Abstract
Background
Chondrosarcoma is the second most frequent primary sarcoma of bone and frequently occurs in the pelvis. Surgical resection is the primary treatment with the two main operative modalities being limb-sparing resection and amputation. Contemporary management has trended toward limb-sparing procedures; however, whether this approach has an adverse effect on long-term survival is unclear.
Questions/purposes
(1) What are the 5- and 10-year survival rates after limb-sparing surgery and amputation? (2) What factors are associated with survival after contemporary surgical management of pelvic chondrosarcoma?
Methods
The 2004-2014 National Cancer Database, a nationwide registry that includes approximately 70% of all new cancers in the United States with requirement for 90% followup, was reviewed for patients diagnosed with pelvic chondrosarcoma who had undergone limb-sparing surgery or amputation. To compare survival, patient demographics, tumor attributes, and treatment characteristics were used to generate one-to-one propensity score-matched cohorts. Other factors associated with survival were determined through multivariable Cox regression. Three hundred eighty-five patients (75%) underwent limb-sparing surgery and 131 (25%) underwent amputation. Propensity score matching resulted in two balanced cohorts of 131 patients.
Results
With the numbers available, we could not demonstrate a difference in overall survival between limb-sparing procedures and amputation. The 5-year survivorship was 70% (95% confidence interval [CI], 62%-79%) for limb-sparing surgery versus 70% (95% CI, 62%-79%) for amputation. The 10-year survivorship was 60% (95% CI, 48%-75%) for limb-sparing surgery versus 59% (95% CI, 48%-72%) for amputation. Kaplan-Meier survival analysis did not demonstrate a difference in survival (p = 0.9). Older age (hazard ratio [HR] = 1.029, p < 0.001), higher Charlson/Deyo comorbidity score (HR = 3.039, p = 0.004), higher grade (HR = 2.547, p = 0.005 for Grade 2; HR = 7.269, p < 0.001 for Grade 3; HR = 10.36, p < 0.001 for Grade 4), and positive surgical margins (HR = 1.61, p = 0.039) were associated with decreased survival.
Conclusions
Our findings support the trend toward increased use of limb-sparing surgery for patients with pelvic chondrosarcoma. Orthopaedic oncologists may use our results when counseling their patients regarding treatment options. However, the choice of limb-sparing surgery versus amputation for these challenging patients should still be a highly individualized decision with careful discussion between the patient and the surgeon.
Level of Evidence
Level III, therapeutic study.
Introduction
Chondrosarcoma is a malignant primary bone tumor with cartilaginous differentiation. It is the second most common primary bone tumor in adults and is located in the pelvis in 22% to 39% of patients [12, 29]. Pelvic bone neoplasms are difficult to resect with wide surgical margins as a result of their location, local extension, large size, and complex regional and local anatomy [18]. Affected patients have a poorer survivorship than has been observed with similar neoplasms in the extremities [2, 4, 8, 13, 14, 41].
Numerous reasons have been identified for this poor survivorship [4, 8, 12, 14, 18, 19, 29, 35, 37, 39, 41]. There is a frequent delay in diagnosis, as tumors can grow to a large size before causing symptoms due to the absence of major anatomic barriers to tumor extension in the pelvis [13]. The large size of these lesions, which itself is a well-known prognostic factor for worse survival, leads to poor compartmentalization in the pelvis [4, 35]; they may cross the sacroiliac joint or involve the surrounding neurovascular and visceral structures [8, 12]. This contributes to the difficulty of achieving adequate margins and can also limit the ability to establish reconstructions that are durable and allow maximum patient function [8, 19]. Resections are also complicated by high rates of infection, local recurrence, and poor wound healing [12].
Chondrosarcoma is distinct from other primary bone tumors because it is both radioresistant and chemoresistant; its primary treatment is therefore surgical resection involving either limb-sparing surgery or amputation [35]. As a result of the complexity of pelvic anatomy, most pelvic chondrosarcomas were treated with amputation in the past [4, 12], which was associated with reduced function [41, 42]. Recent advances in imaging and surgical planning have expanded indications for limb-sparing surgery [4]. However, as a result of the low incidence of chondrosarcoma, studying the impact of limb-sparing surgery on survival has involved small retrospective series, often spanning periods of > 20 years and with limited ability to match for confounding prognostic variables such as grade [4, 9, 12, 13, 22, 29].
Therefore, the primary purpose of this investigation was to use a nationwide oncology registry to compare overall survival between limb-sparing surgery and amputation for pelvic chondrosarcoma. We asked two questions: (1) What are the 5- and 10-year survival rates of limb-sparing surgery and amputation after controlling for potentially confounding variables? (2) What factors are associated with survival after surgical management of pelvic chondrosarcoma?
