Abstract
Background
Deep venous thrombosis (DVT) and pulmonary embolus (PE) remain an important cause of morbidity and mortality after THA and TKA. Prior recommendations have advocated for more aggressive prophylaxis for patients with obesity, whereas the evidence supporting these recommendations is conflicting and often based on underpowered studies.
Questions/purposes
(1) What is the association between obesity and DVT and PE after primary and revision THA and TKA? (2) Is there a body mass index (BMI) threshold beyond which DVT and PE risk is elevated?
Methods
We reviewed the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database from 2008 to 2016 to evaluate the reported 30-day rates of DVT, PE, and combined venous thromboembolism (VTE) after primary THA, primary TKA, revision THA, and revision TKA according to BMI as a continuous variable and a categorical variable as defined by the World Health Organization cutoffs for underweight, normal weight, overweight, and obesity. This database is risk-adjusted and designed to capture complications after surgery, thus making it ideal for this study. The diagnosis of DVT and PE is included in the ACS-NSQIP database for any DVT or PE requiring treatment. Proximal versus distal DVT is not specified within the database. Multivariate logistic regression was performed to determine if obesity was independently associated with DVT and PE risk by controlling for age, sex, race, American Society of Anesthesiologists score, diabetes, hypertension, smoking status, general anesthesia, and hypoalbuminemia.
Results
After controlling for potential confounding variables such as medical comorbidities and procedure type, patients undergoing primary and revision THA and TKA with World Health Organization classification as underweight (BMI < 18.5 kg/m2), overweight (BMI 25-29.9 kg/m2), Class I obese (BMI 30-34.9 kg/m2), Class II obese (BMI 35-39.9 kg/m2), or Class III obese (BMI ≥ 40 kg/m2) did not demonstrate an association with increased risk of DVT compared with patients classified as normal weight (BMI 18.5-25 kg/m2). Compared with patients undergoing primary THA classified as normal weight, the risk of PE was elevated in patients with Class II obesity (odds ratio [OR], 2.36; 95% confidence interval [CI], 1.23-4.50; p = 0.009) and all heavier categories. Compared with patients undergoing TKA classified as normal weight, the risk of PE was elevated in patients classified as overweight (OR, 1.56; 95% CI, 1.03-2.36; p = 0.035) and all heavier categories.
Conclusions
This large administrative database study suggests that patient classification as overweight or obese is associated with increased risk of development of PE but not DVT after primary THA or TKA. Because aggressive pharmacologic anticoagulation regimens can decrease the DVT rate but have not been shown to affect the rate of PE or death, the data do not currently support increased anticoagulation in patients with obesity without other risk factors for VTE undergoing THA or TKA. Additional studies are required to refine VTE prophylaxis protocols to reduce PE risk while maintaining acceptable postoperative bleeding risk.
Level of Evidence
Level III, therapeutic study.
Introduction
Venous thromboembolic disease (VTE) including deep venous thrombosis (DVT) and pulmonary embolus (PE) remains an important cause of morbidity and mortality after THA and TKA [19]. As a result, VTE prevention is a universal quality improvement initiative and a focus of the Surgical Care Improvement Project (SCIP) [21]. Pharmacologic prophylaxis remains the most common strategy to minimize VTE postoperatively [7, 19–21, 25, 26]. However, orthopaedic surgeons must balance the need to prevent DVT and PE and their associated consequences ranging from the more common lower extremity postthrombotic syndrome [13] to (rarely) death with the risks of prophylaxis including bleeding, hematoma, infection, and subsequent return to the operating room [12]. Unfortunately, the optimal individual DVT and PE prophylaxis after THA/TKA remains undefined.
Patients with obesity undergoing THA and TKA may be at increased risk for DVT or PE as a result of increased operative times, lower postoperative mobility, ineffectiveness of mechanical prophylaxis, and elevations in procoagulant inflammatory markers [35]. Although some authors have found increased rates of VTE in superobese patients (body mass index [BMI] > 50 kg/m2) after TKA [15, 22, 34], most institutional reviews have not demonstrated a correlation between VTE risk and BMI as a result of a small sample size and the rarity of symptomatic VTE events [11, 23].
Therefore, the purpose of this study was to use a large administrative database to answer the following questions: (1) What is the association between obesity and DVT and PE after primary and revision THA and TKA? (2) Is there a BMI threshold beyond which DVT or PE risk is elevated?
