Abstract
Objective:
Xenotransplantation using pig organs could end the donor organ shortage for transplantation, but humans have xenoreactive antibodies that cause early graft rejection. Genome editing can eliminate xenoantigens in donor pigs to minimize the impact of these xenoantibodies. Here we determine whether an improved crossmatch and chemical immunosuppression could result in prolonged kidney xenograft survival in a pig-to-rhesus preclinical model.
Methods:
Double xenoantigen (Gal and Sda) knockout (DKO) pigs were created using CRISPR/Cas. Serum from rhesus monkeys (n=43) was cross matched with cells from the DKO pigs. Kidneys from the DKO pigs were transplanted into rhesus monkeys (n=6) that had the least reactive cross matches. The rhesus recipients were immunosuppressed with anti-CD4 and anti-CD8 T cell depletion, anti-CD154, mycophenolic acid, and steroids.
Results:
Rhesus antibody binding to DKO cells is reduced, but all still have positive CDC and flow crossmatch. Three grafts were rejected early at 5, 6, and 6 days. Longer survival was achieved in recipients with survival to 35 days, 100 days, and 435 days. Each of the three early graft losses was secondary to IgM antibody mediated rejection. The 435-day graft loss occurred secondary to IgG antibody mediated rejection.
Conclusions:
Reducing xenoantigens in donor pigs and chemical immunosuppression can be used to achieve prolonged renal xenograft survival in a preclinical model, suggesting that if a negative crossmatch can be obtained for humans that prolonged survival could be achieved.
Mini-Abstract
Xenotransplantation is hampered by the presence of pre-formed antibodies. Here we reduced xenoantigen expression on pig kidneys through genetic engineering and identified ideal donor-recipient pairs through cross-matching. The outcomes of six pig to non-human primate life-sustaining kidney xenotransplants are presented.
Introduction
Kidney transplantation is the preferred therapy for end-stage renal disease. The paradigm of identifying appropriate donor recipient pairs through histocompatibility testing and then using chemical immunosuppression to prevent T cell mediated rejection revolutionized renal transplantation(1, 2). The development of calcineurin inhibition and antiproliferative based immunosuppression regimens improved one-year patient and graft survival rates to more than 90%(3). The success of this paradigm gives rise to the major limitation of kidney transplantation, the shortage of human kidney donors. More than 100,000 patients are currently on the UNOS waitlist and many of those patients will die never receiving a transplant(4).
Xenotransplantation using pig organs could eliminate this shortage since pigs are anatomically similar to humans, have a short gestation period (114 days), reach adult sizes quickly (3–4 months), and are amenable to genome editing(5–10). Xenotransplantation has not progressed to the clinic mainly because humans have preformed antibodies that bind to pig cells and cause early antibody mediated rejection (AMR), leading to graft failure(11, 12). Initial efforts to eliminate xenoreactive antibody as a barrier to xenotransplantation focused on the development of GGTA1 knockout (KO) pigs, eliminating the xenoantigen to which 70–90% of xenoreactive antibodies bound. GGTA1 KO pig kidneys transplanted into NHPs treated with RATG, tacrolimus, mycophenolic acid, and steroids, were rejected by AMR over 6–17 days(13). The search for additional xenoantigens that could serve as targets for further genetic modification in donor pigs, revealed the glycan antigen Sda to be xenoreactive for both humans and primates (14, 15). Sda is produced by the B4GALNT2 enzyme that is present in pigs and deleted in humans. An additional xenoantigen for humans and not primates is N-glycolylneuraminic acid, which is produced by the CMAH gene.
Nuclease based genome editing has made it possible to perform multiplex genome editing in the pig so that multiple genes can be deleted in s single reaction(10, 21). We used CRISPR/Cas to delete three genes that produced enzymes responsible for the production of glycan xenoantigens: a-gal (GGTA1), N-glycolylneuraminic acid (CMAH), and Sda antigen (B4GALNT2). CDC and flow cytometric crossmatches with TKO PBMCs and serum from patients on the kidney transplant waitlist showed that 50% of unsensitized patients had a negative crossmatch to the TKO pig(10, 22, 23).
Recent efforts in preclinical xenotransplantation have focused on the use of pigs with GGTA1 KO and human complement regulatory transgenes (CD46/55) and thromboregulatory transgenes(16–19). Recently we showed that GGTA1 KO/CD55 transgenic pig kidney can provide prolonged survival (>300 days) using T cell depletion and chemical immunosuppression. Graft failure was still due to AMR, suggesting that xenoreactive antibodies were still the limiting factor in xenograft survival(20).