Materials and Methods
Data from 2004 to 2014 were acquired from the bone and joint sarcoma participant use file of the National Cancer Database (NCDB), a collaborative effort between the American Cancer Society and the American College of Surgeons’ Commission on Cancer. Established in 1989, the NCDB is a nationwide cancer registry that includes information on approximately 70% of all new cancer diagnoses in the United States from > 1500 Commission on Cancer-approved centers. Hospitals submitting patient data to the NCDB are required to maintain a followup rate of 90%, although there have been variations to how well this is followed [52]. Compared with other national cancer registries such as the Surveillance, Epidemiology, and End Results (SEER) database, the NCDB records several key variables including systemic adjuvant therapies, margin status, surgical approach, hospital identifiers (blinded), and hospital characteristics [33]. Although the NCDB currently contains the largest number of patients compared with other cancer registries, the requirement to be an accredited cancer center may restrict its generalizability to the entire population [33]. Despite this potential limitation, the NCDB has been utilized for an increasing number of investigations in sarcoma [11, 16, 20, 21, 28, 31, 36, 40, 51] as a result of the detailed information it collects on treatment variables, which facilitates its use as a benchmark for improvement in performance [33]. Because the NCDB does not contain any patient-identifying information and therefore does not constitute human subjects research, our study was granted an exemption from institutional review board review.
We included patients diagnosed with pelvic chondrosarcoma between 2004 and 2014 who had undergone limb-sparing surgery or amputation. Exclusion criteria were missing vital status (unknown whether deceased or alive), missing margin status, and multiple primary tumors.
Limb-sparing surgery (radical resection with limb salvage or internal hemipelvectomy) and amputation (hindquarter amputation and hemipelvectomy, used synonymously in the NCDB) were identified using previously validated definitions [40]. Relevant patient demographic information (age, sex, race, Charlson/Deyo comorbidity score, zip code-level median household income, percentage of population with a high school degree, primary payer), tumor attributes (size, metastasis, grade), and treatment variables (hospital treatment volume, surgical margins, adjuvant radiation and chemotherapy) were extracted. Because the NCDB prohibits the reporting of parameter counts fewer than 10 (for example, “seven patients with Grade 4 tumors”), all such parameters were combined with other counts so that reported totals were > 10. This was the case for Charlson/Deyo score higher than 1, Grade 3 or 4 tumors, Stage 3 or 4 tumors, and radiation/chemotherapy. However, inferential statistics such as Cox regression were performed on the original separate levels (that is, Grade 3 and 4 tumors were separate covariates in the regression).
Of the 726 patients with pelvic chondrosarcoma managed with limb-sparing surgery or amputation identified within the NCDB, 516 patients met inclusion criteria (Fig. 1), and 210 were excluded as a result of missing vital status, margin status, and multiple primary tumors. Of these, 385 (75%) underwent limb-sparing surgery and 131 (25%) underwent amputation. Median followup was 49 months (interquartile range [IQR], 25-79) for patients undergoing limb-sparing surgery and 43 months (IQR, 20-82) for patients undergoing amputation. Mean age of the entire cohort was 50 years (SD 16) with patients being predominantly male (63%). Average tumor size was 10.3 cm (SD 6.0) with 24% of tumors being Grade 3 or 4 and 5% of patients presenting with American Joint Committee on Cancer Stage 3 or 4 cancer [3]. Surgical margins were positive in 15% of patients, and a minority of patients received adjuvant therapy (9%).
Fig. 1.
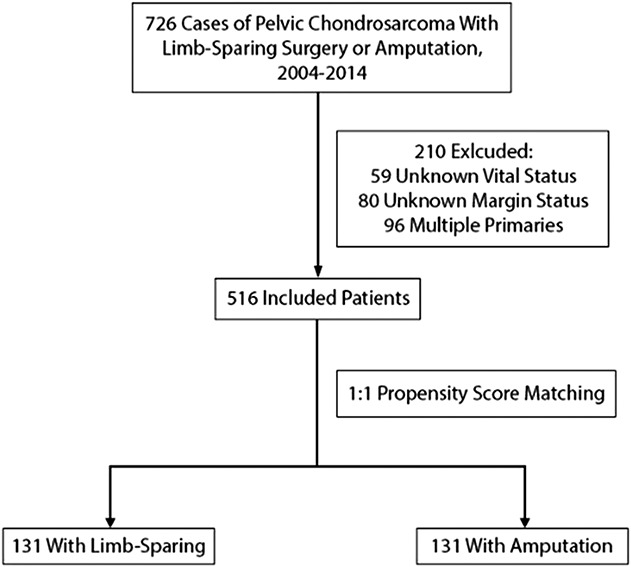
The CONSORT diagram demonstrates patient inclusion-exclusion criteria and propensity score matching.
