Abstract
Background
Although surgical resection or amputation has been the mainstay of localized chondrosarcoma management for many decades, its efficacy in patients with metastatic chondrosarcoma remains unknown, and likewise we do not know whether there are any tumor- or patient-related factors associated with better survival after surgery for metastatic chondrosarcoma.
Questions/purposes
(1) Is resection of the primary tumor associated with improved survival in patients with metastatic chondrosarcoma? (2) Which subgroups of patients with chondrosarcoma benefit more from resection in terms of survival?
Methods
We identified 200 of 222 patients with metastatic chondrosarcoma in the Surveillance, Epidemiology, and End Results (SEER) database between 1988 and 2014 based on the exclusion criteria. Among those patients, 107 (53.5%) underwent primary tumor resection or amputation. Patient information, including demographics (patient age, gender, race, year of diagnosis), tumor characteristics (primary site, histologic subtype, tumor grade, tumor size), and treatment (record of operation and radiation), was collected and included in the study. Kaplan-Meier analyses, log-rank tests, competing risks framework, multivariable Cox regression modeling, and interaction tests were conducted to assess the association of primary tumor resection and survival in the overall cohort and subgroups.
Results
Resection of the primary tumor was associated with improved overall survival (hazard ratio [HR], 0.481; 95% confidence interval [CI], 0.340–0.680; p < 0.001) and cancer-specific survival (HR, 0.493; 95% CI, 0.343–0.709; p < 0.001) after controlling for confounding variables. After controlling further for age, histologic subtype, and grade, primary tumor resection was associated with a survival advantage in patients with conventional subtype and Grade II chondrosarcoma (conventional subtype: HR, 0.403; 95% CI, 0.260–0.623 for overall survival and HR, 0.396; 95% CI, 0.250–0.627 for cancer-specific survival). However, primary tumor resection was not associated with increased survival in patients with metastatic chondrosarcoma who had the dedifferentiated subtype and Grade III malignancy.
Conclusions
The present study demonstrates a possible favorable association between primary tumor resection and survival in some patients with metastatic chondrosarcoma at initial diagnosis. Specifically, patients with conventional subtypes and Grade II malignancies who underwent primary tumor resection had better survival compared with those patients who did not have primary tumor resection. Thus, there might be a benefit from primary tumor resection in these patients, but given the limitations of this database, further prospective studies or randomized trials are needed to confirm our findings. In the meantime, this information might be helpful to consider when discussing surgical options with patients who have conventional, Grade 2 metastatic chondrosarcoma at diagnosis.
Level of Evidence
Level III, therapeutic study.
Introduction
Chondrosarcoma is a malignant bone tumor characterized by the production of chondroid matrix [8]. After osteosarcoma, it is the second most common primary bone tumor and accounts for 30% of primary bone sarcomas [3]. Approximately 8% of patients with chondrosarcoma have metastasis at initial presentation [10]. Although relatively uncommon, distant metastasis has been identified as one of the most significant prognostic factors that dramatically reduces the survival time of patients with chondrosarcoma [1, 2, 4, 10].
Because radiation therapy and chemotherapy have limited effects, surgical resection or amputation is the mainstay of localized chondrosarcoma management [9, 11]. However, there is no standard treatment for patients with metastatic chondrosarcoma. The favorable effect of resection of the primary tumor has been reported in patients with metastatic renal cancer, colorectal cancer, and breast cancer [7, 18]. As a result of the rarity of metastatic chondrosarcoma, data regarding the potential association of primary tumor resection with longer survival are nonexistent. Therefore, in this study, we collected a nationwide, population-based cohort from the Surveillance, Epidemiology, and End Results (SEER) database to assess the possible association of primary tumor resection in patients with metastatic chondrosarcoma and to explore whether and for whom primary tumor resection should be considered.
We asked the following questions: (1) Is resection of the primary tumor associated with improved survival in patients with metastatic chondrosarcoma? (2) Which subgroups of patients with chondrosarcoma benefit more from resection in terms of survival?
