5G; 0.90±0.05-fold of WT) and no change in VGlut1 (Figure 5H; 1.05±0.09-fold of WT).
Figure 5. Chrdl1 is necessary to increase GluA2 AMPARs at cortical synapses.
(A-H) Chrdl1 regulates synaptic GluA2 levels. (A,E) Example images of WT (top) and Chrdl1 KO (bottom) P14 visual cortex sections immunostained for GluA2 and VGlut2 (A) or VGlut1 (E). (B-D,F-H) Quantification of immunostaining: Chrdl1 KO shows significant decrease in GluA2 at thalamocortical VGlut2 synapses (B) and intracortical VGlut1 synapses (F); significant decrease in GluA2 puncta (C,G); significant decrease in presynaptic VGlut2 puncta (D) with no difference in VGlut1 (H). N=6 WT, 6 KO mice VGlut2 and VGlut1. (I-P) Chrdl1 regulates thalamocortical synapse formation. (I,M) Example images of WT (top) and Chrdl1 KO (bottom) P14 visual cortex sections immunostained for PSD95 and VGlut2 (I) or VGlut1 (M). (J-L,N-P) Quantification of immunostaining: Chrdl1 KO shows significant decrease in colocalization of PSD95 with VGlut2 at thalamocortical synapses (J); no change in colocalization of PSD95 with VGlut1 at intracortical synapses (N); no change in PSD95 puncta (K,O); significant decrease in presynaptic VGlut2 puncta (L) with no difference in VGlut1 (P). N=6 WT, 6 KO mice VGlut2; N=5 WT, 5 KO mice VGlut1. (Q-X) Chrdl1 regulates thalamocortical synaptic GluA1. (Q,U) Example images of WT (top) and Chrdl1 KO (bottom) P14 visual cortex sections immunostained for GluA1 and VGlut2 (Q) or VGlut1 (U). (R-T,V-X) Quantification of immunostaining: Chrdl1 KO shows significant decrease in colocalization of GluA1 with VGlut2 at thalamocortical synapses (R); no change in colocalization of GluA1 with VGlut1 at intracortical synapses (V); no large change in GluA1 puncta (S,W); significant decrease in presynaptic VGlut2 puncta (T) with no difference in VGlut1 (X). N=5 WT, 5 KO mice VGlut2 and VGlut1. Scale bar = 5μm. Bar graphs mean±s.e.m., with individual data points representing mice. Statistics by T-test, significance as stated on graph. See also Figure S4,S5,S6; Tables S2,S3,S4.
To determine if the decreased colocalization of GluA2 with presynaptic markers specifically reflects a decrease in GluA2 at synapses, or if there is also a decrease in synapse number, we used immunohistochemistry to label the postsynaptic density marker PSD95 along with presynaptic markers (Figure 5I,M). At thalamocortical synapses there was a significant ~25% decrease in the amount of PSD95 colocalized with VGlut2 in the Chrdl1 KO (Figure 5J; 0.73±0.06-fold of WT), with no change in PSD95 and a significant decrease in VGlut2 (Figure 5K-L; PSD95 0.93±0.06-fold of WT, VGlut2 0.80±0.05-fold of WT). At intracortical synapses we found no difference in colocalization of VGlut1 with PSD95 in the Chrdl1 KO (Figure 5N; 0.96±0.05-fold of WT), or in PSD95 (Figure 5O; 1.03±0.08-fold of WT) or VGlut1 (Figure 5P; 0.98±0.05-fold of WT). We further asked if absence of Chrdl1 had any effect on synaptic levels of GluA1 (Figure 5Q,U), and found that the effect matched that seen with PSD95, namely a 25% decrease in GluA1 colocalized with VGlut2, with no large alteration in GluA1 levels (Figure 5RT; colocalized 0.73±0.07-fold of WT, GluA1 0.93±0.02-fold of WT, VGlut2 0.72±0.03-fold of WT), and no change in colocalization of GluA1 with VGlut1 (Figure 5V-X; colocalized 0.99±0.04-fold of WT, GluA1 0.98±0.03-fold of WT, VGlut1 1.09±0.06-fold of WT). This shows that the strongest effect of Chrdl1 loss is a decrease in GluA2 at excitatory synapses in the developing cortex, and that Chrdl1 has a more pronounced role in regulating thalamocortical connections. Interestingly, the expression pattern of Chrdl1 matches the thalamocortical innervation zone, being highest in astrocytes in upper cortical layers where these synapses form (Figure 4).
Chrdl1 KO mice have altered kinetics of excitatory synaptic events in cortical neurons
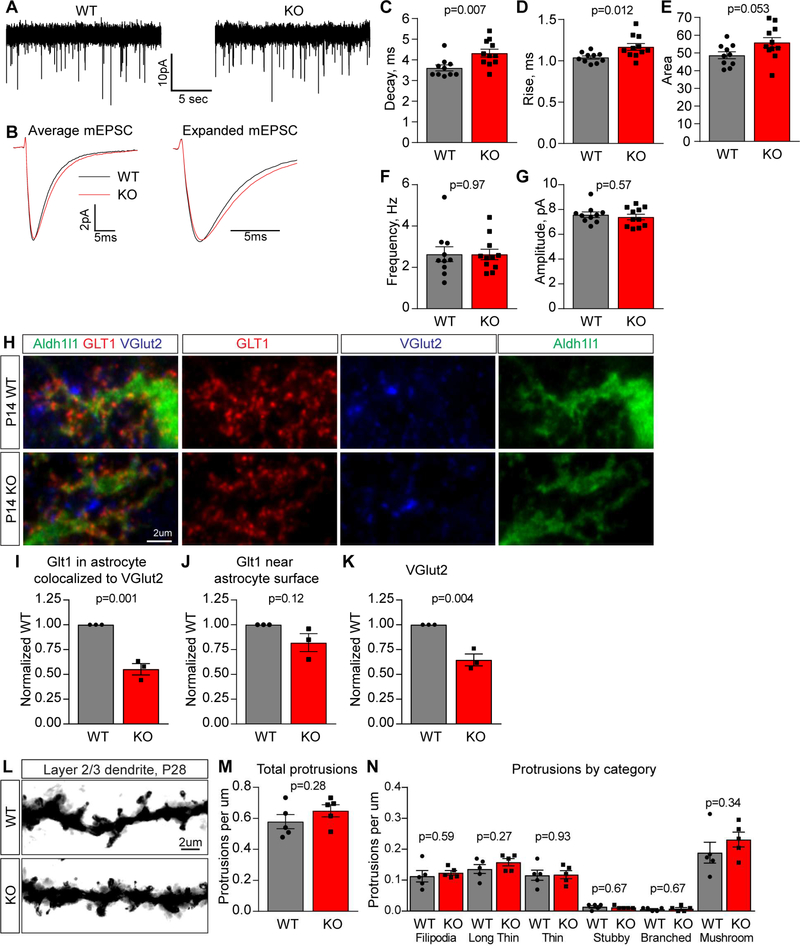
To ask if the decrease in GluA2 at synapses has any impact on synaptic function we performed whole cell patch-clamping from layer 2/3 pyramidal neurons in the visual cortex in acute brain slices from P14 Chrdl1 KO and WT littermate controls. We isolated AMPA-mediated mEPSCs by applying AP5 (NMDA receptor antagonist), bicuculline (GABAA receptor antagonist) and tetrodotoxin (sodium channel blocker) (Figure 6A). We found a significant alteration in the kinetics of mEPSCs, with an increase in the decay time (WT 3.61±0.14ms, KO 4.33±0.19ms), rise time (WT 1.04±0.02ms, KO 1.17±0.04ms) and charge transfer (WT 48.64±1.96, KO 55.87±2.83) at excitatory synapses onto layer 2/3 cortical neurons in the Chrdl1 KO (Figure 6BE, S6A-D), with no change in membrane properties (Figure S6E). We did not detect a significant alteration in the frequency (WT 2.64±0.36Hz, KO 2.63±0.25Hz) or amplitude (WT 7.58±0.22pA, KO 7.39±0.23pA) of mEPSCs (Figure 6F-G), suggesting synapses have formed in the absence of Chrdl1 matching the synaptic staining data (Fig 5I-P).
Figure 6. Chrdl1 regulates mEPSC kinetics at L2/3 synapses in the developing visual cortex.
(A-G) Chrdl1 KO mice display altered mEPSC kinetics in the developing visual cortex at P14. (A) Example mEPSC recordings from layer 2/3 pyramidal neurons in acute visual cortex slices. (B) Average mEPSC from all recordings aligned to rise time (left) and expanded timescale (right). (C-G) Quantification of mEPSCs: Chrdl1 KO mEPSC kinetics are altered, with increased decay time (C), rise time (D) and area (E); mEPSC frequency (F) and amplitude (G) are not altered. N=10 cells WT, 11 cells KO. (H-K) Astrocyte glutamate transporters are not significantly altered at synapses in Chrdl1 KO mice. (H) Example images of WT (top) and Chrdl1 KO (bottom) P14 visual cortex sections immunostained for GLT1 and VGlut2 along with Aldh1l1-GFP to mark astrocytes. (I-K) Quantification of immunostaining: Chrdl1 KO shows significant decrease in presynaptic VGlut2 puncta (K), no change in GLT1 on astrocytes (J) and a significant decrease in GLT1 colocalized with VGlut2 (I). N=3 WT, 3 KO mice. (L-N) No alteration in spine density in layer 2/3 neurons at P28 in Chrdl1 KO. (L) Example images of dendrites and spines visualized using Golgi staining. (M) Quantification of all dendritic protrusions, normalized per μm dendrite. (N) Quantification of spine types, normalized per μm dendrite. N=5 WT, 5 KO mice. Bar graphs mean±s.e.m., with individual data points representing cells (C-G) and mice (I-K, M-N). Statistics by T-test, significance as stated on graph. See also Figure S4,S5,S6; Tables S2,S3,S4.
The increase in mEPSC decay time in the absence of Chrdl1 (Figure 6C) correlates with our findings in vitro, where adding Chrdl1 protein to neurons had the opposing effect and decreased mEPSC decay time (Figure 2G,L). In addition to AMPAR composition and location, which we have demonstrated to be altered in Chrdl1 KO mice (Figure 5), a number of other factors can also alter EPSC kinetics (Jonas, 2000). These include post-translational receptor modifications, interacting proteins, spine morphology and the removal of glutamate from the synaptic cleft (regulated by glutamate uptake transporters on nearby astrocytes). We therefore asked if there was any alteration in spine morphology (see next section below), or in the expression and localization of glutamate transporters in astrocytes in Chrdl1 KO mice, that could contribute to the observed effect on kinetics.