Now that there are pigs with a negative crossmatch to humans, we wanted to determine whether pig kidneys with xenoantigen reduction and no human complement regulatory transgenes could be transplanted into Rhesus monkeys to achieve prolonged survival using chemical immunosuppression.
We created GGTA1/B4GALNT2 KO pigs and transplanted them into Rhesus monkeys who received T cell depletion and chemical immunosuppression. We were able to achieve life-sustaining xenograft survival of 435 days in a Rhesus monkey, with two other grafts surviving 35 and 100 days. Each xenografted kidney was analyzed at explant and revealed that AMR was still the cause of graft failure. These results suggest that simple xenoantigen deletion in donor pigs can allow for long-term xenograft survival if the level of recipient xenoreactive antibody is very low or absent.
Materials and Methods
Animals.
Animal work was performed under IACUC-approved protocols. CRISPR/Cas9 was used to generate GGTA1 and B4GALNT2 gene edited cells as described previously(24). gRNA targets included AATGAATGTCAAAGGAAGAG for in GGTA1. The underlined ATG represents the start codon of the open reading frame. For B4GalNT2, gRNA targeted exon2 (5’-GTGCTTTTGGTCCTGAGCGT-3’ and 5’-CCGATTTCTACGGTGAAAAC-3’), and exon 3 (5’-GCATCAGGAAAGCTAACTT-3’). We used multiple gRNA to increase the efficiency of knockouts. Somatic cell nuclear transfer was performed as described to create GGTA1/B4GALNT2 KO pigs(25).
DNA Sequencing of cloned pigs.
DNA sequencing analysis of gRNA/Cas9 targeted GGTA1 and B4GalNT2 regions in the cloned pig genomic DNA from the cloned pig was extracted using GenElute Mammalian Genomic DNA Miniprep Kit (Sigma, St. Louis MO). PCR was performed with GGTA1 and B4GalNT2-specific primer pairs, respectively. The primers were designed to flank the gRNA/Cas9 target sites and amplified 428 bases GGTA1, and 530 bases of B4GalNT2. Cells with durable homozygous disruptions in all GGTA1 and B4GalNT2 genes were selected.
Preparation and phenotyping of PBMCs
Whole blood was obtained in acid-citrate-dextrose (ACD) and prepared using Ficoll- Paque Plus (GE Healthcare) to isolate PBMCs. Cells were stained with the lectin isolectin Griffonia simplicifolia, GS-IB4, Alexa Fluor 488 (Molecular Probes, Grand Island, NY) and Dolichos biflorus agglutinin. (DBA lectin) labeled with Flourescein (Vector laboratories Burlingame, CA) and compared to cells alone. All cells were suspended at 2 X 106 in HBSS and incubated for 30 minutes at room temperature with appropriate lectin. Cells were washed with blocking buffer. Flow cytometric analysis was completed on BD Accuri C6 flow cytometer with the C6 Software (BD Biosciences, San Jose, CA, USA).
Tissue H&E and immunofluorescence confocal imaging.
Biopsy and nephrectomy specimens were evaluated for pathology using H&E staining, and immunofluorescence using confocal microscopy. Mounted tissues were blocked in Odyssey blocking buffer (LI-Cor Biosciences, Lincoln, NE, USA) in HBSS for 1 h. The slides were then fixed in 4% paraformaldehyde for 10 min. DAPI (Invitrogen) was added to all slides for 1 min as a nuclear stain followed by two 0.1% HBSS washes. Tissues were mounted in ProLong Gold (Invitrogen). Confocal microscopy was performed using an Olympus FV1000 (Olympus America Inc., Center Valley, PA, USA).
Antibody Binding to Porcine PBMCs.
Sera were obtained from Rhesus monkeys in successive batches (total n=43) Yerkes Primate Colony, Atlanta Ga). Pig PBMCs were isolated using Ficoll-Paque Plus (GE Healthcare, Pittsburgh, PA) and suspended in EX-CELL 610-HSF Serum Free Media (Sigma, St. Louis, MO). A 25 % mixture of heat-inactivated serum and 2 X 105 cells in EX-CELL with 0.1% sodium azide was incubated for 30 minutes at 4°C. Cells were washed three times with EX-CELL plus azide and stained with goat anti-human IgG Alexa Fluor 488 and donkey antihuman IgM Alexa Fluor 488 (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for 30 minutes at 4°C. Cells were washed using EX-CELL and azide as above, and flow cytometric analysis completed on BD Accuri C6 flow cytometer.
Pig-to-Rhesus renal transplantation.