The limb-sparing cohort had a higher proportion of females (40.0% versus 29.0% in amputation, p = 0.032), a lower proportion of patients with high-grade cancer (22% Grades 3 or 4 in limb-sparing versus 31% in amputation, p = 0.002), a lower proportion of Stage 3 or 4 cancer (4% versus 10%, p = 0.001), and smaller tumor size (9.6 cm versus 12.4 cm, p = 0.005) (Table 1). The limb-sparing group had a higher proportion of positive margins (17% versus 10%, p = 0.075). Propensity score matching resulted in two cohorts (limb-sparing surgery and amputation ) of 131 patients each with negligible differences in demographic, clinicopathologic, and treatment characteristics (Table 1). Visual inspection of the propensity score jitterplots and histogram (see Appendix, Supplemental Digital Content) demonstrated improved balance. The NCDB requires a 90% followup from its participating hospitals, regardless of a patient’s vital status or disease parameters; combined with multiple imputation to address missing data, we made the assumption that there was minimal differential loss to followup between limb-sparing and amputation cohorts, which would potentially introduce bias into our matched cohorts.
Table 1.
Demographic, clinicopathologic, and treatment characteristics of patients pre- and postpropensity score matching
Our primary outcome, overall survival, was defined as the patient’s vital status at last followup. All patients with unknown vital status (unknown whether dead or alive) at last contact were excluded from the analysis. The NCDB reports only overall survival, not cause-specific survival. Therefore, to remove causes of mortality from other malignancies, patients with primary malignancies other than pelvic chondrosarcoma were excluded following previously validated methodology [31]. To determine low-, intermediate-, and high-volume centers, the total volume of patients with pelvic chondrosarcoma between 2004 and 2014 for each facility was calculated. Those in the first 25% were labeled low-, between 25% and 75% intermediate-, and the top 25% high-volume centers. Within the NCDB, the margin status of surgical procedures is recorded as no residual tumor, residual tumor not otherwise specified, microscopic residual tumor, or macroscopic residual tumor. For the purpose of this analysis, a report of any residual tumor was considered a positive margin. All patients with missing margin status were excluded from analysis, because we defined margin status as an outcome and did not want to introduce uncertainty by imputing values considered an outcome.
To provide a comprehensive review, all histologic subtypes of chondrosarcoma were analyzed in the study (Appendix 1), which included conventional, juxtacortical, myxoid, mesenchymal, clear cell, and dedifferentiated chondrosarcomas. The sarcoma data in the NCDB are divided into two sections: (1) soft tissue; and (2) bone and joint. Because we wanted to focus specifically on osseous chondrosarcomas of the pelvis, our data query was strictly limited to the bone and joint section of the NCDB. Therefore, all patients with chondrosarcomas in our data set, including myxoid and mesenchymal chondrosarcoma, were osseous tumors, not soft tissue sarcomas. Case reports of myxoid chondrosarcoma and mesenchymal chondrosarcoma of bone exist in the literature but are rare [17, 53], and their frequencies were correspondingly low in our data set (83% conventional; 11% dedifferentiated; and 4% juxtacortical, myxoid, mesenchymal, and clear cell combined). In addition, the goal of our analysis was to compare survival between limb-sparing surgery and amputation, not between histologic subtypes. Through propensity score matching, we ensured that the distribution of subtypes was not different between the limb-sparing and amputation cohorts so that the results were not biased by a preponderance of a specific subtype.
A composite socioeconomic score (SES) was derived with methodology derived from the literature [15, 31]. The NCDB records median household income and percentage of the population with only a high school degree, scaled to 2012 national census data, and categorizes them into quartiles (1-4).
In particular, the NCDB estimates household income for each patient's area of residence by matching the zip code of the patient recorded at the time of diagnosis against files derived from the 2012 American Community Survey data, spanning years 2008 to 2012 and adjusted for 2012 inflation. A score of “1” corresponds to the lowest median household income and highest percentage of population with only a high school degree. We added the scores for median household income and percentage of population with a high school degree to calculate a composite SES score ranging from 2 to 8. These scores were then further categorized into four quartiles (2-3, 4-5, 6-7, 8 into SES 1-4).
Statistical Analysis
Missing variables were addressed through multiple imputation with number of imputations based on the proportion of missing variables [23, 47]. In the overall data set, the proportion of missing variables was 5.2% for tumor size 8.5% for stage, and 6.4% for grade. All other values needing imputation were missing for < 3% of patients. After obtaining a set of complete, imputed data sets, propensity scores for limb-sparing surgery or amputation were derived from variables with different frequencies as well as from those that were deemed relevant through review of other studies [4, 8, 9, 11-13, 19, 26, 29, 35, 45, 46, 48] and clinical judgment (age, gender, race, SES, Charlson/Deyo score, facility volume, grade, stage, tumor size). Matching was specifically not done for surgical margins, because we determined that margins are a surgical outcome, not a factor to be used to balance cohorts. Scores from each imputed data set were pooled according to methods from Mitra and Hill [24, 32]. Using these pooled scores, patients were matched one to one with a greedy nearest neighbor algorithm without replacement, which matches a treated to a control subject based on the closest propensity score [5]. Once a treated subject has been matched, that subject is no longer eligible for future matches for other control subjects [5]. Final cohort balance was assessed through visual inspection of propensity score jitterplots/histograms, which measure the distribution of propensity scores for control and treated subjects. If balanced, these distributions should appear similar [25]. A Kaplan-Meier survival analysis was then performed on the matched cohorts, and 5- and 10-year survival rates were determined for limb-sparing surgery and amputation. A stratified log-rank test was used for survival comparison to address matching, a methodology that has been validated for survival analysis in propensity score-matched cohorts [6].