We found no difference in mRNA level for the glutamate transporters GLT1 (Slc1a2) and GLAST (Slc1a3) between Chrdl1 WT and KO visual cortex at P14 (Table S4). We then asked if the localization of GLT1 near synapses was altered, by performing super-resolution imaging of GLT1 and VGlut2, in Aldh1l1-EGFP mice to label astrocytes, at P14 (Figure 6H, S6G). We found no significant difference in the amount of GLT1 colocalized with the astrocyte surface between Chrdl1 KO and WT (Figure 6J; 0.82±0.09-fold of WT). We did detect a significant decrease in VGlut2 puncta (Figure 6K; 0.65±0.06-fold of WT) repeating the finding we had in the analysis of synapse number (Figure 5). There was a significant decrease in the colocalization of GLT1 present on the astrocyte surface with nearby VGlut2 (Figure 6I; 0.55±0.06-fold of WT), mainly reflective of the decrease in total VGlut2 puncta present in the KO. This suggests glutamate transporters are present at synapses at similar levels between the Chrdl1 KO and WT. We further found no difference in spine morphology between Chrdl1 KO and WT at P28 (see below and Figure 6L-N). Overall these data demonstrate that in the Chrdl1 KO cortex synapses are present with altered properties, the exact cause of which remains to be determined, that contain less GluA2 AMPARs.
Chrdl1 KO mice display increased plasticity in the visual cortex
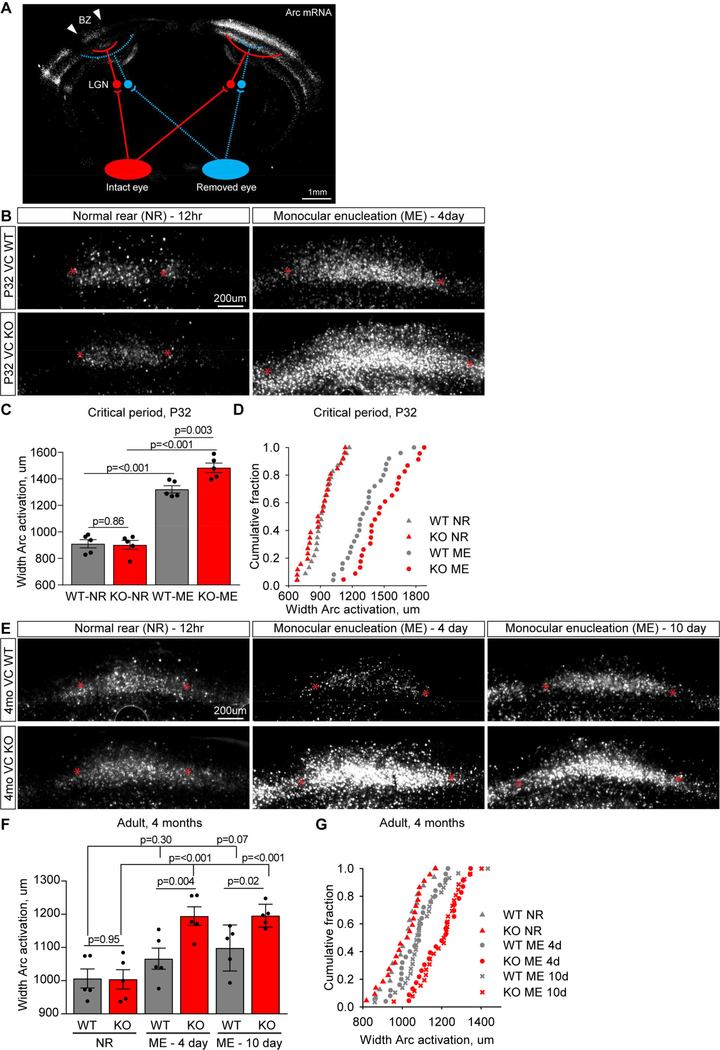
In the absence of Chrdl1 synapses in the visual cortex contain less GluA2, suggestive of immature synapses (Figure 5,6). Based on this we predicted that Chrdl1 KO mice would show enhanced plasticity and remodeling in the visual cortex in response to altered sensory input. To test this we used monocular enucleation (ME), either during the critical period when plasticity is normally present (P28-P32), or in the adult when there is little plasticity (4 months) (Espinosa and Stryker, 2012). In this paradigm one eye is removed, and during the critical period this leads to characteristic remodeling of synaptic inputs to the binocular zone (BZ) of the visual cortex – a loss of drive from the removed eye and an increase in drive from the intact eye. This can be visualized by stimulating the intact eye with light to activate neurons in the BZ, and detecting activated neurons by expression of the immediate early gene Arc (Figure 7A) (Syken et al., 2006). The width of the activated zone of neurons is a readout of the level of plasticity, with a wider activation zone meaning more remodeling and plasticity has occurred.
Figure 7. Chrdl1 KO mice show enhanced visual cortex plasticity in response to monocular enucleation.
(A) Monocular enucleation (ME) assay, schematic adapted from (Djurisic et al., 2013). Removing one eye, and stimulating the intact eye with bright light for 30 minutes, activates only neurons projecting from the intact eye (red) to the binocular zone (BZ), assayed by induction of the immediate early gene Arc. When visual deprivation continues for 4 days during the mouse critical period (P28 to P32), synaptic inputs from the 2 eyes in the BZ of visual cortex undergo remodeling. Width of BZ activation (white arrows) on the side ipsilateral to the intact eye is a measure of plasticity. Representative image is a mosaic of 143 stitched tiles. (B-D) Chrdl1 KO demonstrate enhanced remodeling during the critical period. (B) Example images from WT and Chrdl1 KO BZ at P32, from normal rear (NR – 12 hour visual deprivation) and ME (4 day visual deprivation), showing active neurons marked by mRNA for Arc (white). * mark boundaries of activated neurons. Representative image is cropped from a mosaic of 143 stitched tiles. (C) Analysis of the width of zone of activated neurons in layer 4 of the BZ shows enhanced plasticity occurs in response to ME in the Chrdl1 KO during the critical period. (D) Cumulative distribution plots of width of BZ activation during the critical period, each point represents an individual brain section. Statistical comparison of distribution by Kolmogorov-Smirnov test: WT vs KO NR, p=0.36; WT vs KO ME, p=0.02; WT NR vs ME, p=<0.0001; KO NR vs ME, p=<0.0001. N=5 WT, 5 KO mice for both NR and ME. (E-G) Chrdl1 KO demonstrate enhanced remodeling in the adult (4 months). (E) Example images from WT and Chrdl1 KO BZ at 4 months, from normal rear (NR – 12 hour visual deprivation) and ME (4 day or 10 day visual deprivation), showing active neurons marked by mRNA for Arc (white). * mark boundaries of activated neurons. Representative image is cropped from a mosaic of 143 stitched tiles. (F) Analysis of the width of zone of activated neurons in layer 4 of the BZ shows enhanced plasticity occurs in response to 4 days and 10 days of ME in the Chrdl1 KO in the adult. (G) Cumulative distribution plots of width of BZ activation in the adult, each point represents an individual brain section. Statistical comparison of distribution by Kolmogorov-Smirnov test: WT vs KO NR, p=0.98; WT vs KO 4 day ME, p=0.008; WT vs KO 10 day ME, p=0.001; WT NR vs 4 day ME, p=0.1; WT NR vs 10 day ME, p=0.04; KO NR vs 4 day ME, p=0.0001; KO NR vs 10 day ME, p=<0.0001. N=5 WT, 5 KO mice for NR, ME 4 day and ME 10 day. Scale bar = 200μm. Bar graph mean±s.e.m., with individual data points representing mice. Statistics by two-way ANOVA, significance as stated on graph.
We performed ME at P28 and measured neuronal activation to the intact eye in the BZ 4 days later (at P32), a time period sufficient to allow remodeling. In both the Chrdl1 KO and WT littermate control there was a significant increase in the width of the BZ activated by the intact eye following ME, showing remodeling has occurred as expected during the critical period (Figure 7B-D). Importantly in the Chrdl1 KO the increase in the size of the activation zone was significantly larger than in the WT, showing that more remodeling had happened in the absence of Chrdl1. Chrdl1 KO does not affect the developmental targeting of axons to the BZ of the visual cortex, as the width of activation in the normal rear (NR) condition (visual deprivation for 12 hours) is no different from WT (Figure 7B-D; WT NR 911±31μm, KO NR 902±34μm, WT ME 1321±27μm, KO ME 1484±36μm).
In mice where neuronally-expressed plasticity-limiting molecules are absent, for example PirB KO, there is an increase in the density of dendritic spines present at P28 in the absence of plasticity induction, that is thought to act as a substrate for enhanced plasticity potential (Djurisic et al., 2013). We therefore asked if a similar effect is present in Chrdl1 KO mice, by using Golgi staining to visualize dendrites and spines in layer 2/3 neurons at P28 (Figure 6L). Quantification of the total protrusions per μm of dendrite found no difference between Chrdl1 KO and WT (Figure 6M; WT 0.58±0.05 per μm, KO 0.65±0.04 per μm). We further asked if there was any alteration in the proportion of spine classes present, by classifying spines based on morphology and again found no significant differences between Chrdl1 KO and WT (Figure 6N, (Risher et al., 2014b)). This similarity in spine density between Chrdl1 KO and WT fits with the similarity in synapse number present between genotypes (Figure 5).
We next asked if enhanced plasticity in the absence of Chrdl1 is restricted to development and the critical period, or is also present in the adult brain at P120 when Chrdl1 remains expressed (Figure 4D, (Boisvert et al., 2018)). We performed ME at 4 months of age and analyzed remodeling 4 days and 10 days later, and included the 10 day timepoint as this is reported to be sufficient time to allow minimal remodeling in the WT in the adult (Sato and Stryker, 2008). As in the critical period, we found that more remodeling occurred in the Chrdl1 KO cortex compared to WT in response to ME in the adult. After 4 days no remodeling had occurred in the WT, whereas there was a significant increase in the width of the activation zone in the Chrdl1 KO, showing the absence of Chrdl1 allowed rapid remodeling to occur (Figure 7E-G). After 10 days of deprivation there was a trend towards an increase in remodeling in the WT adult, which was 2-fold larger and significant in the Chrdl1 KO (Figure 7E-G; WT NR 1007±29μm, KO NR 1004±29μm, WT 4 day ME 1066±32μm, KO 4 day ME 1194±28μm, WT 10 day ME 1099±31μm, KO 10 day ME 1196±15μm). The magnitude of the enhanced plasticity present both during the critical period and in the adult is a similar size to that detected in mice where neuronal plasticity limiting molecules are removed, such as PirB KO (Djurisic et al., 2013; Syken et al., 2006). This shows that in the absence of Chrdl1 more remodeling occurs in response to altered sensory input, demonstrating that synapses in the visual cortex have a higher level of plasticity that is maintained into adulthood.
DISCUSSION
In this study we identified a secreted protein, Chrdl1, which is specifically made by astrocytes in the cortex, and is sufficient to increase GluA2 AMPARs at synapses, induce synapse maturation, and inhibit plasticity (Figure S7). We further identified that Chrdl1 carries out these functions through an apparently BMP-independent mechanism.