A GGTA1/B4GalNT2 KO pig underwent bilateral nephrectomy on the day of transplantation. Rhesus macaques then underwent bilateral nephrectomies followed by life-sustaining renal transplantation using a GGTA1/B4GalNT2 KO pig kidney. Operations were performed in accordance with Institutional Animal Care and Use Committee regulations. Postoperatively, graft function was monitored with daily urine output assessment and weekly serum chemistries. Quantitative cytomegalovirus (CMV) were measured using a polymerase chain reaction (PCR)-based assay. Ultrasound- guided biopsies were performed postoperatively.
Treatment regimen.
Recipients underwent T-cell depletion using anti-CD4 and anti-CD8 mAb plus costimulation blockade with anti-CD154, and daily mycophenolic acid and steroids. T cell depletion began 3 days prior to transplantation with a one-time dose of anti-CD4 50 mg/kg IV (cloneCD4R1; NIH Nonhuman Primate Reagent Resource, Boston, MA, USA). Anti- CD8 (clone M-T807R1; NIH Nonhuman Primate Reagent Resource, Boston, MA, USA) was given on postoperative day 0 at 50 mg/kg IV. Anti-CD154 (5c8; NIH Nonhuman Primate Reagent Resource, Boston, MA) was given on days 0, 7, 14, and then biweekly at 20 mg/kg IV. Prophylactic enrofloxacin, fluconazole, and ganciclovir were started on the day of transplant. Fluconazole and enrofloxacin were discontinued when flow cytometry demonstrated sustained T cell reconstitution. Ganciclovir was continued until CMV titers were negative on two consecutive measurements. CMV assays wereperformed as previously described(18). Weekly subcutaneous Epogen injections were started for hemoglobin less than 9.5 g/dL and discontinued when hemoglobin reached 12 g/dL.
Results
Production of GGTA1/B4GALNT2 KO pigs
GGTA1 and B4GALNT2 genes are responsible for production of xenoantigens α-gal and Sda, which are involved in humoral rejection of pig xenografts. Fetal fibroblasts were transfected with plasmids encoding the Cas9 endonuclease and gRNA specific for GGTA1 and B4GALNT2 genes. Fetal fibroblasts that were confirmed to be homozygous for GGTA1 and B4GALNT2 deletions were used in SCNT. Twenty-eight embryo transfers have resulted in 22 pregnancies for a pregnancy rate of 79%. 13 of the 22 pregnancies were carried to term and resulted in 48 total piglets with 40 piglets surviving (83% viability).
The regions encompassing the GGTA1 and B4GALNT2 target sites were evaluated using DNA sequence analysis and also PCR originating at cut site (Figure 1A-B). PBMCs from a single GGTA1/B4GALNT2 KO pig were subjected to phenotypic analysis, and wild type pig PBMCs were used as a control. PBMCs of the GGTA1/B4GALNT2 KO were completely devoid of α-gal or Sda, while the controls were strongly positive for both antigens (Figure 1C).
FIGURE 1.
Genotype and phenotype of double KO pig. (A) GGTA1 and (B) B4GalNT2 gene PCR product noted in wild type, lane 3 (arrow), but absent following Cas9 disruption, lanes 1 and 2. Top band shows internal control, actin PCR product, along top and alone in lane 4. (C) To demonstrate absence of targeted gene activity, flow cytometric PBMC phenotype of double KO pig is stained with IB4 lectin (binding αGal) and DBA lectin (binding SDa antigen). The bottom pair of histograms demonstration of overlay with cells alone controls. (D) Confocal microscopy of renal tissue from wild type and double KO tissue. Top row in wild type and KO pigs respectively, show IB4 and DBA stains alone and bottom row shows overlay with dapi nuclear stain. Images capture a glomerulus. White bar indicates 50uM.
GGTA1/B4GALNT2 KO pig kidneys are devoid of xenoantigens α-gal and Sda.
In order to minimize xenoreactive binding to a pig renal xenograft, the GGTA1/B4GALNT2 KO pigs must not express the two deleted xenoantigens α-gal and Sda. Pig kidneys from wild type and GGTA1/B4GALNT2 KO pigs were evaluated for expression of xenoantigens α-gal and Sda using lectin staining and confocal microscopy. Wild-type pig kidneys express α-gal produced by GGTA1, and Sda produced by B4GALNT2 ubiquitously. The GGTA1/B4GALNT2 KO pig kidney was completely devoid of both α-gal and Sda (Figure 1D).
Rhesus monkeys have reduced anti-pig PBMC antibodies to GGTA1/B4GALNT2 KO compared to GGTA1 KO or GGTA1/CMAH/B4GALNT2 KO PBMCs.