Patients and Methods
Database and Patient Cohort
The SEER database is the largest publicly available cancer data set, consisting of 18 cancer registries and covering approximately 28% of the total US population. The SEER database has been widely used in clinical cancer research of different cancer types, including chondrosarcoma [6, 10, 14, 17, 18]. We used SEER*Stat software (Version 8.3.4; National Cancer Institute, Bethesda, MD, USA) to extract information from the database.
To be included, 222 patients in total had to have high-grade chondrosarcoma with distant metastasis present at initial diagnosis, chondrosarcoma as the first primary malignancy, be diagnosed between 1988 and 2014 and with no missing data on survival time, have their diagnosis confirmed by histology, and have their primary site limited to a bone only. We excluded patients for the following reasons: if the primary site was limited to the skull or facial bones (n = 6) resulting from the rarity in this subgroup, if they had mesenchymal chondrosarcoma (n = 12) resulting from the rarity in this subgroup, or if it was unknown whether a primary tumor operation or radiation was performed. Finally, 200 of 222 patients with metastatic chondrosarcoma were included in the study (see Figure, Supplemental Digital Content).
Patient Characteristics
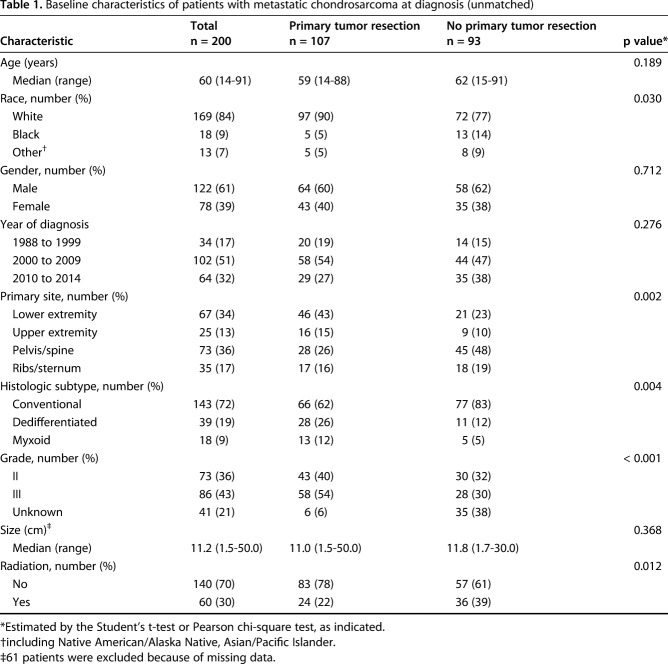
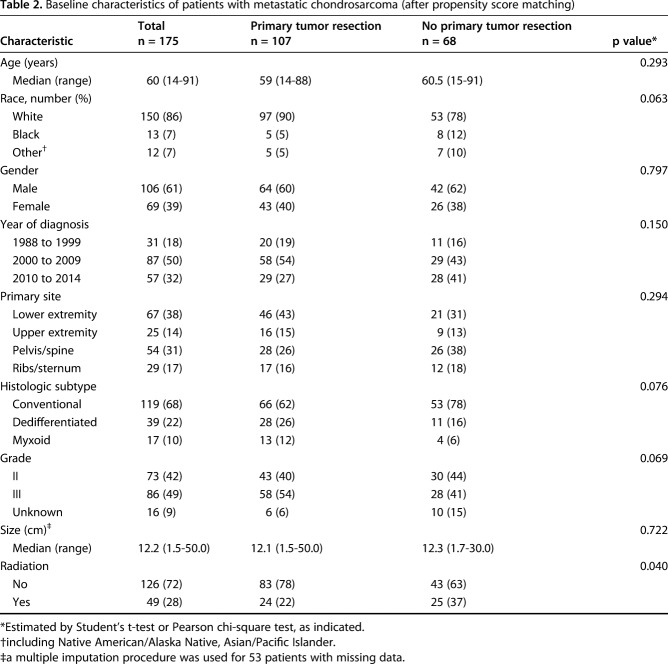
A total of 200 patients with metastatic chondrosarcoma at the time of initial diagnosis were included in our study (Fig. 1). Of the 200 patients, 107 (53%) underwent primary tumor resection or amputation (surgery group), whereas 93 (47%) did not (nonsurgery group). The median age of all patients was 60 years (range, 14-91 years). In all, 92 patients (46%) had a primary lesion in their extremities, and 73 patients (36%) had a primary lesion in the pelvis or spine. The overall cohort was divided according to whether patients had undergone primary tumor resection. The median survival was 13 months (range, 1-206 months) and 7 months (range, 1-200 months) in the surgery and nonsurgery groups, respectively. We found that patients who had surgery were more likely to be white, to have a tumor located in the appendicular skeleton, to have a dedifferentiated subtype, and were less likely to undergo radiotherapy (Table 1). To account for these baseline differences between the surgery and nonsurgery groups, we performed propensity score matching. With propensity score matching, imbalance regarding race, primary site, histologic subtype, and grade was mitigated (Table 2).