Astrocytes orchestrate sequential development of synapses
We demonstrate that astrocytes can increase synaptic GluA2 AMPARs and induce synapse maturation via the release of Chrdl1. The increase in calcium-impermeable GluA2 AMPARs is an important step in synapse maturation, and occurs at defined times in the developing cortex (Brill and Huguenard, 2008). We previously showed that astrocyte-secreted glypican 4 and 6 induce nascent immature synapses to form that contain calcium-permeable GluA1 AMPARs (Allen et al., 2012; Farhy-Tselnicker et al., 2017). Both Chrdl1 and glypican 4 are sufficient to induce active synapses to form between neurons in vitro, however neither factor by itself fully recapitulates the effect of astrocytes on synaptic function. In future it will be important to determine what combinations of these factors, including other synaptogenic molecules such as thrombospondin, are required to fully mimic the astrocyte effect. Expression of glypicans 4 and 6 in astrocytes peaks in the first 2 postnatal weeks, matching the time when immature synapses are forming, whereas expression of Chrdl1 peaks after this time when synapse maturation is occurring (Cahoy et al., 2008). This suggests that astrocytes can regulate the subtype of AMPAR present at the synapse via the release of different signals. The mechanisms that control the temporal expression of these distinct synaptogenic signals in astrocytes are not known and will be important to identify. For example, are neurons signaling to astrocytes to regulate this switch in synaptogenic potential?
Chrdl1 has a heterogeneous expression pattern in astrocytes
Chrdl1 has a heterogeneous expression pattern, being enriched in cortical and striatal astrocytes, and within the cortex enriched in astrocytes in upper layers. Our in vivo data showed a significant decrease in GluA2 at excitatory synapses in upper cortical layers in Chrdl1 KO mice, with a stronger reduction at thalamocortical synapses compared to intracortical synapses. Thalamocortical synapses predominantly form in upper layers of the cortex where Chrdl1 expression is highest (Cruz-Martin et al., 2014; Seabrook et al., 2017), and undergo plasticity following visual deprivation (Espinosa and Stryker, 2012), which is enhanced in Chrdl1 KO mice. Other astrocyte-secreted synaptogenic molecules such as hevin and thrombospondin have differential effects on intracortical compared to thalamocortical synapses, however these molecules have a homogeneous expression pattern throughout the cortical layers in contrast to the restricted expression of Chrdl1 (Eroglu et al., 2009; Risher et al., 2014a). The factors that determine the heterogeneous expression of Chrdl1, and correlation with thalamocortical innervation zones, will be important to determine. Further, the restricted spatial expression pattern of astrocyte Chrdl1 in the cortex and striatum suggests that in other brain regions additional as yet unidentified astrocyte-secreted factors may be regulating synapse maturation. Alternatively, Chrdl1 may be enriched in brain regions where once developmental remodeling has occurred, the circuit needs to remain stable for correct function. For example, the end of the critical period in the visual cortex marks the time when binocular matching is complete, and it would be detrimental if changes in visual input altered this connectivity. Of note, Chrdl1 is absent from the hippocampus, a region of the brain where synapses continue to remodel throughout life, so we hypothesize that its absence allows this remodeling to occur.
Chrdl1 regulates synapse maturation independently of BMP signaling
Identifying the mechanism of how Chrdl1 regulates synaptic levels of GluA2 and synapse maturation will give important insight into the mechanism of plasticity regulation. Chrdl1 was identified as a secreted antagonist of BMP signaling, however we found that Chrdl1-induced recruitment of GluA2 AMPARs to synapses appears to occur in a BMP-independent manner, and that other secreted BMP antagonists such as noggin and chordin do not mimic its effect. Although BMP signaling is not required, the CR repeats/vWFC domains, which bind BMPs, are required for Chrdl1-induced GluA2 recruitment. vWFC domains are present in a number of extracellular proteins, including chordins, integrins, collagens and thrombospondins, and allow complex formation and interaction with multiple proteins (Hunt and Barker, 1987). An example are integrins which bind a number of vWFC containing proteins including collagens and thrombospondins (Humphries et al., 2006), and integrins themselves can regulate GluA2 synaptic recruitment (Park and Goda, 2016). The secreted BMP inhibitor Brorin, which also contains CR repeats, has been identified in proteomic studies as an interactor of GluA2 AMPAR complexes, and not GluA1 AMPAR complexes (Koike et al., 2007; Schwenk et al., 2012). As Chrdl1 specifically regulates GluA2 AMPARs, an intriguing possibility is that it is doing so by physically interacting with GluA2 complexes.
Chrdl1 represses plasticity
Previous work has demonstrated a role for glial-secreted molecules such as Hevin and Tnfα in enabling plasticity to occur in the visual cortex in response to visual deprivation (Kaneko et al., 2008; Singh et al., 2016). We now show that astrocytes can induce the opposite effect, via release of Chrdl1 to repress plasticity. This suggests that astrocytes, through the release of distinct signals, have the ability to regulate the neuronal environment to either allow or repress synaptic plasticity. In the future it will be important to determine how removal of Chrdl1 allows enhanced plasticity to occur. For example, neuronal molecules that repress plasticity e.g. PirB, when knocked out show altered long term potentiation (LTP), long term depression (LTD), and increased spine density in the visual cortex (Djurisic et al., 2013). We found no effect on spine density in Chrdl1 KO mice, however, arguing against a deficit in synapse elimination being responsible for the enhanced plasticity. Identifying the molecular mechanism of how Chrdl1 increase synaptic GluA2 will give important insight into the mechanism of altered plasticity.
In humans null mutations in Chrdl1 cause X-linked megalocornea 1 (MGC1), associated with enlarged corneas and increased risk of cataracts with age (Webb et al., 2012). Interestingly, cognitive testing of MGC1 patients revealed a superior verbal IQ, verbal memory and executive skills (Webb et al., 2012). As Chrdl1 KO mice show enhanced plasticity in the visual cortex, it would be interesting to determine if a similar mechanism is contributing to the enhanced cognitive performance seen in MGC1. Further, transcriptomic profiling of cortical samples from patients with schizophrenia and bipolar disorder identified significantly upregulated Chrdl1 mRNA in both disorders, suggesting the absolute level of Chrdl1 is important for regulating synaptic function (Gandal et al., 2018). The actions of Chrdl1 in inhibiting plasticity are not restricted to development, but continue throughout life into the adult. This is particularly important when considering situations where it would be beneficial to reopen plasticity in the adult brain, for example to encourage synaptic repair after injury such as a stroke. Manipulating Chrdl1 could provide a new avenue of investigation for post-injury treatments, especially at older ages when plasticity declines (Burke and Barnes, 2006; Henley and Wilkinson, 2013). Taken together this study demonstrates that astrocytes, via the release of Chrdl1, are essential for synapse maturation, and play important roles in regulating plasticity in the brain.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the led contact, Nicola J. Allen (nallen@salk.edu).
EXPERIMENTAL MODELS
Animals.
All animal work was approved by the Institutional Animal Care and Use Committee (IACUC) of the Salk Institute for Biological Studies.
Rats.
Sprague Dawley rats (Charles River stock number 001) were housed in the Salk Institute animal facility at a light cycle of 12 hours light: 12 hours dark, with access to water and food ad libitum. Rat pups of both genders were used for preparing astrocyte and neuron cell culture experiments, as described below.
Mice.
Mice were housed in the Salk Institute animal facility at a light cycle of 12 hours light: 12 hours dark, with access to water and food ad libitum.
Wild type (WT) mice.
WT C57BL/6J (Jax stock number 000664) mice were used for analysis of developmental expression of Chrdl1 (qRT-PCR and in situ hybridization, see below), and to breed to Chrdl1 KO mice. Mice of both genders were used.
Chrdl1 germline knock out (KO) mice.
Chrdl1 KO mice were generated using CRIPSR-cas9 technology to introduce a 2 nucleotide deletion in exon 3 of the gene, introducing a premature stop codon (see below for detailed procedure). Mice carrying the mutation were backcrossed to C57BL/6J (Jax stock number 000664) for at least three generations before experiments. Chrdl1 is on the X chromosome, so for the generation of experimental mice heterozygous (+/−) Chrdl1 females were bred to WT (+/y) C57BL/6J males. All experiments were performed using male Chrdl1 KO (-/y) and WT (+/y) littermates.
Astrocyte-specific Ribotag mice.
To obtain HA-tagging of ribosomes in astrocytes to enable purification of astrocyte-enriched mRNA, B6N.129-Rpl22tm1.1Psam/J (Jax stock number 011029) were crossed to B6.Cg-Tg(Gfap-cre)73.12Mvs/J (Jax stock number 012886). Male mice heterozygous for flox-Rpl22-HA and hemizygous for cre (astrocyte-ribotag) were used to purify mRNA.
Aldh1l1-EGFP × Chrdl1 KO mice.
Tg(Aldh1l1-EGFP)OFC789Gsat/Mmucd mice express Aldh1l1-eGFP in astrocytes (011015-UCD). Male Aldh1l1-eGFP mice were bred to Chrdl1 heterozygous (+/−) females. Chrdl1 KO and WT male mice expressing EGFP in astrocytes were used for experiments.
Generation of Chrdl1 knockout mice using Cas9/gRNAs
Procedures for Generating sgRNAs Expressing Vector.
Addgene plasmid 42230 (pX330U6-Chimeric_BB-CBh-hSpCas9), bicistronic expression vector expressing Cas9 and sgRNA was obtained from Addgene and digested with BbsI. Two targeted gRNAs were selected from Chrdl1 exon 3 using the web-tools from Feng Zhang lab (http://www.genome-engineering.org/crispr/). Targeting constructs were created by combining BbsI digested vector (Addgene plasmid 42230) with a pair of annealed oligos (for each targeting site). The sequences of the 2 sgRNA located in Chrdl1 exon3 are CTACCTGGAACCGTATGGAC and AAACCAGTCCATACGGTTCC.
One-Cell Embryo Injection.
B6D2F1 (C57BL/6 × DBA2) female mice and CD1 mouse strains were used as embryo donors and foster mothers, respectively. Superovulated female B6D2F1 mice (3–4 weeks old) were mated to B6D2F1 stud males, and fertilized embryos were collected from oviducts. Cas9 mRNAs (from 30ng/ul) and sgRNAs (15ng/ul) were mixed and injected into zygotes at the pronuclei stage. The injected zygotes were transplanted into pseudopregnant CD1 females (foster mother).
T7 endonuclease Assay for Genome Modification.
Genomic DNA from each transgenic mouse and control wildtype mouse were isolated and purified from tails. Two steps of PCR (nested PCR strategy, two sets of primers around the mutation site) were performed to get a single PCR product (a single band checked on the gel). 4 primer sequences are: Chrdl1-p-5a: GTTCTCTGGAAGACAGGAAGTAC; Chrdl1-p-5b: CAACCTGGGTTTCAGTATCATC; Chrdl1-p3a: GCTGTGGTTTCCATTCAAGG and Chrdl1-p-3b: CTATCCTCTATGCCCTGTGC. Primers Chrdl1-p-5a and Chrdl1-p-3a will generate 1166 bp PCR product, and primers Chrdl1-p-5b and Chrdl1-p-3b will produce 644 bp PCR product. 644 bp PCR products were cleaned up by DNA Clean and Concentrator-25 kit (Zymo Research 11–305) then 1 ug of 644 bp PCR products from each pup were denatured, annealed, and treated with T7 endonuclease (NEB M0302). Digested PCR products were separated on an ethidium bromide-stained agarose gel (2%). For DNA sequencing, PCR products were cloned using the CloneJet PCR Cloning Kit (Thermo Scientific K1231), and mutations were identified by sequencing to identify the actual mutations (deletion or point mutations).