Rhesus monkeys were crossmatched with PBMC from GGTA1 KO, GGTA1/B4GALNT2 KO and GGTA1/CMAH/B4GALNT2 KO PBMCs (Figure 2A). In each monkey, the crossmatch revealed less IgM and IgG binding to GGTA1/B4GALNT2 KO than GGTA1 KO, or GGTA1/CMAH/B4GALNT2 KO PBMCs (Figure 2B). None of the Rhesus had a negative IgM or IgG flow crossmatch (Figure 3A), and every Rhesus tested had a CDC positive crossmatch.
FIGURE 2.
Rhesus Macaque IgG and IgM antibody binding to pig PBMC. (A) Flow cytometric histograms showing IgG, top row, and IgM, bottom row, binding to three genetically modified pig backgrounds. Each representative histogram is overlaid on secondary alone in gray. (B) Flow cytometric MFI of IgG and IgM antibody binding of a group of normal Rhesus Macaque animals (n=14) is shown along X axis and Y axis respectively. GGTA1, GGTA1/B4GalNT2, and lastly GGTA1/CMAH/B4GalNT PBMC antibody binding is demonstrated. Box in left lower corner demonstrates region of estimated negative crossmatch.
FIGURE 3.
Flow cytometric crossmatch and immunosuppression protocol employed in transplants. (A) IgG and IgM flow cytometric MFI and demonstrated for six recipients compared to a subset of screened Rhesus Macaques. All recipients were selected for low pre-transplant IgG antibody binding, but none achieved negative crossmatch as demonstrated by black dotted line. (B) Immunosuppression regimen used in all of the recipients.
Survival of recipients of GGTA1/B4GALNT2 KO pig kidneys
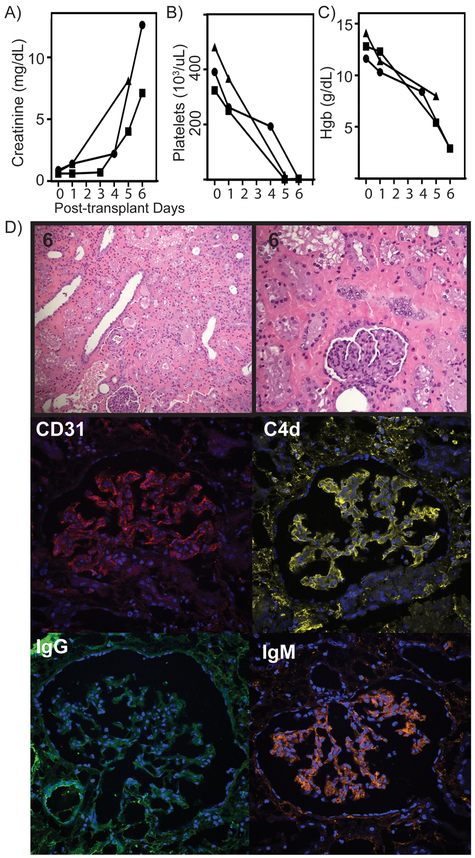
Since the Rhesus had the best crossmatch results with the GGTA1/B4GALNT2 KO pigs, these pigs were used as the kidney donors for this series of six transplants (Figure 3B). Three recipients rejected their kidneys in the first week post-transplant (5,6,6 days) characterized by elevated creatinine, thrombocytopenia, and severe anemia (Figure 3A-C). One recipient rejected at 35 days, and another rejected at 100 days (Figure 5A). The sixth recipient rejected the kidney at 435 days (Figure 6A). None of the three long-term survivors experienced significant anemia or thrombocytopenia. Although, all six recipients regardless of outcome, had elevated creatinine and some degree of thrombocytopenia during first week post-transplant. (Figure 5B, 6B and 6D).
FIGURE 5.
Laboratory and tissue analysis of grafts surviving 35-100 days. (A) Creatinine is shown to be well controlled until acutely rising at time of graft failure. (B) Following initial challenge, there is minimal significant thrombocytopenia noted during course of the transplants. (C) H&E of 100 day explant graft tissue. Confocal microscopy showing tissue CD31, C4d, IgG and IgM deposition in the (D) 35 day explant and (E.) and 100 day explant.
FIGURE 6.
Life-supporting renal function and histology for graft living 435 days. (A) Creatinine was adequately cleared for over one year following transplant and then trended upward. (B) Following first week thrombocytopenia, normal platelet levels were maintained over course of graft following. (C) Unlike early graft failure recipients, this graft did not encounter anemia and shown by hemoglobin levels. (D) H&E biopsies from day 170, 240 and explanted tissue at day 435 are shown. Confocal microscopy at time of explant shows CD31, C4d, IgG and IgM staining. (E.) Proteinuria was not encountered as shown by urine protein.