Materials and Methods
We retrospectively analyzed data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database for the period of 2008 to 2016 to evaluate the reported rates of symptomatic DVT and PE after primary and revision THA and TKA. The ACS-NSQIP is a large, nationally validated, risk-adjusted, outcome-based program to measure and improve the quality of surgical care. Because it is designed specifically to capture adverse events after surgery, it is the ideal tool to study complications after THA and TKA [1]. In addition, because only symptomatic DVT and PE requiring treatment are included in this database, it provides an accurate and clinically relevant portrayal of this problem.
Patients from ACS-NSQIP participating sites were enrolled into the database according to the database-reporting requirements. Patient-specific data and risk factors were collected, and clinical outcomes were reviewed for 30 days after surgery. Patients were identified by Current Procedural Terminology codes for primary THA (27130), primary TKA (27447), revision THA (27134, 27137, 27138), or revision TKA (27486, 27487). Patients were excluded if the admission International Classification of Diseases (ICD) code included a diagnosis code of fracture or a history of clotting or bleeding disorders. ICD-9 or ICD-10 diagnosis codes (depending on year of admission) for fracture included 733.X, 820.X, 821.X, 822.X, 823.X, M80.X, M81.X, M84.X, M96.6, and S72.X. Bleeding and clotting disorder history are variables within the ACS-NSQIP database (Table 1).
Table 1.
Risk factors included for multivariable regression analysis of DVT and PE among patients undergoing primary THA, primary TKA, revision THA, and revision TKA
There were 5,244,805 potentially eligible individuals in the ACS-NSQIP database from 2008 to 2016. After application of the inclusion and exclusion criteria noted previously, 131,579 patients undergoing primary THA, 218,997 patients undergoing primary TKA, 12,913 patients undergoing revision THA, and 15,286 patients undergoing revision TKA were retained for the final analysis. All patients had 30-day followup data available (Fig. 1).
Fig. 1.
Flow diagram describing each stage in exclusion among the ACS-NSQIP patient population to the final cohort for analysis. FAI = femoroacetabular impingement.
Rates of DVT and PE were calculated among each category of obesity within each arthroplasty type. The primary outcome was DVT or PE resulting in treatment within 30 days of surgery. This was determined by the ACS-NSQIP administrator at each participating institution on chart review. Patients were subdivided into the various BMI categories according to the World Health Organization obesity classifications: < 18.5 kg/m2 (underweight), 18.5 to 24.9 kg/m2 (normal weight), 25 to 29.9 kg/m2 (overweight), 30 to 34.9 kg/m2 (Class I obese), 35 to 39.9 kg/m2 (Class II obese), and ≥ 40 kg/m2 (Class III obese). Subsequent analysis was performed for comparison treating BMI as a continuous variable. Potential confounders included for analysis included age, sex, race, American Society of Anesthesiologists (ASA) score, diabetes, hypertension, smoking status, general anesthesia, hypoalbuminemia, and procedure type. Type of postoperative chemoprophylaxis agent was not available from the ACS-NSQIP database.
Statistical Analysis
Univariate logistic regression compared the predictive value of obesity category on incidence of DVT, PE, and combined VTE using the normal weight (BMI 18-24.9 kg/m2) category as the control group. All patients had BMI data available. Multivariate logistic regression modeling was performed separately for primary THA, primary TKA, revision THA, and revision TKA, which controlled for age, sex, race, ASA score, diabetes, hypertension, smoking status, general anesthesia, and hypoalbuminemia. Additionally, weight and 1/height2 were analyzed as predictors independent from BMI. These analyses did not yield stronger predictive ability compared with BMI as a continuous variable. Patient characteristics were compared among obesity categories using chi-square or Fisher’s exact test, where appropriate, for categorical variables and analysis of variance for continuous variables. Pearson chi-square testing was performed to evaluate goodness of fit for the logistic regression model. All statistical calculations were performed using Stata Version 14.2 (StataCorp, College Station, TX, USA).
A priori power analysis was performed to determine the number of patients required to reach 80% power to detect a minimal clinically important difference change in VTE of 2% [24]. However, for this level of precision, approximately 10.6 million patients would need to be included for analysis, which was deemed prohibitive. A 0.1% rate difference was selected given the low baseline rate of symptomatic VTE in the current population with current prophylaxis methods. Using a known DVT rate of 0.74% in the NSQIP database, a minimum sample size of 246,062 patients was required for 80% power to detect a 0.1% increase in DVT rate to 0.84% or greater in the comparison group of patients with obesity with α set at 0.05.