Founder mice and validation of Chrdl1 mutation.
A male mouse carrying a 2 nucleotide deletion in exon 3 of Chrdl1 was identified by DNA sequencing (see below). As Chrdl1 is on the X chromosome, this male was crossed to female C57BL/6J to generate Chrdl1 +/− heterozygous females that contain a single mutation. Chrdl1 +/− females were crossed to WT C57BL/6 males for a minimum of 3 generations before experiments. Chrdl1 mutations in the genomic DNA were identified using custom probes designed by Transnetyx.
Mouse Chrdl1 cDNA sequence
Transcript ID: ENSMUST00000112878.8
Cas9/gRNA target Exon 3: ENSMUSE00000207884
Underlined: Exon 3, target exon
Bold: Guide RNA target region
Italics: Altered bases in Chrdl1 KO, deletion of 2 bases and frame-shift: 158GA159 → --
Mouse Chrdl1 WT Protein Sequence (477 aa)
*: Stop codon
MDGMKYIISLFFIFVFLEGSKTEQVKHSDTYCVFQDKKYRVGEKWHPYLEPYGLVYCVNCICSENGNVLCSRVRCPSLHCLSPVHIPHLCCPRCPDSLPPVNNKVTSKSCEYNGTTYQHGELFIAEGLFQNRQPNQCSQCSCSEGNVYCGLKTCPKLTCAFPVSVPDSCCRVCRGDAELSWEHADGDIFRQPANREARHSYLRSPYDPPPNRQAGGLPRFPGSRSHRGAVIDSQQASGTIVQIVINNKHKHGQVCVSNGKTYSHGESWHPNLRAFGIVECVLCTCNVTKQECKKIHCPNRYPCKYPQKIDGKCCKVCPEEPPSQNFDSKGSFCGEETMPVYESVFMEDGETTRKVALETERPPQVEVHVWTIQKGILQHFHIEKISKRMFGELHHFKLVTRTTLNQWKLFTEGEAQLSQMCSSQVCRTELEDLVQVLYLGRPEKDHC*
Mouse Chrdl1 mutant predicted protein translation
*: Premature stop codons
Bold and underlined: Altered protein sequence
MDGMKYIISLFFIFVFLEGSKTEQVKHSDTYCVFQDKKYRVGEKWHPYLEPYAGLLCELHLL*EWECALQPSQMSKSSLPFTRAYSSSLLPPLPRLLTTSEQ*GDQQVMRIQWNHLPTWRTVHS*RALSEPATQSVQSV*LLGGECILWSQDLPQTDLCIPSLCSRFLLPSMQRGCRIIVGTCGW*YLPATCQQRSKTFLPPFPLRSSTKQTSWRSSPLSWEQKSPGSCYRFPASIRDHRADCHQ*QAQTWTSVCFQWKDLLSWRVLAPKSTSIWHCGMCTMHL*CHQARM*ENPLPQSIPLQVSSKNRWKVLQGVPRRTSKPKL*QQRFLLWRRNHACI*VCVHGGWRDNQKSSTGDRETTSSRGPRLDYSKGHSPALPH*EDFQEDVWGAPSFQASYSDHLEPVEALH*RRSSAQPDVLKSGVQNRAGRFSPGFVPGET*KGPLL
Cell cultures
HEK293 cell cultures.
HEK293 cells (ATCC CRL-1573) were maintained in a humidified incubator at 37°C and 5% CO 2. Growth media contained DMEM (Thermo Fisher Scientific 11960044), 10% heat inactivated fetal bovine serum (Thermo Fisher Scientific 10437028), 1% Penicillin-Streptomycin (Thermo Fisher Scientific 15140–122), 1% glutamax (Thermo Fisher Scientific 35050–061), and 1% sodium pyruvate (Thermo Fisher Scientific 11360–070).
Retinal ganglion cell neuron cultures.
Retinal ganglion cell neurons (RGC) were isolated from P5–7 Sprague Dawley rat retinas (Charles River) as previously described (Ullian et al., 2001). RGCs were plated on glass coverslips (12mm diameter, Carolina Biological Supply 633029) coated with poly-D-lysine (Sigma P6407) and laminin (R&D 340001001) at a density of 30,000 cells/well. RGC growth media contained: 50% DMEM (Thermo Fisher Scientific 11960044), 50% Neurobasal (Thermo Fisher Scientific 21103049), Penicillin-Streptomycin (LifeTech 15140–122), glutamax (Thermo Fisher Scientific 35050–061), sodium pyruvate (Thermo Fisher Scientific 11360–070), N-acetyl-L-cysteine (Sigma A8199), insulin (Sigma I1882), triiodo-thyronine (Sigma T6397), SATO (containing: transferrin (Sigma T-1147), BSA (Sigma A-4161), progesterone (Sigma P6149), putrescine (Sigma P5780), sodium selenite (Sigma S9133)), B27 (see (Winzeler and Wang, 2013) for recipe), BDNF (Peprotech 450–02), CNTF (Peprotech 450–13), and forskolin (Sigma F6886). RGC cultures were maintained in a humidified incubator at 37°C and 10% CO 2.
Cortical astrocyte cultures.
Astrocytes were obtained from the cerebral cortex of P1–2 Sprague Dawley rats (Charles River) as previously described (Allen et al., 2012; McCarthy and de Vellis, 1980). Astrocytes were plated on 75cm2 tissue culture flasks coated with poly-D-lysine (Sigma P6407) and maintained in culture for 4 days in growth medium (DMEM (Thermo Fisher Scientific 11960044) supplemented with 10% heat inactivated FBS (Thermo Fisher Scientific 10437028), Penicillin-Streptomycin (Thermo Fisher Scientific 15140–122), glutamax (Thermo Fisher Scientific 35050–061), sodium pyruvate (Thermo Fisher Scientific 11360–070), hydrocortisone (Sigma H0888), and N-acetyl-L-cysteine (Sigma A8199)). Flasks were then shaken vigorously to remove cells on overgrown layers. Two days later AraC (working concentration 10μM; Sigma C1768) was added for 48 hours in order to inhibit rapidly proliferating cells. Astrocytes were then plated on 15cm tissue culture dishes and maintained in growth media. They were passaged once a week and cultures were maintained for no longer than 4 weeks. Depending on the experimental purpose, astrocytes were plated on inserts (Thermo Fisher Scientific 353104) 4 days before they were applied to RGC cultures at a density of 50,000 cells per insert or plated on 10cm cell culture dishes at a density of 500,000 cells per dish to collect secreted proteins as conditioned media. Astrocyte cultures were maintained in a humidified incubator at 37°C and 10% CO 2.
METHOD DETAILS
Collection of astrocyte or neuron secreted proteins
Astrocytes grown in 10cm tissue culture dishes were washed 3 times with warm DPBS (HyClone SH30264) to remove the serum-containing growth media, and the growth media was replaced by minimal low protein conditioning media (50% DMEM, 50% Neurobasal, penicillinstreptomycin, glutamax and sodium pyruvate). Astrocytes were conditioned in low protein media for 4 days in a tissue culture incubator at 37°C 10 % CO2. The astrocyte conditioned media (ACM) was collected and concentrated 100-fold using Vivaspin 20 centrifugal concentrators with a MW cut-off of 10kDa (Sartorius 14558502). Protein concentration of the ACM was assessed by Bradford protein assay (Bio-Rad 500–0006).
For Western blots in Fig S1B, astrocytes or RGC neurons were grown in 6-well plates in appropriate growth media as detailed above. Cultures were washed 3 times with warm DPBS and the growth media was replaced by minimal low protein conditioning media (50% DMEM, 50% Neurobasal, penicillin-streptomycin, glutamax, sodium pyruvate, N-acetyl cysteine, forskolin, BDNF, CNTF) for 4 days, and collected as outlined above. Western blotting of conditioned media was used to compare secretion of Chrdl1 from neurons to secretion of Chrdl1 from astrocytes.
Column fractionation and mass spectrometry analysis
Astrocyte conditioned media (ACM) was collected from rat astrocyte cultures and concentrated (see above). Concentrated ACM underwent column fractionation as previously described, using an anion exchange column (HiTrap Q HP, GE Healthcare 17–1153-01) (Allen et al., 2012). GluA2 live staining (see below) was performed on RGC cultures after treatment with the different fractions for 6 days, to assess which fraction contained the factor that recruited GluA2 AMPAR subunits to the neuronal surface. The positive fraction (unbound proteins from an anion exchange column) was analyzed by the Stanford University Mass Spectrometry core facility. Detected peptides were searched against the canonical _rattus norvegicus database and assigned to proteins using Byonic Viewer from Protein Metrics.
RGC neuron treatments
RGCs were cultured for 7–10 DIV before treatment to allow process outgrowth. Unless stated in the text, all treatments were applied for 6 days before RGCs were analyzed. In all experiments there was a negative control condition, untreated RGCs (alone condition; treated with buffer), and in some experiments a positive control condition, RGCs cultured with an astrocyte insert feeder layer (astrocyte condition). ACM was added to cultures at 50 μg/ml.
Candidate proteins identified by column fractionation were initially tested at multiple concentrations: 0.004, 0.02, 0.1, 0.5 and 1 μg/ml. The candidates were Chrdl1 (R&D 1808NR/CF at 200 μg/ml in 0.1% BSA 4 mM HCl), chitinase 3 like 1 (R&D 2649-CH-050 at 200 μg/ml in PBS) and thrombospondin 4 (R&D 7860-TH-050 at 200 μg/ml in PBS). In all subsequent experiments Chrdl1 was used at 1 μg/ml.
For the nuclear phospho-SMAD recruitment experiments, RGCs were treated with 0.25 μg/ml BMP4 (R&D 314-BP/CF at 100 μg/ml in 0.1% BSA 4 mM HCl) or 1 μg/ml Chrdl1 for 1 or 6 days. pSMAD staining was performed as outlined below.
For the BMP-block experiments RGCs were treated with 1μg/ml Chrdl1, 0.25μg/ml BMP4, 0.25μg/ml BMP7 (R&D 354-BP/CF at 100μg/ml in 4mM HCl), or 1μg/ml Chrdl1 + 0.25μg/ml either BMP. GluA2 surface expression was analyzed by live staining as described below. For testing other secreted antagonists of BMP signaling RGCs were treated with 1 or 2.5 μg/ml Chrdl1, 2.5 μg/ml Chordin (R&D 758-CN/CF at 200 μg/ml in 0.1% BSA), or 2.5 μg/ml Noggin (R&D 6057-NG/CF at 200 μg/ml in 0.1% BSA) for 6 days. GluA2 surface expression was analyzed by live staining as described below.
HEK cell treatment
For immunostaining experiments HEK293 cells were plated on 12mm diameter glass coverslips (Carolina Biological Supply 633029) coated with poly-D-lysine at 30,000 cells/well. 24 hours before treatment cells were switched to a serum-free media (DMEM (Thermo Fisher Scientific 11960044), Penicillin-Streptomycin (LifeTech 15140–122), glutamax (Thermo Fisher Scientific 35050–061), sodium pyruvate (Thermo Fisher Scientific 11360–070), N-acetyl-L-cysteine (Sigma A8199), insulin (Sigma I1882), triiodo-thyronine (Sigma T6397), SATO (containing: transferrin (Sigma T-1147), BSA (Sigma A-4161), progesterone (Sigma P6149), putrescine (Sigma P5780), sodium selenite (Sigma S9133)). HEK293 cells were treated with BMP4 at 5 ng/ml alone, or BMP4 plus 2.5 μg/ml Chrdl1, 2.5 μg/ml Chordin, or 2.5 μg/ml Noggin for 1 hour. pSMAD staining was performed as outlined below.