Pathophysiology of graft failure
Pathological analysis of the three kidneys that were rejected at 5, 6, and 6 days was similar and revealed interstitial hemorrhage, IgG, IgM, and C4d in the glomeruli. There was little to no cellular infiltrate in the early rejectors (Figure 4D). The two kidneys rejected at 35 and 100 days had signs of AMR with glomerular plugging and thrombotic microangiopathy (Figure 5C). In addition, there was deposition of IgG, IgM, and C4d in the glomeruli (Figure 5D-E). The graft rejected at 435 days had normal biopsy at 170 days, but by day 270 days signs of glomerulopathy were preset with thickened glomerular membranes. The rejected explant showed advanced glomerulopathy with weak, diffusely positive IgG, IgM, and C4d staining in the glomeruli (Figure 6D). The renal xenograft did not experience significant proteinuria, suggesting that failure to properly filter and reabsorb protein may be due to immunologic failure, not a physiologic incompatibility (Figure 6E).
FIGURE 4.
Laboratory and tissue analysis of kidney recipients with graft failure in less than one week. (A) Creatinine shown for three recipients of this group showing elevation and graft failure by day 6. (B) Significant thrombocytopenia was developed in all recipients as shown by platelet count. (C) Severe anemia developed quickly in this subset of recipients as shown by hemoglobin levels during first week. (D) H&E stain at low and high magnification of representative tissue. Confocal microscopy of representative glomeruli showing, CD31 endothelial marker, C4d complement activation product, IgG and IgM binding. Significant IgM and C4d binding was noted in all grafts.
Discussion
Renal transplantation has revolutionized the treatment of ESRD with regards to patient quality and duration of life. The shortage of donor organs has limited the availablility of this therapy to patients with ESRD. Xenotransplantation using pig organs could eliminate this shortage, but our inability to combat the AMR that occurs with the transplantation of crossmatch positive pig kidneys has prevented progression to the clinic.
The introduction of GGTA1 KO pigs eliminated hyperacute rejection, but baboons immunosuppressed with RATG, tacrolimus, mycophenolate, and steroids, were rejected in 6–16 days by non-α-gal xenoreactive antibodies(13). Since there were no known xenoantigens to delete in the pig, investigators added human complement regulatory transgenes and later human thromboregulatory transgenes to combat the impact of AMR(26–28). McGregor showed that incorporation of the human CD55 transgene eliminated early graft failure and reduced complement activation in cardiac xenografts transplanted into baboons but failed to prolong graft survival(28, 29). We used GGTA1 KO/CD55 transgenic pig kidneys transplanted into rhesus monkeys immunosuppressed with immunosuppression either identical or very similar to the regimen used in the monkeys in this manuscript and found that prolonged survival was dependent upon selecting recipients with low levels of xenoreactive antibodies, and CD4 T cell depletion(20).
Generally stated, the results from the GGTA1 KO/CD55 transgenic pig kidney transplant series show that recipients with low levels of xenoreactive antibody can achieve prolonged survival with chemical immunosuppression. These results suggest that xenotransplantation could follow the paradigm developed for prolonged survival in allotransplantation, namely the identification of appropriate donor:recipient pairs with histocompatibility testing, followed by appropriate chemical immunosuppression. It should be feasible to use genome editing to delete xenoantigens to create donor pigs to which recipients have no preformed antibodies. The identity of the remaining xenoantigens on the pig cell to which primate antibodies bind is unknown, but is a subject of active investigation in our laboratory.
In order to develop appropriate donor pigs for transplantation into humans, we used CRISPR/Cas to create triple xenoantigen KO pigs. We deleted enzymes producing α-gal, N-glycolylneuraminic acid, and Sda antigen to produce the GGTA1/CMAH/B4GALNT2 KO pig(10). Crossmatch results of over 800 patients on the kidney transplant waitlist showed that more than 30% of patients had no detectable xenoreactive antibodies to PBMCs from these new pigs(22). These new pigs provide the opportunity to test the transplant paradigm of transplanting crossmatch negative pig kidneys into patients and then using chemical immunosuppression to achieve prolonged post-transplant survival(23). The studies described in this manuscript represent the initial attempt to test the paradigm of using histocompatibility testing and chemical immunosuppression in a preclinical pig-to-NHP model. The GGTA1/CMAH/B4GALNT2 KO pig cannot be tested in the rhesus model because the crossmatch was significantly worse than the GGTA1/B4GALNT2 KO crossmatch. The reasons for this are unclear, but the rhesus has a functional CMAH gene and as such it expresses human xenoantigen Neu5Gc.