Fig. 1.
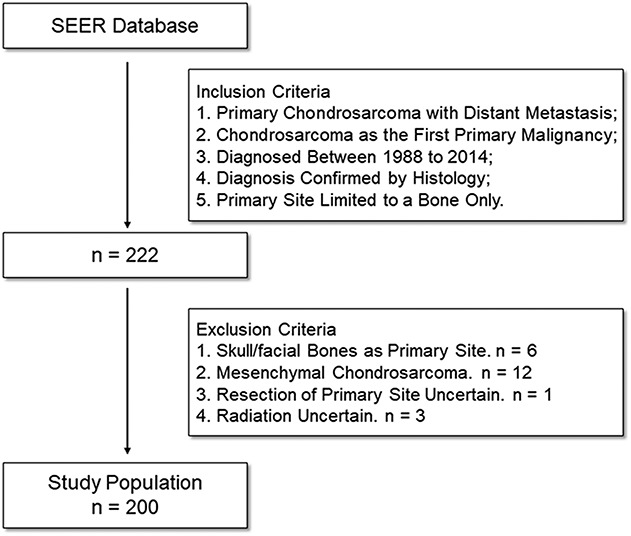
The flowchart shows the process of collecting patients. Overall, 200 patients with metastatic chondrosarcoma were included.
Table 1.
Baseline characteristics of patients with metastatic chondrosarcoma at diagnosis (unmatched)
Table 2.
Baseline characteristics of patients with metastatic chondrosarcoma (after propensity score matching)
Covariates and Outcomes
We collected data on demographics (patient age, gender, race, year of diagnosis), tumor characteristics (primary site, histologic subtype, tumor grade, tumor size), and treatment (record of primary tumor resection and radiation). Because the anatomic site information in the SEER database is relatively nonspecific—for example, the femur, tibia, and fibula were recorded as long bones of the lower extremities without distinction—primary sites were categorized into four groups: lower extremity, upper extremity, pelvis/spine, and ribs/sternum. Primary tumor resection referred to resection of the primary lesion. Because metastasectomy was performed in only two patients, this variable was not included in our study. Eligible patients were grouped according to whether they had undergone primary tumor resection.
The major endpoints of interest were overall survival (OS) and cancer-specific survival (CSS). OS was defined as the time from diagnosis until death from all possible causes or last followup. CSS was defined as the time from diagnosis until death attributed to chondrosarcoma or last followup.
Statistical Analysis
We compared between-group differences with the Student’s t-test for continuous variables and Pearson’s chi-square test for categorical variables. We used a multiple imputation method to analyze the missing data for tumor size. Survival curves were drawn and compared using the Kaplan-Meier method and log-rank analysis. Cumulative incidence curves were drawn and compared under a competing risk framework. The initial Cox regression analysis involved a simple univariate model for each variable, including primary tumor resection, age, race, gender, year of diagnosis, primary site, histologic subtype, grade, size, and radiation. We created a multivariable Cox regression model by selecting only those variables with a substantial measure of association (< 0.10). The first multivariable model included primary tumor resection, age, histologic subtype, and grade. Then, we created a second multivariable model that included all collected variables to verify the stability of our findings.