SDS-PAGE and Western blot
Samples were prepared with reducing sample buffer (Thermo Fisher Scientific 39000) and boiled for 5 minutes at 95°C. Proteins were separat ed on NuPAGE 4–12% Bis-Tris protein gels (Thermo Fisher Scientific NP0322BOX) or Bolt 12% Bis-Tris Plus gel (NW00120BOX) for 1hour at constant voltage of 180V. Proteins were transferred onto Immobilion-FL PVDF membranes (Millipore IPFL00010), for 1.5h at constant current of 0.4A. Membranes were blocked for 1h at room temperature in gentle shaking with blocking buffer (1% casein, TBS). Membranes were incubated overnight at 4°C in gentle shaking with t he primary antibody in blocking buffer, mouse anti-Chrdl1 (Millipore MABN453) at a dilution 1:1000 or rabbit anti-Chrdl1 (Sigma HPA000250) at a dilution 1:250. Membranes were washed with TBS+0.1% Tween and incubated for 1h at room temperature with secondary antibody goat anti-mouse Alexa 680 (Thermo Fisher Scientific A21058) or goat anti-rabbit Alexa 680 (Thermo Fisher Scientific A21109). Membranes were washed with TBS+0.1% Tween, and immediately imaged on Odyssey Infrared Imager (Li-Cor) and Image Studio software (Li-Cor).
Electrophysiology from cultured RGC neurons
Miniature excitatory postsynaptic currents (mEPSCs) were recorded from RGCs in whole cell voltage-clamp mode at room temperature. The extracellular solution contained (in mM): NaCl 140, HEPES 10, Glucose 10, KCl 2.5, CaCl2 2.5, MgCl2 2, NaH2PO4 1, pH 7.4, supplemented with 0.5 μM tetrodotoxin (TTX) to block action potentials. The intracellular solution contained (in mM): K-Gluconate 120, KCl 10, HEPES 10, NaCl 6, MgCl2 1, EGTA 10, MgATP 2, NaGTP 0.3, pH 7.3. Cells were clamped at −70 mV using a Multiclamp 700A, data was filtered at 2 kHz and acquired at 10 kHz using pClamp 10. Before analysis data was low-pass filtered at 1 kHz. Analysis was performed using Minianalysis software (Synaptosoft) as previously described (Allen et al., 2012). Data from 3 independent experiments (separate RGC cultures) were pooled for analysis.
Immunodepletion of Chrdl1
Protein G magnetic beads (Thermo Fisher Scientific 88848) were incubated with 20μg of antiChrdl1 (Millipore MABN453), or no antibody (mock immunodepletion), for 2h in gentle rotation at room temperature. Beads were then washed with PBS three times, using the magnetic stand DynaMag-2 Magnet (Thermo Fisher Scientific 12321D). Protein G magnetic beads conjugated to anti-Chrdl1 or unconjugated magnetic beads were incubated with ACM overnight with gentle shaking at 4°C. Magnetic beads were separated from the ACM in the magnetic stand, ACM was collected, and added to the media to treat RGCs. ACM, Chrdl1-immunodepleted ACM or mockimmunodepleted ACM was added to RGCs at 50μg/ml. GluA2 live staining was performed as below. Bolt 12% Bis-Tris Plus gel (NW00120BOX) were used for SDS-PAGE and proteins were transferred as described previously. Rabbit anti-Chrdl1 (Sigma HPA000250) at a dilution 1:250 was used for immunoblotting in order to prevent detection of the heavy and light chain of the mouse anti-Chrdl1 used in the immunoprecipitation.
GluA2 live staining RGC neurons
RGCs were incubated with mouse anti-GluA2 (Millipore MAB397) at a 1:50 dilution and CellTracker Red CMTPX dye (Invitrogen C34552) at a 0.5 μM concentration in RGC growth medium for 30 minutes at 37°C, 10% CO 2. Cells were then washed with DPBS and fixed with pre-warmed (34°C) 4% PFA (EMS 50980487) for 5 minut es. After washing the coverslips with PBS they were blocked (without permeabilizing) for 30 minutes at room temperature in a humidified chamber with 50% goat serum/50% antibody buffer (150 mM NaCl; 50 mM Tris; 100 mM L-lysine; 1% BSA; pH 7.4). Coverslips were washed with PBS and incubated with secondary antibody for 1 hour at room temperature, goat anti-mouse Alexa 488 (Thermo Fisher Scientific A11029) at a 1:500 dilution in 10% goat serum in antibody buffer. Coverslips were washed with PBS and mounted on microscope slides (Fisherfinest 12–544-2) with SlowFade gold antifade mountant with DAPI (Thermo Fisher Scientific S36939). Samples were imaged on an epifluorescence microscope (Zeiss AxioImager.Z2) using the 63× oil-immersion objective. A total of 30 cells per condition were imaged across three coverslips in each independent experiment. A minimum of 3 independent experiments were run. Cells were located using CellTracker Red CMTPX in the red channel. Exposure acquisition was set according to the positive control (RGCs + rat astrocyte inserts) within each independent experiment. 16 bit images at 1388×1040 pixels were acquired using AxioCam HR3 camera (Zeiss). GluA2 puncta on the soma and proximal dendrites of RGCs were analyzed using MetaMorph software (Molecular devices) as previously described (Allen et al., 2012).
Synaptic staining RGC neurons
RGCs were fixed with pre-warmed (34C) 4% PFA for 10 mins. Coverslips were washed with PBS and blocked and permeabilized with 50% antibody buffer/50% goat serum and 0.2% Triton X-100 for 30 minutes at room temperature. Coverslips were washed with PBS and incubated with primary antibodies, mouse anti-PSD95 (Pierce MA1–045) at 1:500 dilution and rabbit antiBassoon (Synaptic systems 141–002) at 1:1,000 dilution overnight at 4°C. Coverslips were washed with PBS and incubated at room temperature with secondary antibodies, goat antimouse Alexa 488 (Thermo Fisher Scientific A11029) at 1:1,000 dilution and goat anti-rabbit Alexa 594 (Thermo Fisher Scientific A11037) at 1:1,000 dilution for 1 hour at room temperature. Coverslips were washed with PBS and mounted on microscope slides (Fisherfinest 12–544-2) with SlowFade gold antifade mountant (ThermoFisher Scientific S36939). A total of 30 cells per condition were imaged across three coverslips in each independent experiment. A total of 10 independent experiments were run (separate RGC cultures). Samples were imaged with an epifluorescence microscope (Zeiss AxioImager.Z2) using the 63× oil-immersion objective, and an AxioCam HR3 camera for acquisition of 16 bit images at 1388×1040 pixels. Exposure times for image acquisition were set according to the positive control (RGCs + rat astrocyte inserts) within each independent experiment. For analysis of synapses all cells within the same experiment were equally thresholded, and images were analyzed with the ImageJ (NIH) puncta analyzer plug-in as previously described (Allen et al., 2012; Ippolito and Eroglu, 2010).
Phospho-SMAD (pSMAD) immunostaining of RGC neurons and HEK cells
After treatment of RGCs or HEK cells as described, growth medium was removed and cells were washed 1× with DPBS (warmed to 34°C). Cells we re fixed with 4% PFA for 10 minutes (warmed to 34°C). Cells were washed 3 times with DP BS, then blocked and permeabilized in 50% antibody buffer/50% goat serum and 0.4% Triton X-100 for 30 minutes at room temperature. Cells were washed 1× with DPBS and incubated with primary antibody rabbit Phospho-Smad1/5/9 (CST 13820) at 1:200 dilution, overnight at 4°C in antibody buffer and 10% goat serum. Cells were washed 3 times with DPBS and incubated with secondary antibody, goat anti-rabbit Alexa 594 (Thermo Fisher Scientific A11037) at 1:1000 dilution in antibody buffer and 10% goat serum for 1 hour at room temperature. Cells were washed 3 times with DPBS and mounted on microscope slides with SlowFade gold antifade mountant with DAPI. Cells were imaged using an epifluorescence microscope (Zeiss AxioImager.Z2). For RGCs a total of 30 cells per condition were imaged across three coverslips in each independent experiment. Three independent experiments were run. For HEK cell experiments 10 fields of view (each containing multiple cells) from multiple coverslips were imaged per condition. Acquisition of 16 bit images at 1388×1040 pixels was done using the 63× oil-immersion objective, and an AxioCam HR3 camera, using set exposure conditions determined from the positive control (BMP4 treated). Expression of pSMAD in the nucleus was measured using ImageJ software (NIH). DAPI was used to create a mask. pSMAD expression was measured as mean intensity within the area of the DAPI mask.
Tissue collection and preparation
Mice were anesthetized with intraperitoneal injection of 100 mg/kg Ketamine (Victor Medical Company) and 20 mg/kg Xylazine (Anased) mix and transcardially perfused. To obtain fresh frozen tissue, mice were transcardially perfused with PBS. Brains were collected and embedded in OCT (Scigen 4583) and frozen in dry ice/ethanol, and stored at −80°C until use. Fresh frozen tissue was used for in situ hybridization experiments. To obtain fixed tissue, mice were first transcardially perfused with PBS followed by 4% PFA. Brains were removed and kept in 4% PFA at 4°C overnight. Brains were washed three time s with PBS and cryoprotected in 30% sucrose at 4°C. Brains were frozen in TFM (General data healthcare TFM-5) in dry ice/ethanol and kept at −80°C until use. Fixed tissue was used for immunohistochemistry experiments.
Immunohistochemistry, cell counting and cortical thickness measurement.
Littermate male mice (Aldh1l1-EGFP x Chrdl1 KO or WT) were used for these experiments. Fixed sagittal sections were collected on Superfrost Plus micro slides (VWR 48311–703) at 22 μm thickness (1.80–2.16 mm lateral from midline). Sections were blocked and permeabilized for 1 hour at room temperature in a humidified chamber. Blocking buffer contained 5% goat serum, 0.3% Triton X-100 in PBS. Primary antibody mouse anti-NeuN (Millipore MAB377), a neuronal marker, was incubated in 5% goat serum, 100 mM lysine in PBS at a 1:100 dilution in the humidified chamber overnight at 4°C. Slices were wa shed three times with 0.3% Triton X-100 in PBS and incubated for 2 hours at room temperature with secondary antibody goat anti-mouse Alexa 594 (Thermo Fisher Scientific A11032) at a 1:500 dilution, in 5% goat serum, 100 mM lysine in PBS. Sections were washed with 0.3% Triton X-100 in PBS three times and mounted with SlowFade gold antifade mountant with DAPI. Coverslips 22 mm X 50 mm 1.5 thickness (Fisher Scientific 12–544-D) were placed on top of the sections and sealed with nail polish (Electron Microscopy Sciences 72180). Negative controls included slices where NeuN antibody was omitted.