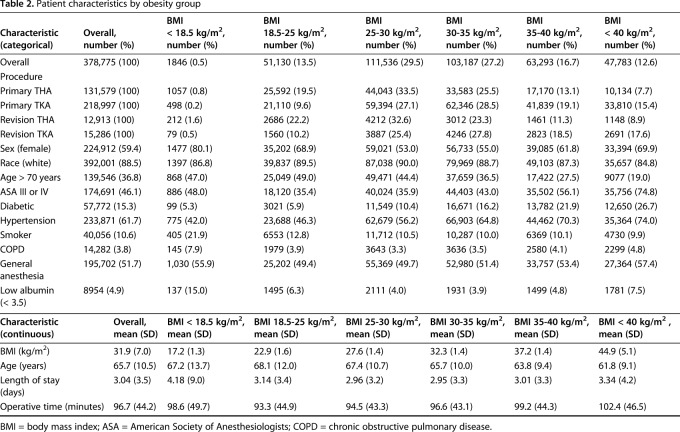
BMI data were available on all included patients (Table 2). The mean BMI of the entire cohort was 31.9 kg/m2 (range, 10.0-173.1 kg/m2). A total of 4407 VTE events (1.2%) were recorded within 30 days of THA or TKA among 378,775 patients: 2624 DVT (0.69%) and 1783 PE (0.47%) events. Of these, 337 patients were reported to have both DVT and PE that resulted in treatment. Patient demographics and preoperative risk factors varied among obesity groups (Table 1). Patients classified as normal weight made up 19.5% of primary THAs, 9.6% of primary TKAs, 22.2% of revision THAs, and 10.2% of revision TKAs (p < 0.001). Mean operative time was 96.7 minutes (SD = 44.2, p < 0.001) and increased across obesity categories, except for patients classified as underweight, who had a mean operative time of 98.6 minutes (SD = 49.7). Mean length of stay was 3.04 days (SD = 3.5, p < 0.001), with patients classified as Class III obese (mean = 3.34 days, SD = 4.2) and underweight (mean = 4.18, SD = 9.0) demonstrating the longest length of stay. Mean age was 65.7 years (SD = 10.5, p < 0.001), with patients classified as Class III obese (mean = 61.8, SD = 9.1) demonstrating the youngest age (Table 2).
Table 2.
Patient characteristics by obesity group
The 30-day rates of DVT were 0.39% for primary THA, 0.87% for primary TKA, 0.65% for revision THA, and 0.83% for revision TKA. The 30-day rates of PE were 0.25% for primary THA, 0.61% for primary TKA, 0.37% for revision THA, and 0.41% for revision TKA (Supplemental Tables 1, 2 [Supplemental materials are available with the online version of CORR®.]).
Pearson chi-square goodness-of-fit testing demonstrated acceptable goodness of fit for multivariate models performed on primary THA and TKA.
To determine a BMI threshold for DVT and PE beyond which risk is increased, a receiver operating characteristic (ROC) curve was developed to graph sensitivity as a function of 1-specificity of BMI as a continuous variable to test for risk of DVT and PE. The area under the curve (AUC) was assessed to determine the accuracy with which BMI can be used to predict DVT and PE with the following accuracy classifications: excellent (0.90-1.00), good (0.80-0.89), fair (0.70-0.79), poor (0.60-0.69), and fail (0.50-0.59). An optimal cut point threshold was determined as the point on the ROC curve that maximizes the product of sensitivity and specificity.
Results
Association Between DVT or PE and Obesity
Our results suggest an association between increasing BMI and increased risk for PE among patients undergoing primary THA and primary TKA. This study did not find an association between obesity and increased risk for DVT resulting in treatment after primary or revision THA or TKA. No association was seen between obesity and increased risk for PE after revision THA or TKA. After controlling for potential confounding variables such as age, sex, race, ASA score, diabetes, hypertension, smoking status, type of anesthesia, and albumin level, we found that the rate of DVT that resulted in treatment within 30 days of surgery was not associated with obesity classification or with BMI as a continuous variable (Table 3).
Table 3.