The DKO pig kidney had a very improved crossmatch when compared to the GGTA1 KO pig, but it was still positive based on the presence of anti-pig IgM. The xenoreactive IgM played a significant role in early graft injury, as each of the recipients experienced significant graft injury at 5–6 days post-transplant, manifested by elevated creatinine, thrombocytopenia, and severe anemia. Three of the six grafts did not recover, and were rejected early (5–6 days). The other three grafts were able to recover and provide more prolonged graft survival, but each of the remaining three grafts was eventually lost due to AMR. Our results regarding AMR as the cause of renal xenograft failure are consistent with the findings of our recent experience with long surviving renal xenografts with GGTA1 KO/CD55 transgenic pig kidneys, where all of the grafts were lost to some form of AMR either early or late. Cooper has also had prolonged renal xenograft survival (8 months in two recipients) using GGTA1 KO pigs with multiple human transgenes as donors, but explant pathology showed signs of chronic AMR with significant transplant glomerulopathy, tubular atrophy, and interstitial fibrosis. Taken together, these results suggest that xenoreactive antibodies dominate the pathophysiology of graft failure, and the addition of human complement regulatory, thromboregulatory, or other transgenes fails to alter the pathophysiology of graft failure.
The facts that human transgenes have not altered graft survival or pathophysiology, and that xenoreactive antibodies are still the cause of xenograft failure, have two important implications for clinical implementation of xenotransplantation. The first implication is that strict adherence to crossmatching principles where only recipients that have a negative crossmatch are considered for initial transplants is likely to be critical to success in achieving prolonged renal xenograft survival in human patients. Starting with no-preformed antibodies in allotransplantation has been a successful strategy for more than 40 years, and now clear guidelines are emerging for preventing early T cell mediated rejection that is required for the formation of de novo donor specific antibodies(30). De novo donor specific antibodies in renal allotransplantation are largely directed against class II HLA(31–33), and recently we have shown that anti-HLA DQ antibodies cross-react with SLA class II DQ in an epitope-restricted pattern(34). Given this information it seems likely that if too little chemical immunosuppression is used in a renal xenograft that the recipient would develop anti-SLA-DQ antibodies. Robust data from the FKC-008 study establishes the trough levels of tacrolimus required in conjunction with mycophenolate mofetil and steroids are required to prevent subclinical T cell mediated rejection and severely minimize the development of de novo donor specific antibody(35).
In order to improve the survival of pig kidneys transplanted into rhesus monkeys using only antigen deletion, two approaches come to mind, the first is to identify other candidate xenoantigens in the donor pig that could be eliminated through genome editing to create a crossmatch negative pig with regards to the rhesus monkey. The other option would be to try complement inhibition for the early post-transplant period so that the IgM barrier could be overcome during the critical first few weeks post-transplant. In clinical renal allotransplantation, plasmapheresis with IVIG is used to desensitize patients(36, 37), but this will be difficult in xenotransplantation in the human as well as the primate since IVIG contains many xenoreactive antibodies that will attack the xenograft.
Another consideration for implementation of clinical renal xenotransplantation is whether it is necessary to use tacrolimus in an immunosuppressive regimen for the preclinical model rather than anti-CD154 prior to attempting clinical cases. Currently there are no rhesus monkeys that have a negative crossmatch to the GGTA1/B4GALNT2 KO pigs, which is not reflective of the situation for the GGTA1/CMAH/B4GALNT2 KO pig. The use of conventional tacrolimus based allograft immunosuppression has been very successful in renal allotransplantation for crossmatch negative transplantation, but far less effective for crossmatch positive transplantation(37, 38). Conventional immunosuppression has been combined with desensitization protocols in clinical allotransplantation(37, 38), which are not practical for the pig-to-NHP model, especially in light of the fact that the use of IVIG is precluded in xenotransplantation since it will provide a source of additional xenoreactive antibodies and will likely hasten the onset of AMR (personal unpublished observations).
This study is an important landmark for clinical implementation of renal xenotransplantation because it shows that the simplest genome editing approach, namely using xenoantigen deletion alone, can result in prolonged graft survival in a preclinical NHP model. The GGTA1/CMAH/B4GALNT2 KO pig kidney has a negative crossmatch negative for many patients and could be used in clinical pilot trials(22, 23). The use of a xenoantigen reduction strategy without the use of transgenes that interfere with the recipient immune system’s ability to interact with the xenograft keeps the protective immunity within the xenograft intact, so that the recipient immune system is able to fight off infection should it occur. This consideration will be particularly important for the initial cases of clinical xenotransplantation, where public safety particularly with regards to zoonotic risk will be a primary concern for all interested parties(41–43). The simplistic approach to genome editing for the initial clinical trials will also reduce concerns over evaluating the potential issue of off target effects of genome editing that will come with the use of more complicated genome editing strategies(44).