Layer 2/3 of the visual cortex was imaged using a fluorescent microscope Zeiss Axio Imger.Z2. using the Apotome.2 module (Zeiss). Images were taken at 20X magnification, as 16 bit images of 4 tiles with 10% overlap, z-stacks of 10 steps with a total thickness of 10μm. Exposure acquisition was consistent across WT and KO samples from the same experiment. Imaging was performed on 6 sections (technical replicates) from 3 mice (biological replicates) per genotype. Cell counting was performed using IMARIS software (Bitplane). DAPI stained nuclei were defined as spots with an average diameter ranging between 7 and 9 pixels. NeuN expressing neurons (channel 594) and astrocytes (EGFP) were defined as surfaces with an average diameter of 14 and 9 pixels, respectively. One cell was counted as co-localization of a DAPI nucleus embedded in a surface, defining co-localization as proximity from the center of the spot to the surface that was equal or less than 1/3 of the assigned average diameter of the spot. Representative images are maximum orthogonal projections of the full z-stack, obtained with ZEN blue software.
To measure cortical thickness the DAPI channel of the visual cortex of Chrdl1 KO or WT sagittal sections were imaged using a fluorescence microscope Zeiss Axio Imager.Z2 at 10X magnification as 16 bit images of 3 tiles with 10% overlap, image size 895.26 μm x 1880 μm. The cortex from the top of layer 1 (pia) to the bottom of layer 6 (corpus callosum), was measured using the Zen blue edition software (Zeiss) distance tool.
RNA isolation and qRT-PCR from visual cortex
RNA was extracted from brain tissue using the RNeasy mini kit (QIAgen 74104). Tissue from the visual cortex of the mouse brain at different ages (P7, 12, 21, 30 and 120) was homogenized with RLT buffer (provided in the kit) and β-mercaptoethanol. The homogenate was loaded in a QIAshredder (QIAgen 79656) for complete homogenization. RNA was isolated following manufacturer’s instructions. 200 ng of RNA was used for cDNA synthesis by RT-PCR using Superscript VILO master mix (Thermo Fisher Scientific 11755050) according to manufacturer’s instructions. 1 μl of the obtained cDNA was used for qPCR reaction using SYBR green PCR mastermix (Life Supply 4309155). All samples were run in triplicates (technical replicates) and at least three mice (biological replicates) were analyzed. Primer pairs (Integrated
DNA Technologies) used: GAPDH (forward TGCCACTCAGAAGACTGTGG, and reverse GCATGTCAGATCCACAATGG) and Chrdl1 primer 1 (forward GACCGAGAGACCACCTCAAG, reverse AGCTTCCACTGGTTCAAGGTG). Additionally, two different pairs of primers were used to confirm knock out of Chrdl1: Chrdl1 primer 2 (forward TGGCACCCAAATCTACGAGC, and reverse TTTGGCTTGGAGGT TCTTCTGG) and Chrdl1 primer 3 (forward GTGCCCAGCTTTAGTCCACC, and reverse CCATACGGTTCCAGGTAGGG). To determine Chrdl1 mRNA level specifically in astrocytes in the developing visual cortex, astrocyte mRNA was purified from GFAP-cre x Ribotag visual cortex at P7 and P14, following the procedure in (Sanz et al., 2009). See (Boisvert et al., 2018) for validation of mouse model. qRT-PCR was then performed as outlined above. Data was analyzed using SDS 2.4 software (Applied Biosystems). Ct values of Chrdl1 were normalized to GAPDH and to the control condition of the experiment.
RNA-sequencing (RNA-seq)
To determine if Chrdl1 regulates expression of genes in neurons in vitro, RGCs were treated for 12 hours with 1ug/ml Chrdl1, and compared to buffer-treated RGCs as a control.
To determine if Chrdl1 regulates gene expression in the brain in vivo, visual cortex was collected from WT and Chrdl1 KO mice at P14.
RNA was isolated from RGCs or brain tissue using RNeasy mini kit (QIAgen 74104). Libraries were prepared with TrueSeq Stranded mRNA Library Prep Kit (Illumina). Poly(A) mRNA was isolated from total RNA samples, followed by mRNA fragmentation, first strand cDNA synthesis, second cDNA synthesis and adaptor ligation, and isolation and amplification of cDNA fragments. Illumina HiSeq 2500 instrument was used for sequencing. For the analysis, sequenced reads were quality-tested using FASTQC and aligned to the mm10 mouse genome (KO vs WT mouse experiment) and rn6 rat genome (RGC neuron in vitro experiment) using the STAR aligner version 2.4.0k. Mapping was carried out using default parameters (up to 10 mismatches per read, and up to 9 multi-mapping locations per read). The genome index was constructed using the gene annotation supplied with the mm10 or rn6 Illumina iGenomes collection and sjdbOverhang value of 100. Normalized gene expression (Fragments per kilobase per million mapped reads, FPKM) was quantified with HOMER v4.8.3 across all gene exons using the top-expressed isoform as proxy for gene expression.
Electrophysiology in acute visual cortex brain slices
Experiments were carried out on littermate WT and Chrdl1 KO mice at P14. Miniature excitatory postsynaptic currents (mEPSCs) were recorded from layer 2/3 pyramidal neurons in acute coronal slices of P14 visual cortex in whole cell voltage-clamp mode, following the procedures in (Brill and Huguenard, 2008). A chilled slicing solution was used for dissection and slicing and contained (in mM): Sucrose 234, NaHCO3 24, Glucose 11, KCl 2.5, MgCl2 2, NaH2PO4 1.25, CaCl2 0.5, pH 7.4. Coronal sections at 400 μm were made on a Leica vibratome (VT1200 S), and recovered in ACSF at 34°C for 1 hour, and moved to room temperature before recording. ACSF contained (in mM): NaCl 126, NaHCO3 26, Glucose 10, KCl 2.5, MgCl2 2, NaH2PO4 1.25, CaCl2 2, pH 7.4. mEPSCs were recorded from slices in oxygenated ACSF with a flow rate of 2 ml/min at room temperature (20–22°C) and a cocktail of drugs was added to isolate AMPA currents: 40 μM bicuculline methochloride, 50 μM D-AP5, 0.5 μM TTX. The intracellular solution contained (in mM): CsMeSO3 130, HEPES 10, NaCl 4, EGTA 5, CaCl2 0.5, QX314-chloride 5, MgATP 4, NaGTP 0.5, pH 7.2. Cells were clamped at −60 mV using a Multiclamp 700 A, recordings were made for 5 minutes, data was filtered at 2 kHz and acquired at 10 kHz using pClamp 10. Before analysis data was low-pass filtered at 1 kHz. Analysis was performed using Minianalysis software (Synaptosoft) as previously described (Allen et al., 2012). Acquisition and analysis were carried out blind to genotype.
In situ hybridization
Sagittal brain sections were obtained from WT C57BL/6J mice from different ages (P7, P12, P30, and P120) to analyze developmental expression of Chrdl1, or from WT and Chrdl1 KO mice at P14 to study Chrdl1 expression. Coronal sections were obtained from WT or Chrdl1 KO mice that underwent monocular enucleation to analyze Arc expression. All sections were made using a cryostat (Hacker Industries OTF5000) at a slice thickness of 16 μm (1.80–2.40 mm lateral from midline for sagittal sectioning, 3.40 mm posterior to Bregma for coronal sectioning). Brown (ACDbio 322300) and fluorescent (FISH) (ACDbio 320850) in situ hybridizations were performed following manufacturer’s instructions for fresh frozen tissue, with the following modifications. Protease pre-treatments of the slices depended on the age of the brain. The following criteria were used: P7 was pre-treated with protease plus for 17 minutes, P12–14 was pre-treated with protease 4 for 15 minutes and P30 and P120 were pre-treated with protease 4 for 30 minutes. SlowFade gold antifade mountant with DAPI was applied to every section. Coverslips 22 mm × 50 mm 1.5 thickness were placed on top of the sections and sealed with nail polish.
Probes used were Aldh1l1 (ACDbio 405891-C2), Tubb3 (ACDbio 423391-C3), Chrdl1 (ACDbio 442811), Slc1a3/GLAST (ACDbio 430781-C2) and Arc (ACDbio 316911). In control experiments a negative control probe was used (ACDbio 310043) to determine the level of background signal. For fluorescent RNAscope, Aldh1l1 or Slc1a3 were imaged in channel 488, Tubb3 was imaged in channel 647, and Chrdl1 was imaged in channel 550. A minimum of 3 animals were used per condition, and 3 brain sections per animal. The upper layers of the visual cortex were imaged at 20X magnification at 1024 × 1024 pixels as z-stack of 4 slices with a total thickness of 5 μm. Zoomed cells in example images were imaged at 63X magnification at 1024 × 1024 pixels as z-stacks of 4 slices with a total thickness of 5 μm. Cells co-expressing Chrdl1 and Aldh1l1 (astrocyte-specific expression), or Chrdl1 and Tubb3 (neuron-specific expression) were manually counted using the Zen blue edition software (Zeiss) events tool, and expressed as the proportion of Chrdl1 positive total cells in the upper layers of the visual cortex region of interest. When imaging all layers of the visual cortex images were taken from the dentate gyrus as the deepest imaging point. 16 bit images were taken at 1024 × 1024 pixels as z-stack of 4 slices with a total thickness of 5 μm, and 14 tiles with 10% overlap using 20X magnification in a Zeiss LSM 710 confocal microscope. Representative images are maximum orthogonal projections of the z-stack. Images of the whole sagittal section were acquired using fluorescence microscope Zeiss Axio Imager.Z2 at 10X magnification as 16 bit images of 126–130 tiles with 10% overlap. For Arc visualization, coronal sections were imaged using fluorescence microscope Zeiss Axio Imager.Z2 at 10X magnification as 16 bit images of 143 tiles with 10% overlap. Arc was imaged in channel 550. A minimum of 5 animals were used per condition. Brown RNAscope samples were imaged in brightfield using 2.5X magnification in a Zeiss Axio Imager.Z2 microscope.
Immunohistochemistry and puncta analysis.
Littermate Chrdl1 KO and WT male mice were used for experiments, and 3–6 independent experiments were run (biological replicates), each with imaging and analysis of 3 independent brain sections (technical replicates). Sagittal sections at a 16 μm thickness (1.80–2.16 mm lateral from midline) were obtained from PFA fixed WT or Chrdl1 KO mouse brains using the cryostat, and immediately processed for immunohistochemistry. Sections were on Superfrost Plus micro slides (VWR 48311–703). Sections were blocked and permeabilized for 1 hour at room temperature in a humidified chamber with 5% goat serum, 0.3% Triton X-100 in PBS. Primary antibodies were incubated in 5% goat serum, 0.3% Triton X-100, 100 mM lysine in PBS, at 4°C overnight in a humidified chamber. Prim ary antibodies used were rabbit anti-GluA2 (Millipore AB1768-I) at 1:500 dilution, anti-GluA1 (Millipore AB1504) at 1:400 dilution, PSD-95 (Thermo Fisher Scientific 51–6900) at 1:400, and guinea pig anti-VGlut1 (Millipore AB5905) at 1:2000 dilution or guinea pig anti-VGlut2 (Millipore AB2251) at 1:1000 dilution. Slices were washed with PBS 3 times for 5 minutes. Slices were then incubated with secondary antibodies for 2 hours at room temperature. All secondary antibodies were at 1:500 dilution. Secondary antibodies used were goat anti-guinea pig Alexa 488 (Thermo Fisher Scientific A11073) to label VGlut1 or VGlut2, goat anti-rabbit Alexa 594 (Thermo Fisher Scientific A11032) to label GluA2, GluA1 or PSD-95. As negative controls, the primary antibodies were omitted and the brain slices were incubated with just secondary antibodies: goat anti-guinea pig Alexa 488 and goat anti-rabbit Alexa 594.