Unadjusted and multivariate regression for risk of DVT among a total joint arthroplasty population by obesity category
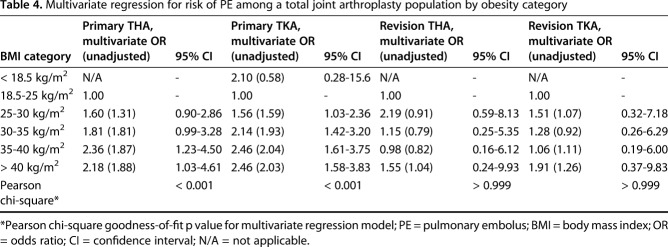
On the other hand, we found that the rate of PE that resulted in treatment within 30 days of surgery was associated with obesity classification for patients with Class I obesity (odds ratio [OR], 1.76; 95% confidence interval [CI], 1.03-3.03; p = 0.04) or higher among patients undergoing primary THA. BMI as a continuous variable was associated with an OR of 1.03 for each increase in kg/m2 (95% CI, 1.01-1.06; p = 0.01).
Similarly, we found that the rate of PE that resulted in treatment within 30 days of surgery was associated with obesity classification for patients classified as overweight (OR, 1.64; 95% CI, 1.11-2.45; p = 0.01) or higher among patients undergoing primary TKA. BMI as a continuous variable was associated with an OR of 1.03 for each increase in kg/m2 (95% CI, 1.01-1.04; p < 0.001) (Table 4).
Table 4.
Multivariate regression for risk of PE among a total joint arthroplasty population by obesity category
Surprisingly, after controlling for the same potential confounding variables, we found that the rate of PE that resulted in treatment within 30 days of surgery was not associated with obesity classification or BMI as a continuous variable for patients undergoing revision THA or revision TKA. Finally, the analysis showed that the rate of combined VTE (DVT or PE resulting in treatment) within 30 days of surgery was not associated with BMI as a continuous variable for patients undergoing primary THA, primary TKA, revision THA, or revision TKA.
Is There a BMI Threshold for DVT and PE Risk?
Compared with patients of normal weight, no association with an increased rate of DVT resulting in treatment was identified among patients with obesity among various World Health Organization categories or BMI as a continuous variable in patients undergoing primary THA, primary TKA, revision THA, or revision TKA (Table 3; Fig. 2). Similarly, no association with increased risk of PE resulting in treatment was identified among patients with obesity or by BMI as a continuous variable in patients undergoing revision THA or revision TKA (Table 4; Fig. 3).
Fig. 2.
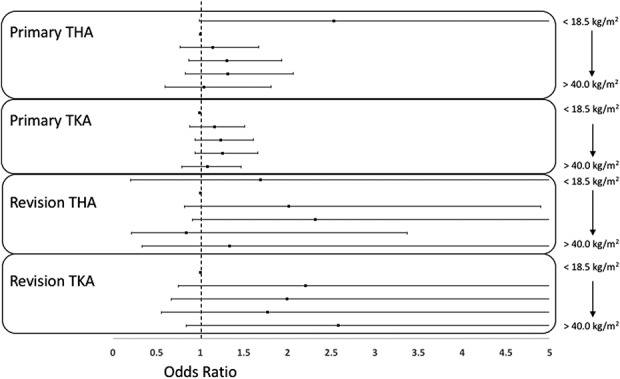
Forest plot shows ORs for DVT among primary THA, primary TKA, revision THA, and revision TKA by obesity categories.
Fig. 3.

Forest plot shows ORs for pulmonary embolism among primary THA, primary TKA, revision THA, and revision TKA by obesity categories.
In contrast, compared with patients of normal weight, an increased rate of PE resulting in treatment was associated with patients classified as Class I obese and heavier in those undergoing primary THA and with patients classified as overweight and heavier in those undergoing primary TKA (Table 4).
Attempts to identify a BMI threshold beyond which PE risk is elevated demonstrated poor accuracy with regard to primary THA and failed to identify a meaningful cut point for primary TKA using ROC curve optimization methods. We were unable to determine a BMI threshold beyond which there was an increased risk of PE after primary THA/TKA. ROC curves were developed for BMI as a continuous variable to determine the accuracy in predicting PE among patients undergoing primary THA and primary TKA. The AUC was 0.61 (poor) for the ROC curve of an association between BMI and PE among patients undergoing primary THA. The optimal cut point for BMI among patients undergoing primary THA to maximize sensitivity and specificity of risk for PE was 29.8 kg/m2. The AUC was 0.58 (fail) for the ROC curve of an association between BMI and PE among patients undergoing primary TKA. The optimal cut point for BMI among patients undergoing primary TKA to maximize sensitivity and specificity of risk for PE was 33.0 kg/m2.