We conducted the Student’s t-test, Pearson’s chi-square test, multiple imputation, univariate and multivariable Cox regression analyses, and interaction test using SPSS 22.0 (IBM Corp, Armonk, NY, USA). The Kaplan-Meier survival curves and log-rank analysis were performed using GraphPad Prism 7.0 (GraphPad Software, San Diego, CA, USA). Propensity score matching, cumulative incidence curves, and Gray tests were conducted in R version 3.3.1 (http://www.r-project.org/). Forest plots summarizing the results of subgroup multivariable analyses were drawn using Stata 14.0 (StataCorp, College Station, TX, USA). All p values were two-sided and p < 0.05 was considered statistically significant except for the interaction test (a threshold at 0.10 was set).
Results
Association Between Resection of the Primary Tumor and Survival in the Overall Cohort
Patients who underwent surgery had improved OS at 2 years compared with those who did not (36.5% [95% confidence interval {CI}, 27.3%-45.7%] versus 9.9% [95% CI, 2.1%-17.7%]; p < 0.001) (Fig. 2A). Likewise, patients who underwent surgery had improved cancer-specific survival at 2 years compared with those who did not (38.0% [95% CI, 28.6%-47.4%] versus 11.3% [95% CI, 2.5%-20.1%]; p = 0.005) (Fig. 2B).
Fig. 2 A-B.
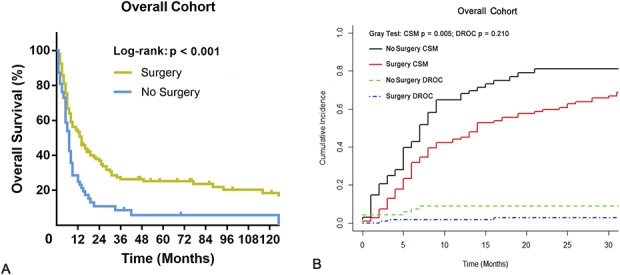