Sections were washed 3 times for 5 minutes with PBS, and SlowFade gold antifade mountant with DAPI was applied to every section. Coverslips 22 mm × 50 mm 1.5 thickness (Fisher Scientific 12–544-D) were placed on top of the sections and sealed with nail polish. Upper layers of the visual cortex of the sections were imaged as 16 bit images at 1420 × 1420 pixels, 0.08 μm × 0.08 μm pixel size on a Zeiss LSM 780 confocal microscope using the 63X oil-immersion objective as a z-stack of 10 slices with a total thickness of 3.51 μm. Exposure acquisition was set according to the WT sample and all sections were imaged within the same session for each independent experiment.
Analysis of synapse number was performed blind to genotype. In order to assess synapses IMARIS software (Bitplane) was used. Synapses were defined as proximity of the postsynaptic GluA2, GluA1 or PSD-95 puncta to presynaptic VGlut1 or VGlut2 puncta. The z-stacks of each condition (WT or Chrdl1 KO) were presented as 3D images. Gaussian filter of 0.0725 μm was applied to both channels in order to smooth the images and reduce noise. Puncta were defined in each channel as spheres of average diameter of 0.5 μm using the spot detection function. The co-localization spots function defined a distance from center to center of spots of 0.7 μm. Spots from different channels that were within 0.7 μm from each other’s center were quantified as a synapse.
To assess changes in glutamate transporters in astrocytes, co-localization of VGluT2 and GLT1 puncta in the upper layers of the mouse visual cortex was analyzed. Littermate Aldh1l1-EGFP × Chrdl1 KO and WT male mice were used. Sectioning and antibody incubations were performed as described above. Primary antibodies used were guinea pig anti-VGlut2 (Millipore AB2251) at 1:1000 dilution, and rabbit anti-EAAT2/GLT1 (Novus Biologicals NBP1–20136SS) at 1:300 dilution. Secondary antibodies anti-rabbit Alexa 555 and anti-guinea pig Alexa 647 were used at 1:500 dilution. Upper layers of the visual cortex were imaged as 16 bit images at 1360 × 1360 pixels, 0.06 μm × 0.06 μm pixel size on a Zeiss LSM 880 Airyscan FAST super-resolution microscope using the 63X oil-immersion objective as a z-stack of 10 slices with a total thickness of 3.51 μm. Exposure acquisition was set according to the WT sample and all sections were imaged within the same session for each independent experiment. Aldh1l1-EGFP was imaged along with GLT1 and VGlut2.
Analysis of co-localization of GLT1 and VGlut2 was performed blind to genotype. The z-stacks of each condition (Aldh1l1-EGFP × Chrdl1 KO or WT) were presented as 3D images, using IMARIS software (Bitplane). Gaussian filter of 0.0725 μm was applied to all channels in order to smooth the images and reduce noise. Astrocytes (Aldh1l1-EGFP) were defined in channel 488 as surface objects with a surface detail of 0.085 μm, and GLT1 and VGlut2 were defined as spheres of 0.5 μm of diameter. GLT1 spheres that were in proximity to the surface of the astrocyte (distance of GLT1 sphere to surface object defined as 0.5 μm) were considered for colocalization to VGlut2 analysis, using the spots closest to surface function. Co-localization was assessed as described previously for analysis of synapse number. Total GLT1 and total VGlut2 puncta referred to the total number of spots detected by the spot detection function in the entire z-stack. All representative images are maximum orthogonal projections of 4 consecutive steps of the z-stack.
Monocular visual enucleation and Arc analysis.
Littermate WT and Chrdl1 KO male mice at P28 (within critical period) or 4 months old (after critical period is closed) were used for these experiments. Mice were anesthetized with 2% isofluorane in oxygen by constant flow via nose cone. The right eye was removed by transecting the optic nerve. Gelfoam (Pfizer 031508) was placed in the empty ocular cavity. Eyelids were sutured using 6–0 silk sutures (Henry Schein 101–2636). Lidocaine 2% and erythromycin 0.5% were applied topically on the sutured eyelids. Normal rearing (NR) mice were collected 12 hours later. Mice for monocular enucleation (ME) analysis were subjected to a 12 hour light/12 hour dark cycle every day until collection 4 days later, at P32, or 4 or 10 days later for the 4 month old adult mice. Prior to collection, mice were exposed to light for 30 minutes in the alert condition to activate neurons in the visual cortex and induce expression of Arc. Mice were anesthetized with 100 mg/kg ketamine (Victor Medical Company) and 20 mg/kg xylazine (Anased) mix and brains removed. Brains were coronally sectioned in half and the posterior brain collected and embedded in OCT (Scigen 4583), frozen in dry ice/ethanol, and stored at 80°C. Coronal sections of the brain were obtained a t 16 μm thickness (3.40 mm posterior to Bregma), and Arc in situ hybridization and imaging were performed as described above. Zen blue edition software (Zeiss) distance tool was used to measure the width of the Arc activated brain region along layer 4 of the visual cortex, contralateral to the removed eye. 4–6 brain sections were measured per mouse. Analysis was performed blind to genotype.
Golgi-cox staining and analysis.
Brains were stained using FD Rapid Golgi Stain Kit (FD Neurotechnologies PK401). Briefly, brains from littermates Chrdl1 KO and WT were collected at age P28–32, rinsed in MilliQ H2O and left in impregnation solution (1:1 mixture of solutions A and B from the kit) at room temperature and protected from light for 2 weeks. Impregnation solution was replaced in the first 24 hours. Brains were transferred to solution C from the kit, and left for 3 days at room temperature and protected from light. Solution C was replaced in the first 24 hours. Coronal slices at 100 μm thickness (3.40 mm posterior to Bregma) were obtained using a HM 430 sliding microtome (Thermo Fisher Scientific 910010). Slices were collected using a glass retriever and drops of solution C, and mounted on gelatin-coated microscope slides (FD Neurotechnologies PO101). Slices were left to dry for at least 4h at room temperature and away from light. All following steps were performed at room temperature and away from light when possible. Slices were rinsed in distilled water for 4 minutes, 3 times, and incubated in a mixture of Solution D + Solution E + MilliQ H2O in a proportion of 1:1:2 for 10 minutes. Slices were rinsed in MilliQ H2O3 times for 4 minutes, and dehydrated by sequential incubations in 50%, 75%, and 95% ethanol for 4 minutes each. Slices were dehydrated in absolute ethanol 4 times for 4 minutes each, and cleared in xylene 3 times for 4 minutes. Slides were mounted using Permount (Fisher Scientific SP15–100). Pyramidal neurons from layers 2/3 within the visual cortex were imaged in brightfield in a Zeiss Axio Imager.Z2 microscope, using the 63X oil-immersion objective as a zstack of 160 slices with a total thickness of 80 μm. Z-stacks were transformed in ImageJ (NIH) to RGB images, and saved as image sequences. Spines located in sections of secondary and tertiary dendrites of pyramidal cortical neurons within the upper layers of the visual cortex were analyzed. Length and width of spines were assessed using Reconstruct software (Fiala, 2005). Analysis and classification of spine morphology was assessed according to (Risher et al., 2014b). A total of 5 littermate Chrdl1 KO and WT mice were analyzed, with 9 neurons per mouse, and 3 dendrites per neuron analyzed.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical tests and graphs were designed using Prism 7 and Sigmaplot, all tests are 2-tailed. Student’s T-test was used to compare two groups. To compare more than two groups, one-way ANOVA with Dunnett’s post-hoc test for multiple comparisons was used. When data did not pass normal distribution test, multiple comparisons were done by Kruskal-Wallis ANOVA on ranks and pairwise comparisons were done with Mann-Whitney Rank Sum test. For analysis of monocular enucleation a two-way ANOVA was used. To compare distributions a Kolmogorov-Smirnov (K-S) test was run in MiniAnalysis (Synaptosoft). Exact P values reported on graphs.
DATA AND SOFTWARE AVAILABILITY
RNA sequencing data have been deposited at GEO: GSE108483 (Chrdl1 KO and WT mouse visual cortex). GSE108484 (Rat RGC neurons +/− Chrdl1 protein treatment).
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
Highlights.
Astrocytes regulate synapse maturation by controlling AMPA receptor subtype
Astrocyte-secreted Chrdl1 increases synaptic levels of GluA2 AMPA receptors
Chrdl1 KO mice have immature synapses
Chrdl1 KO mice show enhanced plasticity in vivo, which is maintained into adulthood
ACKNOWLEDGEMENTS
We thank Cari Dowling for technical assistance and Matt Boisvert for ribotag samples. We thank S. Pfaff, G. Lemke, C. Kintner, R. Daneman, C. Shatz, A.R. Aricescu, the Allen lab, and members of MNL for helpful discussion on the project. We thank Tsung-Chang Sung and Yelena Dayn for making the Chrdl1 KO mouse, and the Biophotonics Core at the Salk Institute for advice with image acquisition and analysis. This work is supported by NIH-NINDS NS105742. Work in the lab of NJA is supported by the Hearst, Pew, Ellison and Whitehall Foundations. EBS was supported by Postdoctoral Fellowships from the Helmsley and Catarina Foundations. TL was supported by a Postdoctoral Fellowship from the Helmsley Foundation. This work was supported by Core Facilities of the Salk Institute: (Functional Genomics, Genome Manipulation, Transgenic, Next Generation Sequencing, Bioinformatics: NIH-NCI CCSG P30 014195), (Biophotonics: NIH-NINDS P30 NS072031, NIH-NCI CCSG P30 014195, Waitt Foundation).