Finally, a review of the regression diagnostics demonstrated an equal or stronger association between age and ASA score with DVT and PE compared with BMI.
Discussion
DVT and PE after primary and revision THA and TKA can result in serious morbidity, mortality, increased length of stay, and increased cost [12, 22]. For this reason, DVT and PE remain an important focus for quality improvement initiatives [6]. Although the current literature is conflicted on the impact of obesity on DVT and PE risk [11, 15, 22, 23, 34], some have advocated a more aggressive anticoagulation prophylaxis regimen in this patient population as a result of longer operative times, decreased postoperative mobility, ineffective mechanical prophylaxis, and elevations in procoagulant markers. Most reports have been limited by sample size and the rarity of symptomatic VTE events after surgery. Thus, the purpose of this study was to determine whether BMI was independently associated with increased risk for DVT or PE after primary and revision THA or TKA compared with controls using a large, national, risk-adjusted and validated administrative database.
This study has several limitations. First, although the NSQIP database is a validated, risk-adjusted outcome program, it only represents a small portion of all the surgical procedures from the contributing institutions and, therefore, this can lead to both selection and sampling bias. In addition, given the occurrence of symptomatic DVT and PE is fortunately rare and the reporting is limited to only 30 days in this database, there is a risk of underestimation. However, because most VTE events after lower extremity arthroplasty have been shown to occur between 5 and 36 days postoperatively [33], approximately three-fourths of DVT and PE would have been captured during the NSQIP 30-day reporting period. Second, this database does not contain variables such as insurance or socioeconomic status or geographic region, which can impact DVT and PE rates resulting from regional practice variability and patient access to pharmacologic VTE chemoprophylaxis. Nevertheless, although this database is not as validated as a nationally representative sample, its trends and observations have been validated and generalizable to most surgical patients over the age of 18 years because the database roughly approximates national race and sex distributions. Third, there was an underreporting of DVT in patients with PE. In this data set, there were only 326 patients with both DVT and PE codes, whereas there were 1783 symptomatic PE cases. A patient who has a PE is also likely to have a DVT and this discrepancy could have impacted our results, preventing us from finding an association between BMI and DVT. To account for this, we developed a sensitivity analysis model to assess the risk of either DVT or PE (all VTE) resulting in treatment to more closely estimate the true incidence of DVT in our patient population. The analysis did not find an association between BMI and increased risk of all-cause VTE requiring treatment.
Additional limitations include a lack of information on personal or family history of VTE, which could be used to further risk-adjust our study population. Furthermore, NSQIP does not include the type of pharmacologic agents used for VTE prophylaxis, which limits the ability to make definitive statements about prophylactic regimens for obese patients undergoing THA or TKA. We assumed that patients with high BMI without a history of VTE received the same VTE prophylaxis as patients with lower BMI. However, if this assumption was incorrect, and patients with obesity systematically received different VTE prophylaxis (that is, more aggressive anticoagulation) compared with controls, it would result in an underestimation of the risk of DVT and PE in this patient population. Even then, these treatment variations would not be expected to cause systematic differences in DVT and PE rates across the various obesity categories. Anticoagulants can decrease the risk of DVT but have not been shown to decrease the risk of fatal PE. Warfarin, fondaparinux, and enoxaparin have not demonstrated superiority to aspirin alone for prevention of DVT or PE after primary TKA [5]. Prospective, large-scale, multicenter studies with well-defined inclusion and exclusion criteria and treatment protocols such as the Comparative Effectiveness of Pulmonary Embolism Prevention After Hip and Knee Replacement (PEPPER) study (NCT02810704) may help to determine which pharmacologic modality and regimen is best suited for various patients undergoing THA and TKA.