The graphs show Kaplan-Meier and cumulative incidence curves of (A) overall and (B) cancer-specific survival according to whether patients underwent primary tumor surgery in the overall cohort. CSM = cancer-specific mortality; DROC = death resulting from other causes.
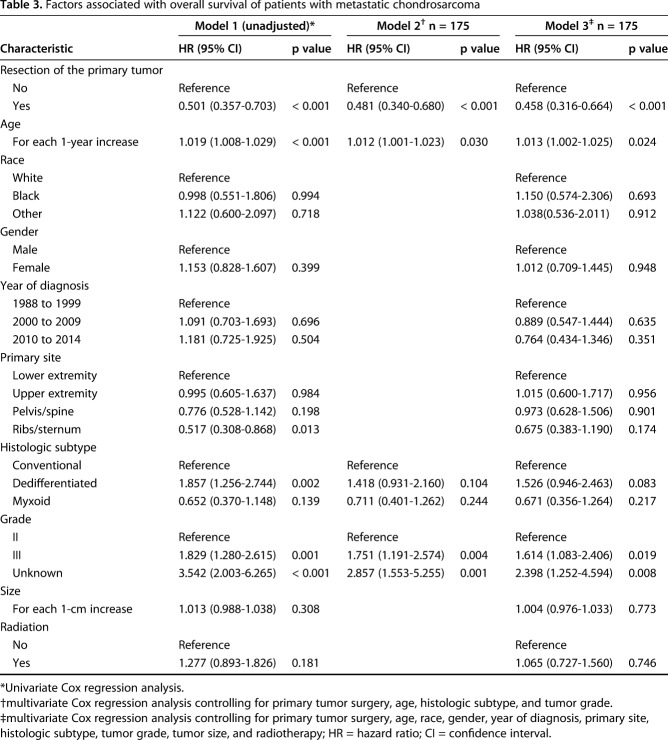
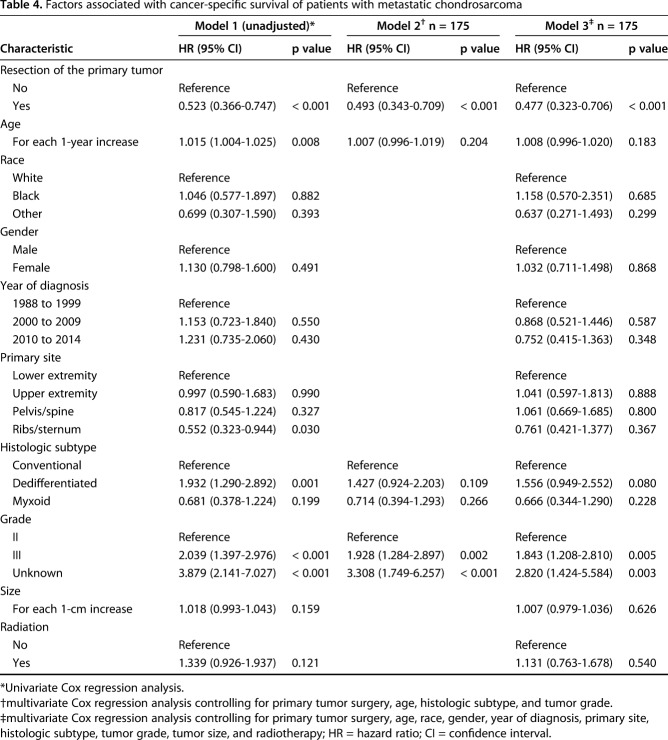
After controlling for resection of the primary tumor, age, histologic subtype, and grade revealed that primary tumor resection was associated with improved OS (hazard ratio [HR], 0.481; 95% CI, 0.340–0.680; p < 0.001) (Table 3). Likewise, primary tumor resection was associated with improved CSS (HR, 0.493; 95% CI, 0.343–0.709; p < 0.001) (Table 4).
Table 3.
Factors associated with overall survival of patients with metastatic chondrosarcoma
Table 4.
Factors associated with cancer-specific survival of patients with metastatic chondrosarcoma
Resection of the Primary Tumor on Survival in Different Subgroups
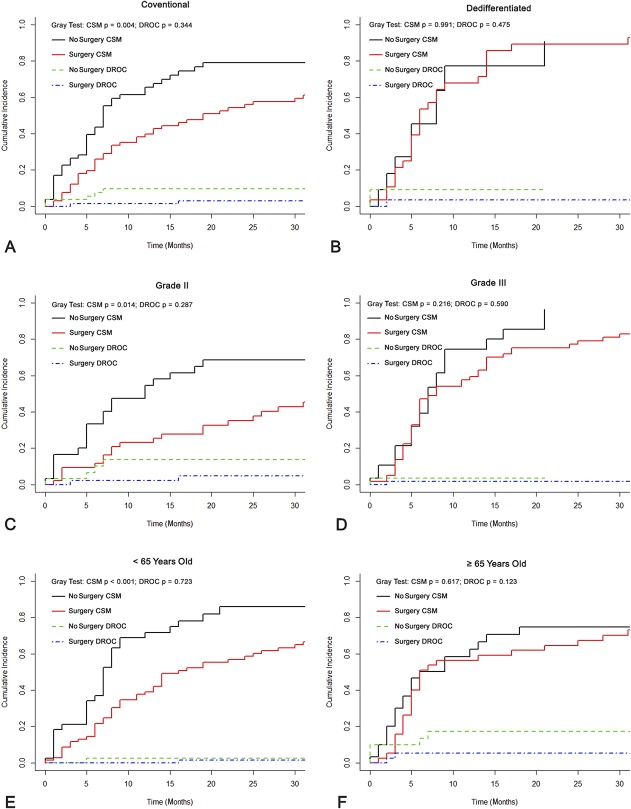
Resection of the primary tumor was associated with improved OS at 2 years in patients with the conventional subtype (40.8% [95% CI, 28.7%-52.9%] versus 11.2% [95% CI, 2.2%-20.2%]; p < 0.001), Grade II malignancy (60.0% [95% CI, 45.2%-74.8%] versus 17.5% [95% CI, 3.6%-31.4%]; p < 0.001), and an age younger than 65 years old (39.9% [95% CI, 28.2%-51.6%] versus 11.3% [95% CI, 0.1%-22.5%]; p < 0.001) (Fig. 3). Likewise, primary