Acknowledgments
Conflicts of Interest and Sources of Funding
This work was funded by grants from the National Institutes of Health (1R01AI26322) and ORIP/OD P51OD011132 and portions were funded by the Indiana University Health Transplant Institute. D.E. Eckhoff and A.J. Tector have had funding from United Therapeutics. A.J. Tector has applied for and been awarded patents related to genetic engineering of pigs for use in xenotransplantation. A.J. Tector has also founded XenoBridge llc. Other authors do not claim conflicts.
References
- 1.Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280(14):735–9. [DOI] [PubMed] [Google Scholar]
- 2.Starzl TE, Marchioro TL, Terasaki PI, Porter KA, Faris TD, Herrmann TJ, et al. Chronic survival after human renal homotransplantation. Lymphocyte-antigen matching, pathology and influence of thymectomy. Ann Surg. 1965;162(4):749–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenthal JT, Hakala TR, Iwatsuki S, Shaw BW Jr., Starzl TE. Cadaveric renal transplantation under cyclosporine-steroid therapy. Surg Gynecol Obstet. 1983;157(4):309–15. [PMC free article] [PubMed] [Google Scholar]
- 4.Wijkstrom M, Iwase H, Paris W, Hara H, Ezzelarab M, Cooper DK. Renal xenotransplantation: experimental progress and clinical prospects. Kidney Int. 2017;91(4):790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper DK, Ekser B, Ramsoondar J, Phelps C, Ayares D. The role of genetically engineered pigs in xenotransplantation research. J Pathol. 2016;238(2):288–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wells KD, Prather RS. Genome-editing technologies to improve research, reproduction, and production in pigs. Mol Reprod Dev. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, Kraner-Scheiber S, Petersen B, Rieblinger B, Buermann A, Flisikowska T, et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci Rep. 2016;6:29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nottle MB, Salvaris EJ, Fisicaro N, McIlfatrick S, Vassiliev I, Hawthorne WJ, et al. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI- dCas9. Sci Rep. 2017;7(1):8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan J, Li H, Xu K, Wu T, Wei J, Zhou R, et al. Highly efficient CRISPR/Cas9-mediated transgene knockin at the H11 locus in pigs. Sci Rep. 2015;5:14253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Estrada JL, Burlak C, Montgomery J, Butler JR, Santos RM, et al. Efficient generation of genetically distinct pigs in a single pregnancy using multiplexed single-guide RNA and carbohydrate selection. Xenotransplantation. 2015;22(1):20–31. [DOI] [PubMed] [Google Scholar]
- 11.Starzl TE, Rao AS, Murase N, Fung J, Demetris AJ. Will xenotransplantation ever be feasible? J Am Coll Surg. 1998;186(4):383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Valdivia LA, Murase N, Demetris AJ, Fontes P, Rao AS, et al. The biological basis of and strategies for clinical xenotransplantation. Immunol Rev. 1994;141:213–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005;11(12):1295–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Byrne GW, Du Z, Stalboerger P, Kogelberg H, McGregor CG. Cloning and expression of porcine beta1,4 N-acetylgalactosaminyl transferase encoding a new xenoreactive antigen. Xenotransplantation. 2014;21(6):543–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne GW, Stalboerger PG, Du Z, Davis TR, McGregor CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91(3):287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwase H, Hara H, Ezzelarab M, Li T, Zhang Z, Gao B, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwase H, Liu H, Wijkstrom M, Zhou H, Singh J, Hara H, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22(4):302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML 3rd, Lewis BG, et al. One- year heterotopic cardiac xenograft survival in a pig to baboon model. Am J Transplant. 2014;14(2):488–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohiuddin MM, Singh AK, Corcoran PC, Hoyt RF, Thomas ML 3rd, Ayares D, et al. Genetically engineered pigs and target-specific immunomodulation provide significant graft survival and hope for clinical cardiac xenotransplantation. J Thorac Cardiovasc Surg. 2014;148(3):1106–13; discussion 13–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higginbotham L, Mathews D, Breeden CA, Song M, Farris AB 3rd, Larsen CP, et al. Pre- transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation. 2015;22(3):221–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz AJ, Li P, Estrada JL, Sidner RA, Chihara RK, Downey SM, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20(1):27–35. [DOI] [PubMed] [Google Scholar]