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, and Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature 486, 410–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball RW, Peled ES, Guerrero G, and Isacoff EY (2015). BMP signaling and microtubule organization regulate synaptic strength. Neuroscience 291, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck HN, Drahushuk K, Jacoby DB, Higgins D, and Lein PJ (2001). Bone morphogenetic protein-5 (BMP-5) promotes dendritic growth in cultured sympathetic neurons. BMC Neuroscience 2, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berke B, Wittnam J, McNeill E, Van Vactor DL, and Keshishian H (2013). Retrograde BMP Signaling at the Synapse: A Permissive Signal for Synapse Maturation and ActivityDependent Plasticity. The Journal of Neuroscience 33, 17937–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert MM, Erikson GA, Shokhirev MN, and Allen NJ (2018). The Aging Astrocyte Transcriptome from Multiple Regions of the Mouse Brain. Cell Reports 22, 269–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragdon B, Moseychuk O, Saldanha S, King D, Julian J, and Nohe A (2011). Bone Morphogenetic Proteins: A critical review. Cellular signalling 23, 609–620. [DOI] [PubMed] [Google Scholar]
- Brill J, and Huguenard JR (2008). Sequential Changes in AMPA Receptor Targeting in the Developing Neocortical Excitatory Circuit. The Journal of Neuroscience 28, 13918–13928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, and Barnes CA (2006). Neural plasticity in the ageing brain. Nature Reviews Neuroscience 7, 30. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, and Barres BA (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. The Journal of neuroscience : the official journal of the Society for Neuroscience 28, 264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, and Barres BA (2013). Emerging roles of astrocytes in neural circuit development. Nature reviews Neuroscience 14, 311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C, Tran U, Larrain J, and De Robertis EM (2001). Neuralin-1 is a novel Chordinrelated molecule expressed in the mouse neural plate. Mechanisms of development 100, 119122. [DOI] [PubMed] [Google Scholar]
- Cruz-Martin A, El-Danaf RN, Osakada F, Sriram B, Dhande OS, Nguyen PL, Callaway EM, Ghosh A, and Huberman AD (2014). A dedicated circuit links directionselective retinal ganglion cells to the primary visual cortex. Nature 507, 358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hübener M, and Shatz CJ (2013). PirB regulates a structural substrate for cortical plasticity. Proceedings of the National Academy of Sciences 110, 20771–20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu Ç, Allen NJ, Susman MW, O’Rourke NA, Park CY, Özkan E, Chakraborty C, Mulinyawe SB, Annis DS, and Huberman AD (2009). Gabapentin Receptor α2δ−1 Is a Neuronal Thrombospondin Receptor Responsible for Excitatory CNS Synaptogenesis. Cell 139, 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa JS, and Stryker Michael P. (2012). Development and Plasticity of the Primary Visual Cortex. Neuron 75, 230–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquenazi S, Monnerie H, Kaplan P, and Le Roux P (2002). BMP-7 and excess glutamate: opposing effects on dendrite growth from cerebral cortical neurons in vitro. Experimental neurology 176, 41–54. [DOI] [PubMed] [Google Scholar]
- Farhy-Tselnicker I, van Casteren ACM, Lee A, Chang VT, Aricescu AR, and Allen NJ (2017). Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron 96, 428–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala JC (2005). Reconstruct: a free editor for serial section microscopy. Journal of microscopy 218, 52–61. [DOI] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, and Budnik V (2012). Integration of a Retrograde Signal during Synapse Formation by Glia-Secreted TGF-β Ligand. Current Biology 22, 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, Schork AJ, Appadurai V, Buil A, Werge TM, Liu C, White KP, Horvath S, and Geschwind DH (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359, 693–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara K, Watanabe K, Chun J, and Yamaguchi Y (2000). Glypican-4 is an FGF2binding heparan sulfate proteoglycan expressed in neural precursor cells. Dev Dyn 219, 353–367. [DOI] [PubMed] [Google Scholar]
- Henley JM, and Wilkinson KA (2013). AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues in Clinical Neuroscience 15, 11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley JM, and Wilkinson KA (2016). Synaptic AMPA receptor composition in development, plasticity and disease. Nature reviews Neuroscience 17, 337–350. [DOI] [PubMed] [Google Scholar]
- Humphries JD, Byron A, and Humphries MJ (2006). INTEGRIN LIGANDS. Journal of cell science 119, 3901–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt LT, and Barker WC (1987). Von Willebrand factor shares a distinctive cysteine-rich domain with thrombospondin and procollagen. Biochemical and biophysical research communications 144, 876–882. [DOI] [PubMed] [Google Scholar]
- Ippolito DM, and Eroglu C (2010). Quantifying Synapses: an Immunocytochemistry-based Assay to Quantify Synapse Number. Journal of Visualized Experiments : JoVE, 2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Agopyan N, Miu P, Xiong Z, Henderson J, Gerlai R, Taverna FA, Velumian A, MacDonald J, Carlen P, Abramow-Newerly W, and Roder J (1996). Enhanced LTP in Mice Deficient in the AMPA Receptor GluR2. Neuron 17, 945–956. [DOI] [PubMed] [Google Scholar]
- Jonas P (2000). The Time Course of Signaling at Central Glutamatergic Synapses. Physiology 15, 83–89. [DOI] [PubMed] [Google Scholar]
- Kalinovsky A, Boukhtouche F, Blazeski R, Bornmann C, Suzuki N, Mason CA, and Scheiffele P (2011). Development of Axon-Target Specificity of Ponto-Cerebellar Afferents. PLoS Biol 9, e1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, and Stryker MP (2008). Tumor Necrosis Factor-α Mediates One Component of Competitive, Experience-Dependent Plasticity in Developing Visual Cortex. Neuron 58, 673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS, and Sofroniew MV (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18, 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike N, Kassai Y, Kouta Y, Miwa H, Konishi M, and Itoh N (2007). Brorin, a Novel Secreted Bone Morphogenetic Protein Antagonist, Promotes Neurogenesis in Mouse Neural Precursor Cells. Journal of Biological Chemistry 282, 15843–15850. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, and Huguenard JR (2002). A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience 22, 3005–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee‐Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, and Attisano L (2004). Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP‐dependent dendritogenesis. The EMBO journal 23, 4792–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, and Niswander LA (2005). Bone morphogenetic protein signalling and vertebrate nervous system development. Nature reviews Neuroscience 6, 945–954. [DOI] [PubMed] [Google Scholar]
- Liu S-QJ, and Cull-Candy SG (2000). Synaptic activity at calcium-permeable AMPA receptors induces a switch in receptor subtype. Nature 405, 454–458. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, and de Vellis J (1980). Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. The Journal of Cell Biology 85, 890–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Han CE, Scully S, Nishinakamura R, He C, Zeni L, Yamane H, Chang D, Yu D, Yokota T, and Wen D (2001). A novel chordin-like protein inhibitor for bone morphogenetic proteins expressed preferentially in mesenchymal cell lineages. Developmental biology 232, 372–387. [DOI] [PubMed] [Google Scholar]
- Park YK, and Goda Y (2016). Integrins in synapse regulation. Nature Reviews Neuroscience 17, 745. [DOI] [PubMed] [Google Scholar]
- Risher WC, Patel S, Kim IH, Uezu A, Bhagat S, Wilton DK, Pilaz LJ, Singh Alvarado J, Calhan OY, Silver DL, Stevens B, Calakos N, Soderling SH, and Eroglu C (2014a). Astrocytes refine cortical connectivity at dendritic spines. eLife 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Ustunkaya T, Singh Alvarado J, and Eroglu C (2014b). Rapid Golgi Analysis Method for Efficient and Unbiased Classification of Dendritic Spines. PloS one 9, e107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuta H, Suzuki R, Takahashi H, Kato A, Shintani T, Iemura S, Yamamoto TS, Ueno N, and Noda M (2001). Ventroptin: a BMP-4 antagonist expressed in a double-gradient pattern in the retina. Science 293, 111–115. [DOI] [PubMed] [Google Scholar]
- Sanz E, Yang L, Su T, Morris DR, McKnight GS, and Amieux PS (2009). Cell-typespecific isolation of ribosome-associated mRNA from complex tissues. Proceedings of the National Academy of Sciences of the United States of America 106, 13939–13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, and Stryker MP (2008). Distinctive Features of Adult Ocular Dominance Plasticity. The Journal of Neuroscience 28, 10278–10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Müller, Catrin S, Bildl W, Baehrens D, Hüber B, Kulik A, Klöcker N, Schulte U, and Fakler B (2012). HighResolution Proteomics Unravel Architecture and Molecular Diversity of Native AMPA Receptor Complexes. Neuron 74, 621–633. [DOI] [PubMed] [Google Scholar]
- Seabrook TA, Burbridge TJ, Crair MC, and Huberman AD (2017). Architecture, Function, and Assembly of the Mouse Visual System. Annual Review of Neuroscience 40, 499–538. [DOI] [PubMed] [Google Scholar]
- Shen W, Finnegan S, Lein P, Sullivan S, Slaughter M, and Higgins D (2004). Bone morphogenetic proteins regulate ionotropic glutamate receptors in human retina. European Journal of Neuroscience 20, 2031–2037. [DOI] [PubMed] [Google Scholar]
- Singh Sandeep K., Stogsdill Jeff A., Pulimood Nisha S., Dingsdale H, Kim Yong H., Pilaz L-J, Kim H Il, Manhaes Alex C., Rodrigues Jr, Wandilson S, Pamukcu A, Enustun E, Ertuz Z, Scheiffele P, Soderling SH, Silver Debra L., Ji R-R, Medina Alexandre E., and Eroglu C (2016). Astrocytes Assemble Thalamocortical Synapses by Bridging NRX1α and NL1 via Hevin. Cell 164, 183–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syken J, Grandpre T, Kanold PO, and Shatz CJ (2006). PirB restricts ocular-dominance plasticity in visual cortex. Science 313, 1795–1800. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, and Dingledine R (2010). Glutamate receptor ion channels: structure, regulation, and function. Pharmacological reviews 62, 405–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, and Sato K (2003). A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 23, 11732–11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, and Barres BA (2001). Control of Synapse Number by Glia. Science 291, 657–661. [DOI] [PubMed] [Google Scholar]
- Webb TR, Matarin M, Gardner JC, Kelberman D, Hassan H, Ang W, Michaelides M, Ruddle JB, Pennell CE, Yazar S, Khor CC, Aung T, Yogarajah M, Robson AG, Holder GE, Cheetham ME, Traboulsi EI, Moore AT, Sowden JC, Sisodiya SM, Mackey DA, Tuft SJ, and Hardcastle AJ (2012). X-linked megalocornea caused by mutations in CHRDL1 identifies an essential role for ventroptin in anterior segment development. American journal of human genetics 90, 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler A, and Wang JT (2013). Purification and culture of retinal ganglion cells from rodents. Cold Spring Harb Protoc 2013, 643–652. [DOI] [PubMed] [Google Scholar]
- Withers GS, Higgins D, Charette M, and Banker G (2000). Bone morphogenetic protein-7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. The European journal of neuroscience 12, 106–116. [DOI] [PubMed] [Google Scholar]
- Xiao L, Michalski N, Kronander E, Gjoni E, Genoud C, Knott G, and Schneggenburger R (2013). BMP signaling specifies the development of a large and fast CNS synapse. Nat Neurosci 16, 856–864. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, Phatnani HP, Guarnieri P, Caneda C, Ruderisch N, Deng S, Liddelow SA, Zhang C, Daneman R, Maniatis T, Barres BA, and Wu JQ (2014). An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. The Journal of Neuroscience 34, 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Esteban JA, Hayashi Y, and Malinow R (2000). Postnatal synaptic potentiation: Delivery of GluR4-containing AMPA receptors by spontaneous activity. Nat Neurosci 3, 1098–1106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA sequencing data have been deposited at GEO: GSE108483 (Chrdl1 KO and WT mouse visual cortex). GSE108484 (Rat RGC neurons +/− Chrdl1 protein treatment).
The data that support the findings of this study are available from the corresponding author upon reasonable request.