To determine other factors associated with long-term survival, a multivariable Cox proportional hazard model was constructed on the complete data set with 121 patients who died. Inferential estimates (hazard ratio and confidence intervals) were pooled from each data set according to Rubin’s rules for inferences after multiple imputation [43]. According to Rubin’s rules, several different values are generated for each missing data point and then analyzed. Results from each analysis are then combined following appropriate criteria to produce overall estimates with confidence intervals and p values. The overall estimates incorporate the missing data uncertainty as well as sampling variation [30]. The proportional hazard assumption of the multivariate Cox regression model was validated by examining Schoenfeld residuals, which must be independent versus time in a valid Cox model [50].
Statistical analyses were performed with packages MatchIt, mice, and survival in R (R Foundation, Vienna, Austria; www.r-project.org) [10, 25, 49] and general data processing was conducted in Python programming language (Python Foundation, www.python.org). All statistical testing was two-sided with a p value < 0.05 considered significant.
Results
With the numbers available for analysis, we could not demonstrate a difference in overall survival between limb-sparing surgery and amputation. The 5-year survivorship was 70% (95% confidence interval [CI], 62%-79%) for limb-sparing surgery versus 70% (95% CI, 62%-79%) for amputation. The 10-year survivorship was 60% (95% CI, 48%-75%) for limb-sparing surgery versus 59% (95% CI, 48%-72%) for amputation. Stratified log-rank test of the Kaplan-Meier survival curve did not demonstrate a difference in survival (p = 0.9; Fig. 2).
Fig. 2.
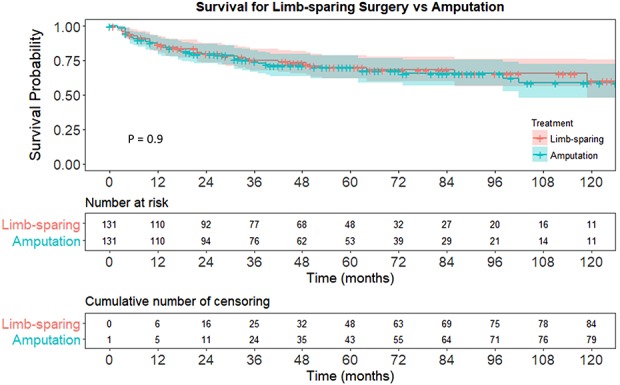
A Kaplan-Meier survival analysis of matched cohorts demonstrates equivalent overall survival between limb-sparing surgery and amputation for pelvic chondrosarcoma.
In a multivariable analysis designed to analyze other independent factors associated with survival, we found that increasing age (hazard ratio [HR], 1.029; 95% CI, 1.014-1.044; p < 0.001), Charlson/Deyo score ≥ 2 (HR, 3.039; 95% CI, 1.39-6.644; p = 0.005), grade (HR, 2.561, 95% CI, 1.318-4.919, p = 0.005 for Grade 2; HR, 7.269, 95% CI, 3.002-17.599, p < 0.001 for Grade 3; HR, 10.36, 95% CI, 4.024-26.669, p < 0.001 for Grade 4), and positive surgical margins (HR, 1.61; 95% CI, 1.026-2.529; p = 0.041) were associated with decreased survival (Table 2).
Table 2.
Multivariable Cox regression analysis of prognostic factors for survival
Discussion
Despite increased utilization of limb-sparing surgery for pelvic chondrosarcoma, assessing its impact on overall survival has been limited by the low incidence of disease. To help counsel patients with this condition, it would help to identify factors associated with survival. Using a contemporary national oncology database, we therefore determined (1) overall survival after limb-sparing surgery compared with amputation using matched cohorts; and (2) factors for long-term survival after surgical management of pelvic chondrosarcoma. With the numbers available, we could not demonstrate a difference in overall survival between limb-sparing surgery and amputation.and identified several parameters associated with decreased survival.