Our data demonstrated that only the risk of PE is elevated in patients with obesity after primary THA and primary TKA. No differences were observed in the revision THA and revision TKA groups compared with patients of normal weight, possibly as a result of a smaller sample size. There was no positive association between DVT and BMI. These results are consistent in part with other published reports. Mantilla et al. [22] performed a retrospective, case-controlled study to identify risk factors for clinically relevant PE and DVT. They found that obesity defined as BMI > 30 kg/m2 imparted a 3.4 times elevated risk of VTE compared with controls. Additionally, in evaluating risk factors for VTE necessitating rehospitalization after THA, White and colleagues [35] demonstrated a 1.8 times increased risk in patients with BMI > 25 kg/m2. Obese patients may be at increased risk for VTE after surgery as a result of lower postoperative mobility, ineffectiveness of mechanical prophylaxis, and elevated procoagulant inflammatory markers. However, most reported case series have failed to demonstrate obesity to be an independent risk factor for VTE [2, 11, 15, 22, 23, 35]. Most of these studies had relatively small sample sizes and, coupled with the relatively rarity of symptomatic VTE events after THA and TKA, were underpowered to detect differences between the groups. Thus, the benefit of an aggressive anticoagulation regimen in this patient population remains unproven. Also, the finding that DVT rates are not increased in patients with obesity further illustrates the complexity and intricacy of chemoprophylaxis. The American Academy of Orthopaedic Surgeons and the 2012 American College of Chest Physicians guidelines recently recommended aspirin as an acceptable VTE chemoprophylaxis agent after THA and TKA in low-risk patients [14, 18]. Furthermore, aspirin has been shown to be not inferior, more cost-effective, and have a lower side effect profile compared with other anticoagulants used for chemoprophylaxis after THA and TKA with a higher rate of DVT but not fatal PE [3-5, 8, 10, 17, 19-21, 26–30]. Hood et al. [16] analyzed the Michigan Arthroplasty Registry and found that aspirin alone was not inferior to other anticoagulants in the postoperative rate of VTE or death. Thus, orthopaedic surgeons have to balance the risks of thromboembolic events versus bleeding and other associated complications and adopt a risk-adjusted anticoagulation protocol. However, our data do not support a more aggressive anticoagulation regimen in patients with isolated obesity as a risk factor.
We could not establish a BMI threshold beyond which there was a predictable increase in the risk of DVT or PE. These results are also consistent in part with previously published reports. Although Werner et al. [34] reported increased risk of VTE in the superobese (BMI > 50 kg/m2) compared with even morbidly obese patients undergoing primary TKA, Friedman and colleagues [11] did not observe a difference in VTE in morbidly obese (BMI > 40 kg/m2) patients compared with controls undergoing primary hip and knee arthroplasty. Furthermore, Tay et al. [31] also reported a low incidence of VTE (0.67%) in Asian patients undergoing THA or TKA and no differences in VTE rates between obese patients compared with controls, although this conclusion must be taken in the context given possible differences in ethnicity and race and other comorbidities compared with our patient sample. However, the inability to establish an optimal BMI cutoff to predict DVT or PE supports the results that increased BMI alone is not an independent predictor of DVT postoperatively and aggressive anticoagulation based on weight is not justified.
This study demonstrates that obesity is associated with increased risk only for PE resulting in treatment after primary THA and primary TKA. However, no optimal BMI threshold was identified beyond which DVT, PE, or combined VTE risk could accurately be predicted. Although recommendations for chemoprophylaxis are not standardized [9, 32], no particular agent has demonstrated superiority in preventing symptomatic PE [3, 5, 29]. Therefore, these data do not support increased anticoagulation in obese patients without a history of DVT or PE undergoing primary THA, primary TKA, revision THA, or revision TKA. Larger randomized, prospective studies are ongoing such as PEPPER, which is enrolling 25,000 patients at sites across the United States to compare aspirin, warfarin, and rivaroxaban postoperative regimens after THA and TKA to refine chemoprophylaxis protocols to reduce DVT and PE risk in this patient population.
Footnotes
One of the authors certifies that he (NS), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in the amount of less than USD 10,000 from Zimmer Orthopaedics (Warsaw, IN, USA), Smith & Nephew (Andover, MA, USA), Medacta (Chicago, IL, USA), and Elsevier (Rockville, MD, USA). One of the authors certifies that he (G-CL), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in the amount of less than USD 10,000 from DePuy/Johnson & Johnson (Warsaw, IN, USA), Ferring Pharmaceuticals (Parsippany, NJ, USA), Heron Therapeutics (San Diego, CA, USA), KCI (San Antonio, TX, USA), Ceramtec (Laurens, SC, USA), Pacira (Parsippany, NJ, USA), CD Diagnostics (Claymont, DE, USA), Cempra Pharmaceuticals (Chapel Hill, NC, USA), Zimmer Orthopaedics (Warsaw, IN, USA), the National Institutes of Health (Rockville, MD, USA), and United Orthopedics (Irvine, CA, USA). One of the authors certifies that he (G-CL), or a member of his immediate family, has received or may receive payments or benefits, during the study period, in the amount of greater than USD 10,000 and less than USD 100,000 from Corin USA (Tampa, FL, USA) and Stryker (Kalamazoo, MI, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
This work was performed at the University of Pennsylvania, Philadelphia, PA, USA.
