Minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) has been a workhorse technique for the treatment of degenerative spine pathology since its introduction more than a decade ago [1]. With advances in surgical technology and fiberoptic illumination, surgeons now use this approach for increasingly complex procedures, including multilevel disease as well as for sagittal- and coronal-plane deformity correction. While the short-term success of MI-TLIF depends primarily on the adequate decompression of stenotic segments and immediate improvement of spinal alignment, long-term pain relief depends on successful fusion [2].
Opponents of minimally invasive spine surgery in general, and MI-TLIF specifically, have questioned the rate at which a solid interbody arthrodesis is achieved using the MI-TLIF technique given the challenges of an adequate discectomy and appropriate end-plate preparation. While pseudoarthrosis rates are expected to be higher earlier in a surgeon’s learning curve, there are certain tips and tricks that may help surgeons of all levels of experience increase the likelihood they will achieve fusion.
The combination of iliac crest autograft plus local bone remains the standard against which alternatives, including allograft and synthetic products, are judged. But since graft-site pain is often cited as the reason to avoid iliac crest graft harvest, focusing attention on minimizing that seems well worthwhile. The approach I use, which is nearly percutaneous, avoids dissecting muscle off of the inner or outer tables of the ilium.
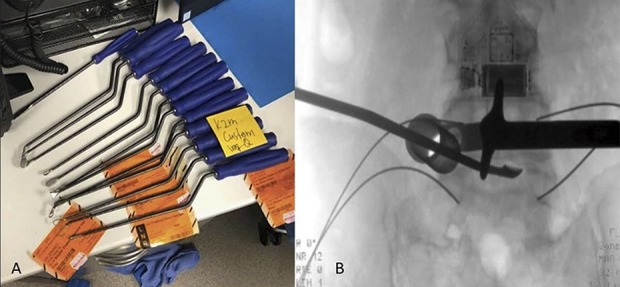
First, a small stab incision is made over the posterior superior iliac spine (Fig 1A). An awl is then tamped between the inner and outer tables of the ilium just deep enough to get into cancellous bone (Fig 1B). Next, a 12-mm reverse reamer is docked onto the track created by the awl (Fig 1C). The reamer is then passed between the inner and outer tables in two or three different directions, or until sufficient graft has been harvested (Fig 1D).
Fig. 1 A-D.