Our analysis has several limitations. As a result of the low incidence of malignant pelvic neoplasms, the majority of studies on this subject are retrospective as is our investigation. Selection and observational bias during data collection may have affected the results, although we attempted to compensate for bias by propensity matching for variables associated with treatment exposure and outcome. Second, our analysis is limited to the data elements within the NCDB, which does not report patterns of recurrence, recurrence-free survival, and disease-specific survival. However, for our primary outcome of overall survival, we excluded patients with multiple primary malignancy to focus on pelvic chondrosarcoma, a methodology followed by the literature. Additionally, some aspects of chondrosarcoma treatment such as extent of surgical resection might be heterogeneous across centers and thus difficult to define. Fourth, the pathologic diagnosis of sarcomas is challenging and might not be uniform across centers (for example, use of different grading systems). We made the assumption in our analysis that such heterogeneity in defining treatment and pathology would be distributed randomly across cohorts without a systematic bias for limb-sparing surgery or amputation. Fifth, the NCDB reports initial treatment, not salvage therapies performed for disease progression or recurrence. Therefore, patients with conversion to amputation after limb-sparing surgery would not be reported, and our design thus reflects an intention-to-treat analysis. However, previous research suggests that the rate of conversion to amputation is small with one investigation reporting 4% after 23 to 38 years of followup [44]. We expect the effects of such conversion, if any exist in the data set, to not skew our results. Sixth, although it would be useful to stratify the results based on exact location in the pelvis (periacetabular encroachment, involvement of the ischium), which would further facilitate propensity score matching, the NCDB does not include information to do so. Such an analysis may become possible with future changes to the NCDB or a large, prospective investigation of pelvic chondrosarcoma. As previously discussed, although the NCDB is the largest cancer registry with requirements for 90% followup from participating centers, the requirement for a participating hospital to be an accredited cancer center may restrict the generalizability of our results to the entire population [33].
Finally, although not statistically significant, our results demonstrated a small difference in overall survival between limb-sparing surgery and amputation at 10 years. Although it is possible that this difference may become significant with a larger sample size, designing a study capable of detecting a 1% difference in survival over 10 years at 80% power would require two cohorts each of over 19,000 patients, an unrealistic sample size even with a national oncology database. The focus of this study was not to suggest that the small difference in mortality would be significant, but rather to show that there was no increased mortality risk seen with limb-sparing approaches as compared with amputation. Our investigation thus primarily serves to support results from recent retrospective cohorts such as the investigation by Bus et al. that examined 162 patients with pelvic chondrosarcomas treated with limb-sparing surgery (135 patients) or amputation (27 patients) and found no difference in survival [9].
With the numbers available, our investigation could not demonstrate a difference in overall survival between limb-sparing surgery and amputation for pelvic chondrosarcoma. Taken together, these results support the increasing use of limb-sparing surgery for pelvic chondrosarcoma. There are a number of findings and observations in this report that merit further discussion. The prevalence of limb-sparing surgery in our cohort (75%) reflects its role as the preferred surgical modality in contemporary management of pelvic chondrosarcoma. Our survival data are consistent with previous investigations of limb-sparing surgery and amputation on overall survival [4, 7-9, 12, 13]. Lee et al., in a study of 32 patients with high-grade pelvic chondrosarcoma managed mostly with limb-sparing procedures between 1972 and 1994, reported that 5-year survival was 63% [29]. Deloin et al., in a series of 57 patients with pelvic chondrosarcoma managed surgically between 1968 and 2003, showed that 5- and 10-year overall survival was 66% at 5 years and 52% at 10 years with no significant difference in survival between limb-sparing surgery and amputation [12]. Surgery-related complications such as infection and revision were higher for pelvic chondrosarcoma managed with endoprosthetic reconstructions in an investigation by Stihsen et al. [48]; however, a competing risk regression analysis demonstrated that these factors did not affect long-term overall survival. The majority of these investigations are restricted to single centers, and reports are often based on outcomes that span periods of > 20 years. In contrast, our investigation takes advantage of a robust national data set to generate the largest sample size on pelvic chondrosarcoma to date while limiting the analysis to a contemporary time period (2004–2014). Our sample size made it possible to carry out a one-to-one matched-cohort analysis, which would have been difficult with the smaller cohorts in previous literature.
In a separate multivariable analysis designed to analyze other independent factors associated with survival, we found that increasing age, higher Charlson/Deyo comorbidity score, higher grade, and positive surgical margins were associated with poorer survivorship. The significance of age and Charlson/Deyo comorbidity score highlights the importance of preoperative health status to surgical risk assessment and is consistent with Ogura et al. who found a similar association between musculoskeletal oncology surgery and comorbidity burden [38]. In particular, the correlation between Charlson/Deyo score and survival is important for risk stratification given that the score is based on administrative coding (International Classification of Diseases, 9th and 10th Revision codes) based on common comorbidities, widely used in clinical practice, and has been validated for musculoskeletal tumor surgery [27, 34, 38]. Our results may therefore enable physicians to assess perioperative risk more accurately and better inform their patients of their overall risk for surgery.
The most prognostic factor for survival was tumor grade. Grading for pelvic chondrosarcoma has been debated, because grading schemes for chondrosarcoma rely on histologic interpretation and radiographic evidence, which can vary across institutions [22, 35]. Therefore, its prognostic value in survival has been found to be controversial in the past [22, 35]. However, the distribution of grade in our cohort of patients (31.4% Grade 1, 45.2% Grade 2, 23.4% Grades 3 or 4) was consistent with those from previous retrospective investigations. Pring et al. [41], in a series of 64 patients with surgical management of pelvic chondrosarcoma between 1975 and 1996, reported 51.6% Grade 1, 35.9% Grade 2, and 12.5% Grade 3 or higher. Similarly, Deloin et al. in their investigation found the distribution to be 11% Grade 1, 61% Grade 2, and 20% Grade 3 or higher [12]. Both authors found higher grade to be significantly prognostic for overall survival, corroborated by Giuffrida et al. in their analysis of the SEER database [22].