References
- 1.American College of Surgeons. ACS NSQIP in the Literature. 2017. Available at: https://www.facs.org/quality-programs/acs-nsqip/. Accessed March 10, 2017. [Google Scholar]
- 2.Andrew JG, Palan J, Kurup HV, Gibson P, Murray DW, Beard DJ. Obesity in total hip replacement. J Bone Joint Surg Br. 2008;90:424–429. [DOI] [PubMed] [Google Scholar]
- 3.Bala A, Huddleston JI, Goodman SB, Maloney WJ, Amanatullah DF. Venous thromboembolism prophylaxis after TKA: aspirin, warfarin, enoxaparin, or factor Xa inhibitors? Clin Orthop Relat Res. 2017;475:2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown GA. Venous thromboembolism prophylaxis after major orthopaedic surgery: a pooled analysis of randomized controlled trials. J Arthroplasty . 2009;24(Suppl):77-83. [DOI] [PubMed] [Google Scholar]
- 5.Cafri G, Paxton EW, Chen Y, Cheetham CT, Gould MK, Sluggett J, Bini SA, Khatod M. Comparative effectiveness and safety of drug prophylaxis for prevention of venous thromboembolism after total knee arthroplasty. J Arthroplasty . 2017;32:3524-3528.e1. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare and Medicaid. Surgical Care Improvement Project (SCIP). 2011. Available at: https://innovation.cms.gov/Files/x/ACE-Quality-Measures.pdf. Accessed August 2, 2018.
- 7.Dager WE. Warfarin for venous thromboembolism prophylaxis after elective hip or knee arthroplasty: exploring the evidence, guidelines, and challenges remaining. DICP. 2012;46:79–88. [DOI] [PubMed] [Google Scholar]
- 8.Deirmengian GK, Heller S, Smith EB, Maltenfort M, Chen AF, Parvizi J. Aspirin can be used as prophylaxis for prevention of venous thromboembolism after revision hip and knee arthroplasty. J Arthroplasty. 2016;31:2237–2240. [DOI] [PubMed] [Google Scholar]
- 9.Eikelboom JW, Karthikeyan G, Fagel N, Hirsh J. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest. 2009;135:513–520. [DOI] [PubMed] [Google Scholar]
- 10.Feldstein MJ, Low SL, Chen AF, Woodward LA, Hozack WJ. A comparison of two dosing regimens of ASA following total hip and knee arthroplasties. J Arthroplasty. 2017;32:S157–S161. [DOI] [PubMed] [Google Scholar]
- 11.Friedman RJ, Hess S, Berkowitz SD, Homering M. Complication rates after hip or knee arthroplasty in morbidly obese patients. Clin Orthop Relat Res. 2013;471:3358–3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:381S-453S. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg JS, Gent M, Turkstra F, Buller HR, MacKinnon B, Magier D, Hirsh J. Postthrombotic syndrome after hip or knee arthroplasty: a cross-sectional study. Arch Intern Med. 2000;160:669-672. [DOI] [PubMed] [Google Scholar]
- 14.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(Suppl):7s-47s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haverkamp D, Klinkenbijl MN, Somford MP, Albers GH, van der Vis HM. Obesity in total hip arthroplasty--does it really matter? A meta-analysis. Act Orthop Scand. 2011;82:417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hood BR, Cowen ME, Zheng HT, Hughes RE, Singal B, Hallstrom BR. Association of aspirin with prevention of venous thromboembolism in patients after total knee arthroplasty compared with other anticoagulants: a noninferiority analysis. JAMA Surg. 2018. Oct 17. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang RC, Parvizi J, Hozack WJ, Chen AF, Austin MS. Aspirin is as effective as and safer than warfarin for patients at higher risk of venous thromboembolism undergoing total joint arthroplasty. J Arthroplasty. 2016;31:83–86. [DOI] [PubMed] [Google Scholar]
- 18.Johanson NA, Lachiewicz PF, Lieberman JR, Lotke PA, Parvizi J, Pellegrini V, Stringer TA, Tornetta P, III, Haralson RH, III, Watters WC., III American Academy of Orthopaedic Surgeons clinical practice guideline on prevention of symptomatic pulmonary embolism in patients undergoing total hip or knee arthroplasty. J Bone Joint Surg Am. 2009;91:1756-1757. [DOI] [PubMed] [Google Scholar]