tumor resection was associated with improved CSS at 2 years in those subgroups (conventional subtype: 42.8% [95% CI, 30.5%-55.1%] versus 13.1% [95% CI, 2.7%-23.5%], p = 0.004; Grade II malignancy: 63.7% [95% CI, 49.7%-77.7%] versus 21.8% [95% CI, 5.2%-38.4%], p = 0.014; younger than 65 years old: 40.8% [95% CI, 28.7%-52.9%] versus 11.7% [95% CI, 0.2%-23.2%], p < 0.001) (Fig. 4). However, in patients with dedifferentiated subtype or Grade III malignancy, resection of the primary tumor did not improve OS at 6 months (dedifferentiated subtype: 42.9% [95% CI, 33.5%-52.3%] versus 45.5% [95% CI, 30.5%-60.5%], p = 0.703; Grade III malignancy: 51.1% [95% CI, 38.2%-64.0%] versus 57.1% [95% CI, 47.7%-66.5%], p = 0.217). It is noteworthy that patients 65 years old or older had prolonged OS but not CSS at 2 years after resection of the primary tumor (OS: 30.0% [95% CI, 22.4%-37.6%] versus 8.0% [95% CI, 2.6%-13.4%], p = 0.040; CSS: 31.8% [95% CI, 23.9%-39.7%] versus 10.9% [95% CI, 3.8%-18.0%], p = 0.123).
Fig. 3 A-F.
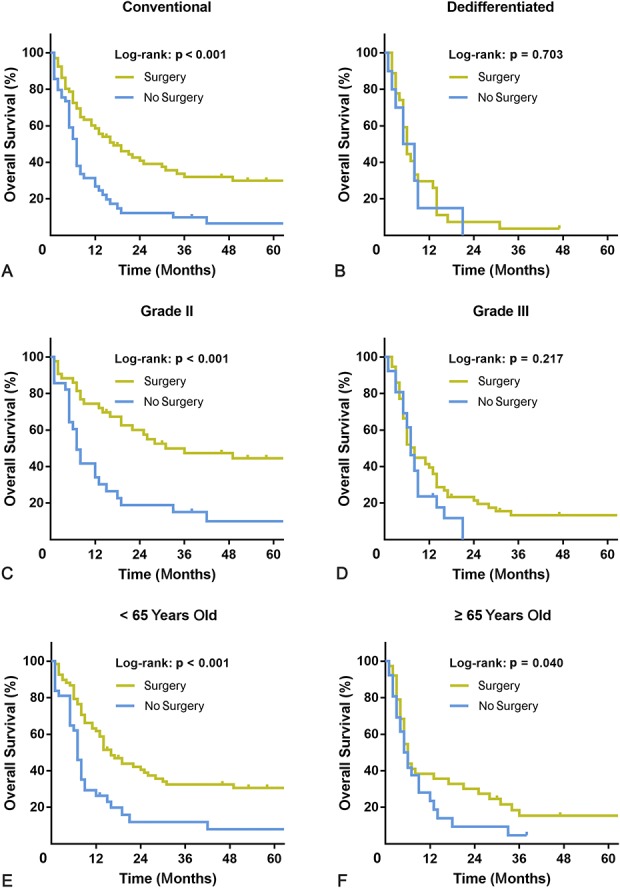
The graphs show Kaplan-Meier curves of overall survival according to whether patients underwent primary tumor surgery in (A) the conventional subtype, (B) dedifferentiated subtype, (C) Grade II malignancy, (D) Grade III malignancy, (E) patients < 65 years old, or (F) patients ≥ 65 years old.
Fig. 4 A-F.