Although multiple authors have found positive surgical margins to be an important prognostic factor for survival, like in our analysis, there has been some debate regarding whether amputation achieves better margins compared with limb-sparing surgery [12, 41, 45, 46]. Our comparison of the two techniques demonstrated that amputation achieved moderately better margins; however, despite the correlation between positive margins and decreased survival, limb-sparing surgery did not decrease overall survival. Donati et al. [13], in a study of 124 pelvic chondrosarcomas over 20 years, and Bus et al. [9], in a recent investigation of 162 patients over 28 years, both reported a higher rate of inadequate margins and local recurrence in patients after limb-sparing surgery. However, neither author found that survival was worse after limb-sparing resection. Further prospective investigation should be performed in this regard, including how better functional and psychologic outcomes after limb-sparing resection impact survival [35, 42].
With the numbers available, our study did not demonstrate a difference in survival between limb-sparing surgery and amputation for pelvic chondrosarcoma. Overall, our findings support the trend toward increased use of limb-sparing surgery for patients with pelvic chondrosarcoma. However, the choice of limb-sparing surgery versus amputation for these challenging patients should still be a highly individualized decision with careful discussion between the patient and the surgeon. Potential future work includes a multicenter investigation with data on functional outcomes, further anatomic stratification, and detailed documentation on type of limb-sparing resection, which would assist in better cohort matching. Although such investigations may be challenging to conduct, they may be facilitated by developments in national oncology registries, possible through modifications in the NCDB to include more detailed and specialized parameters.
Acknowledgments
We thank the American College of Surgeons, American College of Surgeons’ Commission on Cancer, and Julian Kim, Chief Medical Officer for University Hospitals Seidman Cancer Center, for access to the National Cancer Database.
Footnotes
One of the authors (RL) reports other from Orthopediatrics Corp (Warsaw, IN, USA) and other from SIGN Fracture Care International (Richland, WA, USA) outside the submitted work. One of the authors (PG) reports that he is on the medical board of trustees for the Musculoskeletal Transplant Foundation; his involvement with the foundation does not directly influence the submitted work.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his institution waived approval for the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aksnes LH, Bauer HC, Jebsen NL, Folleras G, Allert C, Haugen GS, Hall KS. Limb-sparing surgery preserves more function than amputation: a Scandinavian Sarcoma Group study of 118 patients. J Bone Joint Surg Br. 2008;90:786-794. [DOI] [PubMed] [Google Scholar]
- 2.Aljassir F, Beadel GP, Turcotte RE, Griffin AM, Bell RS, Wunder JS, Isler MH. Outcome after pelvic sarcoma resection reconstructed with saddle prosthesis. Clin Orthop Relat Res. 2005;438:36-41. [DOI] [PubMed] [Google Scholar]
- 3.American College of Surgeons. National cancer database. Available at: http://ncdbpuf.facs.org/taxonomy/term/100. Accessed September 1, 2018.
- 4.Angelini A, Drago G, Trovarelli G, Calabro T, Ruggieri P. Infection after surgical resection for pelvic bone tumors: an analysis of 270 patients from one institution. Clin Orthop Relat Res. 2014;472:349-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin P. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2013;33:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Austin P. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrientos-Ruiz I, Ortiz-Cruz EJ, Peleteiro-Pensado M. Reconstruction after hemipelvectomy with the ice-cream cone prosthesis: what are the short-term clinical results? Clin Orthop Relat Res. 2017;475:735-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bloem JL, Reidsma Bone and soft tissue tumors of hip and pelvis. Eur J Radiol. 2012;81:3793-3801. [DOI] [PubMed] [Google Scholar]
- 9.Bus MPA, Campanacci DA, Albergo JI, Leithner A, van de Sande MAJ, Gaston CL, Caff G, Mettelsiefen J, Capanna R, Tunn PU, Jeys LM, Dijkstra PDS. Conventional primary central chondrosarcoma of the pelvis: prognostic factors and outcome of surgical treatment in 162 patients. J Bone Joint Surg Am. 2018;100:316-325. [DOI] [PubMed] [Google Scholar]