- 19.Kapadia BH, Pivec R, Issa K, Mont MA. Prevention and management of venous thromboembolic disease following lower extremity total joint arthroplasty. Surg Technol Int. 2012;22:251–259. [PubMed] [Google Scholar]
- 20.Knesek D, Peterson TC, Markel DC. Thromboembolic prophylaxis in total joint arthroplasty. Thromb J. 2012;2012:837896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lachiewicz PF. Comparison of ACCP and AAOS guidelines for VTE prophylaxis after total hip and total knee arthroplasty. Orthopedics. 2009;32:74–78. [DOI] [PubMed] [Google Scholar]
- 22.Mantilla CB, Horlocker TT, Schroeder DR, Berry DJ, Brown DL. Risk factors for clinically relevant pulmonary embolism and deep venous thrombosis in patients undergoing primary hip or knee arthroplasty. Anesthesiology. 2003;99:552–560; discussion 5A. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin JR, Lee KR. The outcome of total hip replacement in obese and non-obese patients at 10- to 18-years. J Bone Joint Surg Br. 2006;88:1286–1292. [DOI] [PubMed] [Google Scholar]
- 24.Moretto P, Park J, Rodger M, Le Gal G, Carrier M. A survey of thrombosis experts evaluating practices and opinions regarding venous thromboprophylaxis in patients with active cancer hospitalized with an acute medical illness. Thromb J. 2015;13:10-015-0040–6. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaou VS, Desy NM, Bergeron SG, Antoniou J. Total knee replacement and chemical thromboprophylaxis: current evidence. Curr Vasc Pharmacol. 2011;9:33–41. [DOI] [PubMed] [Google Scholar]
- 26.Parvizi J, Azzam K, Rothman RH. Deep venous thrombosis prophylaxis for total joint arthroplasty: American Academy of Orthopaedic Surgeons guidelines. J Arthroplasty. 2008;23:2–5. [DOI] [PubMed] [Google Scholar]
- 27.Parvizi J, Huang R, Restrepo C, Chen AF, Austin MS, Hozack WJ, Lonner JH. Low-dose aspirin is effective chemoprophylaxis against clinically important venous thromboembolism following total joint arthroplasty: a preliminary analysis. J Bone Joint Surg Am. 2017;99:91–98. [DOI] [PubMed] [Google Scholar]
- 28.Raphael IJ, Tischler EH, Huang R, Rothman RH, Hozack WJ, Parvizi J. Aspirin: an alternative for pulmonary embolism prophylaxis after arthroplasty? Clin Orthop Relat Res. 2014;472:482–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schousboe JT, Brown GA. Cost-effectiveness of low-molecular-weight heparin compared with aspirin for prophylaxis against venous thromboembolism after total joint arthroplasty. J Bone Joint Surg Am . 2013;95:1256-1264. [DOI] [PubMed] [Google Scholar]
- 30.Stewart DW, Freshour JE. Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. DICP. 2013;47:63–74. [DOI] [PubMed] [Google Scholar]
- 31.Tay K, Bin Abd Razak HR, Tan AH. Obesity and venous thromboembolism in total knee arthroplasty patients in an Asian population. J Arthroplasty. 2016;31:2880–2883. [DOI] [PubMed] [Google Scholar]
- 32.Warwick D, Dahl OE, Fisher WD, Forum IST. Orthopaedic thromboprophylaxis: limitations of current guidelines. J Bone Joint Surg Br. 2008;90:127–132. [DOI] [PubMed] [Google Scholar]
- 33.Warwick D, Friedman RJ, Agnelli G, Gil-Garay EA, Johnson K, Fitzgerald G, Turibio FM. Insufficient duration of venous thromboembolism prophylaxis after total hip or knee replacement when compared with the time course of thromboembolic events: findings from the Global Orthopaedic Registry. J Bone Joint Surg Br. 2007;89:799-807. [DOI] [PubMed] [Google Scholar]
- 34.Werner BC, Evans CL, Carothers JT, Browne JA. Primary total knee arthroplasty in super-obese patients: dramatically higher postoperative complication rates even compared to revision surgery. J Arthroplasty. 2015;30:849–853. [DOI] [PubMed] [Google Scholar]
- 35.White RH, Gettner S, Newman JM, Trauner KB, Romano PS. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med. 2000;343:1758–1764. [DOI] [PubMed] [Google Scholar]