The graphs show cumulative incidence curves of cancer-specific survival according to whether patients underwent primary tumor surgery in (A) the conventional subtype, (B) dedifferentiated subtype, (C) Grade II malignancy, (D) Grade III malignancy, (E) patients < 65 years old, or (F) patients ≥ 65 years old. CSM = cancer-specific mortality; DROC = death resulting from other causes.
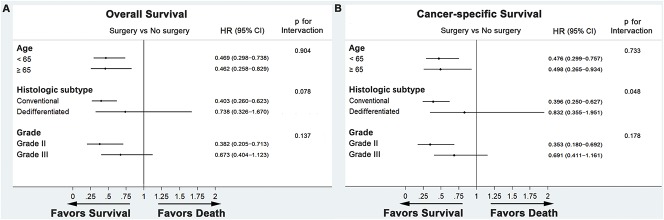
After controlling for age, histologic subtype, and grade, resection of the primary tumor was associated with increased survival in patients with the conventional subtype (OS: HR, 0.403, 95% CI, 0.260–0.623, p < 0.001; CSS: HR, 0.396, 95% CI, 0.250–0.627, p < 0.001) and Grade II chondrosarcoma (OS: HR, 0.382, 95% CI, 0.205–0.713, p = 0.002; CSS: HR, 0.353, 95% CI, 0.180–0.692, p = 0.002), but not in patients with the dedifferentiated subtype (OS: HR, 0.738, 95% CI, 0.326–1.670, p = 0.466; CSS: HR, 0.832, 95% CI, 0.355–1.951, p = 0.673) or Grade III chondrosarcoma (OS: HR, 0.673, 95% CI, 0.404–1.123, p = 0.130; CSS: HR, 0.691, 95% CI, 0.411–1.161, p = 0.162) (Fig. 5). For patients < 65 years old and ≥ 65 years old, respectively, we observed an association between primary tumor resection and improved survival (< 65 years: OS--HR, 0.469, 95% CI, 0.298–0.738, p = 0.001; CSS--HR, 0.476, 95% CI, 0.299–0.757, p = 0.002; ≥ 65 years: OS--HR, 0.462, 95% CI, 0.258–0.829, p = 0.010; CSS--HR, 0.498, 95% CI, 0.265–0.934, p = 0.030). Interaction tests were further carried out to investigate the potential differential effect of resection in different subgroups. With the numbers available, we could not demonstrate a differential surgical effect between conventional and dedifferentiated subtypes (p = 0.078 for OS, p = 0.048 for CSS).
Fig. 5 A-B.
Forest plots summarize the HRs and 95% CIs of (A) overall and (B) cancer-specific survival according to whether patients underwent primary tumor surgery in subgroup analyses.
Discussion
For many decades, because radiotherapy or chemotherapy is not considered effective as a result of the extracellular matrix and poor vascularity, the primary treatment option with curative intent has been surgical resection [9, 10, 15, 20]. However, for patients with metastatic chondrosarcoma, no standard treatment has been established, and whether surgery still confers a survival advantage is worth further investigation. Our investigation demonstrates that there is an association between primary tumor resection in patients with metastatic chondrosarcoma and longer survival based on a large patient cohort. More importantly, the present study also finds that patients with metastatic chondrosarcoma with conventional subtypes and Grade II malignancies who underwent primary tumor resection had better survival compared with those patients who did not have primary tumor resection. This information might be helpful to consider when discussing surgical options with patients who have conventional, Grade 2 metastatic chondrosarcoma at diagnosis.