(A) A small stab incision over the posterior superior iliac spine is shown. (B) An awl tamped between the inner and outer tables of the ilium should be deep enough to get into cancellous bone. (C) A 12-mm reverse reamer is shown docked onto the track created by the awl. (D) The reamer passes between the inner and outer tables in different directions or until an acceptable amount of graft is harvested.
After completion of iliac crest autograft harvest, a facetectomy is performed using a combination of high speed burr and Kerrison rongeurs (Fig 2). The bone shavings produced during burring of the facet are collected using the cup-side of a Penfield 1, and along with bone fragments detached from the facet and lamina using a Kerrison rongeur, are combined with the previously collected crest graft. The autograft is kept in a closed specimen cup in room temperature normal saline and some of the patient’s blood until ready for use.
Fig. 2.
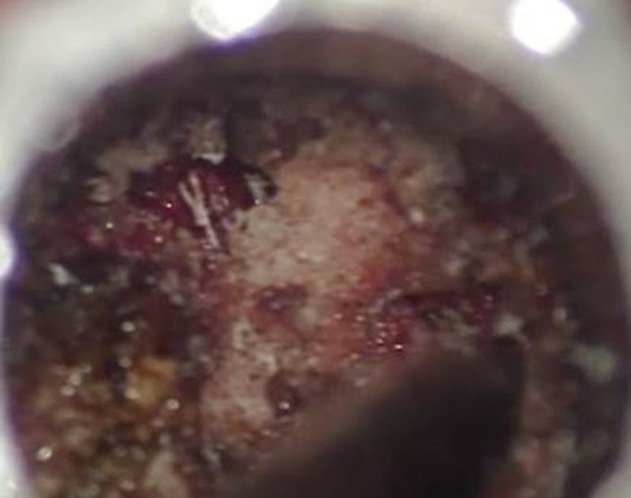
A facetectomy is shown using a combination of high-speed burr and Kerrison rongeurs.
After completion of facetectomy and exposure of the disc space, a wide annulotomy is performed to allow for a thorough discectomy and end-plate preparation. It is important that all instruments are placed in line with the disc space so there is the least likelihood of end plate violation. Passing all instruments into the disc space under fluoroscopic visualization can help with this and also allows for preparation of the entire disc space. Using angled curettes can help get across to the opposite side of the disc space and ensure near-complete disc and end-plate cartilage removal (Fig 3A-B).
Fig. 3 A-B.
(A) A wide annulotomy allows for a thorough discectomy and end-plate preparation. (B) Angled curettes can help get a surgeon to the opposite side of the disc space, ensuring near-complete disc and end-plate cartilage removal.
Once the disc space is prepared, a bone funnel is used to deliver a combination of the previously collected iliac crest and local bone to the anterior aspect of the disc space and placed along the anterior longitudinal ligament (Fig 4). An articulating interbody cage packed with the same combination of bone graft is then placed in the anterior third of the disc space. The largest implant in terms of width should be chosen in order to get the most end plate coverage from pedicle to pedicle (Fig 5A-B). By placing the implant into the anterior part of the disc space, it allows for more bone graft to be placed behind the cage thus surrounding the cage with graft. If there is more bone graft left, the opposite facet is targeted and decorticated and packed with the iliac crest and local bone combination, as well.
Fig. 4.

A bone funnel can deliver a combination of the previously collected iliac crest and local bone to the anterior aspect of the disc space and should be placed along the anterior longitudinal ligament.
Fig. 5 A-B.

(A) An articulating interbody cage packed with the same combination of bone graft should be placed in the anterior third of the disc space. (B) Surgeons should choose the largest implant in terms of width in order to get the most end-plate coverage from pedicle to pedicle.
Solid fusion, which is an important component of long-term success, can be predictably achieved by paying close attention to discectomy and end-plate preparation and by placing a combination of iliac crest autograft and local bone in front, inside, and behind a large interbody cage.
Footnotes
A note from the Editor-in-Chief: We are pleased to present the next installment of “Pearls”, a column in Clinical Orthopaedics and Related Research®. In this column, distinguished surgeons, scientists, or scholars share surgical or professional tips they use to help surmount important or interesting problems. We welcome reader feedback on all our columns and articles; please send your comments to eic@clinorthop.org.
One of the authors certifies that he (SQ) or a member of his or her immediate family, has received or may receive payments or benefits, during the study period, an amount of USD 10,000 to USD 100,000 from Stryker Spine (Kalamazoo, MI, USA), an amount of USD 10,000 to 100,000 from Globus Medical Inc, (Audubon, PA, USA), an amount of less than USD 10,000 from Zimmer Biomet (Warsaw, IN, USA), and an amount of less than USD 10,000 from RTI Surgical Inc (Alachua, FL, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
The opinions expressed are those of the writers, and do not reflect the opinion or policy of CORR® or The Association of Bone and Joint Surgeons®.
References
- 1.Schwender JD, Holly LT, Rouben DP, Foley KT. Minimally invasive transforaminal lumbar interbody fusion (TLIF): Technical feasibility and initial results. J Spinal Disord Tech. 2005;18:S1-6. [DOI] [PubMed] [Google Scholar]
- 2.Singh K, Ahmadinia K, Park DK, Nandyala SV, Marquez-Lara A, Patel AA, Fineberg SJ. Complications of spinal fusion with utilization of bone morphogenic protein: A systematic review of the literature. Spine. 2014;39:91-101. [DOI] [PubMed] [Google Scholar]