- 10.Buuren S, Groothuis-Oudshoorn K. Mice: Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:68. [Google Scholar]
- 11.Damron T, Ward W, Stewart A. Osteosarcoma, chondrosarcoma, and ewing's sarcoma: National Cancer Data Base report. Clin Orthop Relat Res. 2007;459:7. [DOI] [PubMed] [Google Scholar]
- 12.Deloin X, Dumaine V, Biau D, Karoubi M, Babinet A, Tomeno B, Anract P. Pelvic chondrosarcomas: surgical treatment options. Orthop Traumatol Surg Res. 2009;95:393-401. [DOI] [PubMed] [Google Scholar]
- 13.Donati D, El Ghoneimy A, Bertoni F, Di Bella C, Mercuri M. Surgical treatment and outcome of conventional pelvic chondrosarcoma. J Bone Joint Surg Br. 2005;87:1527-1530. [DOI] [PubMed] [Google Scholar]
- 14.Donati D, Giacomini S, Gozzi E, Ferrari S, Sangiorgi L, Tienghi A, DeGroot H, Bertoni F, Bacchini P, Bacci G, Mercuri M. Osteosarcoma of the pelvis. Eur J Surg Oncol. 2004;30:332-340. [DOI] [PubMed] [Google Scholar]
- 15.Du XL, Fang S, Coker AL, Sanderson M, Aragaki C, Cormier JN, Xing Y, Gor BJ, Chan W. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106:1276-1285. [DOI] [PubMed] [Google Scholar]
- 16.Ecker BL, McMillan MT, Datta J, Mamtani R, Giantonio BJ, Dempsey DT, Fraker DL, Drebin JA, Karakousis GC, Roses RE. Efficacy of adjuvant chemotherapy for small bowel adenocarcinoma: a propensity score-matched analysis. Cancer. 2016;122:693-701. [DOI] [PubMed] [Google Scholar]
- 17.Finos L, Righi A, Frisoni T, Gambarotti M, Ghinelli C, Benini S, Vanel D, P P. Primary extraskeletal myxoid chondrosarcoma of bone: report of three cases and review of the literature. Pathol Res Pract. 2017;213:6. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs B, Hoekzema N, Larson DR, Inwards CY, Sim FH. Osteosarcoma of the pelvis: outcome analysis of surgical treatment. Clin Orthop Relat Res. 2009;467:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebert C, Wessling M, Gosheger G, Aach M, Streitburger A, Henrichs MP, Dirksen U, Hardes J. Pelvic reconstruction with compound osteosynthesis following hemipelvectomy: a clinical study. Bone Joint J. 2013;95:1410-1416. [DOI] [PubMed] [Google Scholar]
- 20.Gingrich A, Bateni S, Monjazeb A, Darrow M, Thorpe S, Kirane A, Bold R, Canter R. Neoadjuvant radiotherapy is associated with r0 resection and improved survival for patients with extremity soft tissue sarcoma undergoing surgery: a National Cancer Database analysis. Ann Surg Oncol. 2017;24:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gingrich AA, Bateni SB, Monjazeb AM, Darrow MA, Thorpe SW, Kirane AR, Bold RJ, Canter RJ. Neoadjuvant radiotherapy is associated with r0 resection and improved survival for patients with extremity soft tissue sarcoma undergoing surgery: a National Cancer Database analysis. Ann Surg Oncol. 2017;24:3252-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:10. [DOI] [PubMed] [Google Scholar]
- 23.Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206-213. [DOI] [PubMed] [Google Scholar]
- 24.Hill J. Reducing Bias in Treatment Effect Estimation in Observational Studies Suffering From Missing Data. ISERP Working Papers, 04-01. New York, NY, USA: Institute for Social and Economic Research and Policy, Columbia University; 2004. [Google Scholar]
- 25.Ho DE, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42:29. [Google Scholar]
- 26.Kawai A, Healey JH, Boland PJ, Lin PP, Huvos AG, Meyers PA. Prognostic factors for patients with sarcomas of the pelvic bones. Cancer. 1998;82:851-859. [PubMed] [Google Scholar]
- 27.Kim CY, Sivasundaram L, LaBelle MW, Trivedi NN, Liu RW, Gillespie RJ. Predicting adverse events, length of stay, and discharge disposition following shoulder arthroplasty: a comparison of the Elixhauser Comorbidity Measure and Charlson Comorbidity Index. J Shoulder Elbow Surg . 2018;27:1748-1755. [DOI] [PubMed] [Google Scholar]
- 28.Koch B, Karnell L, Hoffman H, Apostolakis L, Robinson R, Zhen W, Menck H. National Cancer Database report on chondrosarcoma of the head and neck. Head Neck. 2000;22:18. [DOI] [PubMed] [Google Scholar]
- 29.Lee FY, Mankin HJ, Fondren G, Gebhardt MC, Springfield DS, Rosenberg AE, Jennings LC. Chondrosarcoma of bone: an assessment of outcome. J Bone Joint Surg Am. 1999;81:326-338. [DOI] [PubMed] [Google Scholar]
- 30.Marshall A, Altman D, Holder R, Royston P. Combining estimates of interest in prognostic modelling studies after multiple imputation: current practice and guidelines. BMC Med Res Methodol. 2009;9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller BJ, Gao Y, Duchman KR. Does surgery or radiation provide the best overall survival in ewing's sarcoma? A review of the National Cancer Data Base. J Surg Oncol. 2017;116:384-390. [DOI] [PubMed] [Google Scholar]