- 22.Martens GR, Reyes LM, Butler JR, Ladowski JM, Estrada JL, Sidner RA, et al. Humoral Reactivity of Renal Transplant-Waitlisted Patients to Cells From GGTA1/CMAH/B4GalNT2, and SLA Class I Knockout Pigs. Transplantation. 2017;101(4):e86–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cowan PJ, Ierino FL. Reducing the Threshold for Clinical Renal Xenotransplantation. Transplantation. 2017;101(4):692–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22(3):194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estrada J, Sommer J, Collins B, Mir B, Martin A, York A, et al. Swine generated by somatic cell nuclear transfer have increased incidence of intrauterine growth restriction (IUGR). Cloning Stem Cells. 2007;9(2):229–36. [DOI] [PubMed] [Google Scholar]
- 26.Le Bas-Bernardet S, Tillou X, Branchereau J, Dilek N, Poirier N, Chatelais M, et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am J Transplant. 2015;15(2):358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowan PJ, Robson SC. Progress towards overcoming coagulopathy and hemostatic dysfunction associated with xenotransplantation. Int J Surg. 2015;23(Pt B):296–300. [DOI] [PubMed] [Google Scholar]
- 28.McGregor CG, Ricci D, Miyagi N, Stalboerger PG, Du Z, Oehler EA, et al. Human CD55 expression blocks hyperacute rejection and restricts complement activation in Gal knockout cardiac xenografts. Transplantation. 2012;93(7):686–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tazelaar HD, Byrne GW, McGregor CG. Comparison of Gal and non-Gal-mediated cardiac xenograft rejection. Transplantation. 2011;91(9):968–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thaunat O, Koenig A, Leibler C, Grimbert P. Effect of Immunosuppressive Drugs on Humoral Allosensitization after Kidney Transplant. J Am Soc Nephrol. 2016;27(7):1890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, et al. Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant. 2013;13(12):3114–22. [DOI] [PubMed] [Google Scholar]
- 32.Nickerson PW, Rush DN. Begin at the Beginning to Prevent the End. J Am Soc Nephrol. 2015;26(7):1483–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nevins TE, Robiner WN, Thomas W. Predictive patterns of early medication adherence in renal transplantation. Transplantation. 2014;98(8):878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ladowski JM, Reyes LM, Martens GR, Butler JR, Wang ZY, Eckhoff DE, et al. Swine Leukocyte Antigen Class II Is a Xenoantigen. Transplantation. 2018;102(2):249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rush D, Arlen D, Boucher A, Busque S, Cockfield SM, Girardin C, et al. Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. Am J Transplant. 2007;7(11):2538–45. [DOI] [PubMed] [Google Scholar]
- 36.Kahwaji J, Jordan SC, Najjar R, Wongsaroj P, Choi J, Peng A, et al. Six-year outcomes in broadly HLA-sensitized living donor transplant recipients desensitized with intravenous immunoglobulin and rituximab. Transpl Int. 2016;29(12):1276–85. [DOI] [PubMed] [Google Scholar]
- 37.Zachary AA, Montgomery RA, Leffell MS. Factors associated with and predictive of persistence of donor-specific antibody after treatment with plasmapheresis and intravenous immunoglobulin. Hum Immunol. 2005;66(4):364–70. [DOI] [PubMed] [Google Scholar]
- 38.Jordan SC, Choi J, Vo A. Kidney transplantation in highly sensitized patients. Br Med Bull. 2015;114(1):113–25. [DOI] [PubMed] [Google Scholar]
- 39.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365(4):318–26. [DOI] [PubMed] [Google Scholar]
- 40.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med. 2008;359(3):242–51. [DOI] [PubMed] [Google Scholar]
- 41.Fishman JA. Infectious Disease Risks in Xenotransplantation. Am J Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 42.Denner J, Tonjes RR, Takeuchi Y, Fishman J, Scobie L. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 5: recipient monitoring and response plan for preventing disease transmission. Xenotransplantation. 2016;23(1):53–9. [DOI] [PubMed] [Google Scholar]
- 43.Spizzo T, Denner J, Gazda L, Martin M, Nathu D, Scobie L, et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 2a: source pigs-- preventing xenozoonoses. Xenotransplantation. 2016;23(1):25–31. [DOI] [PubMed] [Google Scholar]
- 44.Cowan PJ, Ayares D, Wolf E, Cooper DK. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes--Chapter 2b: genetically modified source pigs. Xenotransplantation. 2016;23(1):32–7. [DOI] [PubMed] [Google Scholar]