- 32.Mitra R, Reiter JP. Propensity Score Matching With Missing Covariates via iterated, Sequential Multiple Imputation. Southampton Statistical Sciences Research Institute Working Paper, M11/06 . Southampton, UK: Southampton Statistical Sciences Research Institute; 2011. [Google Scholar]
- 33.Mohanty S, Bilimoria K. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. 2014;109:2. [DOI] [PubMed] [Google Scholar]
- 34.Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55:8. [DOI] [PubMed] [Google Scholar]
- 35.Morris CD. Pelvic bone sarcomas: controversies and treatment options. J Natl Compr Canc Netw. 2010;8:731-737. [DOI] [PubMed] [Google Scholar]
- 36.Nussbaum DP, Rushing CN, Lane WO, Cardona DM, Kirsch DG, Peterson BL, Blazer DGR. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966-975. [DOI] [PubMed] [Google Scholar]
- 37.O'Connor MI, Sim FH. Salvage of the limb in the treatment of malignant pelvic tumors. J Bone Joint Surg Am. 1989;71:481-494. [PubMed] [Google Scholar]
- 38.Ogura K, Yasunaga H, Horiguchi H, Fushimi K, Kawano H. What is the effect of advanced age and comorbidity on postoperative morbidity and mortality after musculoskeletal tumor surgery? Clin Orthop Relat Res. 2014;472:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozaki T, Flege S, Kevric M, Lindner N, Maas R, Delling G, Schwarz R, von Hochstetter AR, Salzer-Kuntschik M, Berdel WE, Jurgens H, Exner GU, Reichardt P, Mayer-Steinacker R, Ewerbeck V, Kotz R, Winkelmann W, Bielack SS. Osteosarcoma of the pelvis: experience of the Cooperative Osteosarcoma Study Group. J Clin Oncol. 2003;21:334-341. [DOI] [PubMed] [Google Scholar]
- 40.Pollock RE, Karnell LH, Menck HR, Winchester DP. The National Cancer Data Base report on soft tissue sarcoma. Cancer. 1996;78:2247-2257. [PubMed] [Google Scholar]
- 41.Pring ME, Weber KL, Unni KK, Sim FH. Chondrosarcoma of the pelvis. A review of sixty-four cases. J Bone Joint Surg Am. 2001;83:1630-1642. [PubMed] [Google Scholar]
- 42.Puri A, Pruthi M, Gulia A. Outcomes after limb sparing resection in primary malignant pelvic tumors. Eur J Surg Oncol. 2014;40:27-33. [DOI] [PubMed] [Google Scholar]
- 43.Rubin DB. Multiple Imputation for Nonresponse in Surveys . New York, NY, USA: John Wiley & Sons; 2004:15-17. [Google Scholar]
- 44.Sherman C, O'Connor M, Sim F. Survival, local recurrence, and function after pelvic limb salvage at 23 to 38 years of followup. Clin Orthop Relat Res. 2012;470:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sheth DS, Yasko AW, Johnson ME, Ayala AG, Murray JA, Romsdahl MM. Chondrosarcoma of the pelvis. Prognostic factors for 67 patients treated with definitive surgery. Cancer. 1996;78:745-750. [DOI] [PubMed] [Google Scholar]
- 46.Shin KH, Rougraff BT, Simon MA. Oncologic outcomes of primary bone sarcomas of the pelvis. Clin Orthop Relat Res. 1994;304:207-217. [PubMed] [Google Scholar]
- 47.Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stihsen C, Panotopoulos J, Puchner SE, Sevelda F, Kaider A, Windhager R, Funovics PT. The outcome of the surgical treatment of pelvic chondrosarcomas: a competing risk analysis of 58 tumours from a single centre. Bone Joint J. 2017;99:686-696. [DOI] [PubMed] [Google Scholar]
- 49.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model . New York, NY, USA: Springer; 2000:39-77. [Google Scholar]
- 50.UCLA Institute for Digital Research and Education. Testing the proportional hazard assumption in cox models. Available at: https://stats.idre.ucla.edu/other/examples/asa2/testing-the-proportional-hazard-assumption-in-cox-models/. Accessed June 20, 2018.
- 51.Venigalla S, Nead K, Sebro R, Guttmann D, Sharma S, Simone C, Levin W, Wilson R, Weber K, Shabason J. Association between treatment at high-volume facilities and improved overall survival in soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 2018;100:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Winchester D, Stewart A, Phillips J, Ward E. The National Cancer Data Base: past, present, and future. Ann Surg Oncol. 2010;17:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu J, Li D, Xie L, Tang S, Guo W. Mesenchymal chondrosarcoma of bone and soft tissue: a systematic review of 107 patients in the past 20 years. PLoS One. 2015;10:e0122216. [DOI] [PMC free article] [PubMed] [Google Scholar]