We would like to acknowledge the limitations of the present study. First, at baseline, the surgical groups were dissimilar in important ways, including race (surgery was more common in white patients than in nonwhite patients), tumor location (resections were more common in appendicular lesions than axial ones), histologic subtype (resections were more common in dedifferentiated and myxoid subtypes), tumor grade (resections were more common in Grade III cases), and use of radiation (resections were common in patients who did not undergo radiotherapy). In addition, the nature of the SEER registry does not provide information about how the decision on whether to operate was made. To address these issues, we performed propensity score matching, multivariable analyses, and further subgroup analyses with interaction tests to reduce potential confounding. Second, some unidentified prognostic factors such as the extent of metastatic disease, surgical margin, and location of the metastases (only available between 2010 and 2014) were not collected in the SEER registry and may have contributed to the observed results. Nevertheless, considering the use of multiple methods to investigate the association and the large beneficial effect of primary tumor resection on survival, it seems unlikely that such effect was solely the result of unadjusted confounding. Third, 53 patients (30%) had missing tumor size data. However, we accounted for these missing data with a multiple imputation procedure. Based on the assumption that the data were missing at random, multiple imputation could reconstruct the missing values using the information from observed data to decrease bias resulting from missing size. Finally, our study is based on registry data, which is retrospective by nature and lack validated tools to explain not merely whether those patients are surviving, but also whether they are functioning well.
We observed that patients with chondrosarcoma presenting with metastases at diagnosis who had resection of their primary tumor had longer survival than patients who did not have the primary tumor resected. We do not know the reason for this association in these patients, but accumulating studies have been reported to justify primary tumor resections for patients with metastatic cancer. The possible mechanism of the association we found could be that resection of the primary tumor may reduce the tumor burden [12, 13], remove the “seed source” to retard cancer progression [16], reverse systemic inflammation [19], or restore immunomodulation by removing a potential source of immunosuppression from the primary tumor site [5]. However, to our knowledge, these theories have not been corroborated in patients with metastatic chondrosarcoma.
We also found that patients with conventional chondrosarcoma benefited from resection of the primary tumor, but patients with dedifferentiated chondrosarcoma did not. Interaction tests suggest a differential surgical effect might exist between conventional and dedifferentiated subtypes. Because conventional chondrosarcoma constitutes approximately 85% of all chondrosarcomas, our findings are applicable to most patients who have metastatic chondrosarcoma. Primary tumor resection was associated with prolonged survival in patients with Grade II chondrosarcoma but not in patients with Grade III chondrosarcoma. The results could be partially explained by the limited efficacy of primary tumor resection on a higher degree of malignancy. It is also possible that this observation is the result of selection bias because we do not know how patients were chosen to receive resection nor do we know the metastatic extent in these patients. It is possible that patients with higher grade tumors had more extensive metastatic disease and larger tumors; these patients might have been considered less likely to benefit from surgical resection of their primary tumor. Competing risks framework suggests that older patients (≥ 65 years old) did not have prolonged CSS after primary tumor resection. After controlling for confounding variables, including histologic subtype and tumor grade, primary tumor resection was found to be associated with a survival advantage in both younger patients (< 65 years old) and older patients (≥ 65 years old) regarding OS and CSS. Interaction tests showing no differential surgical effect between younger and older groups supports the finding in the subgroup analyses.
In summary, the present study demonstrates an association of primary tumor resection on survival in some patients with metastatic chondrosarcoma at initial diagnosis, especially those with Grade 2 chondrosarcoma. We also found that resection of the primary tumor was not associated with improved survival for high-grade chondrosarcoma. We hope that our study will stimulate prospective studies to confirm our observations because we could not determine metastatic burden or the decision-making considerations for tumor resection in these patients, but our findings might be considered when counseling patients with metastatic chondrosarcoma.
Footnotes
Each author certifies that neither he or she, nor any member of his or her immediate family, has funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution waived approval for the reporting of this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at Huashan Hospital, Shanghai, China.
References
- 1.Andreou D, Ruppin S, Fehlberg S, Pink D, Werner M, Tunn PU. Survival and prognostic factors in chondrosarcoma: results in 115 patients with long-term follow-up. Acta Orthop. 2011;82:749-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelini A, Guerra G, Mavrogenis AF, Pala E, Picci P, Ruggieri P. Clinical outcome of central conventional chondrosarcoma. J Surg Oncol. 2012;106:929-937. [DOI] [PubMed] [Google Scholar]
- 3.Biermann JS, Chow W, Reed DR, Lucas D, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, Didwania A, Fabbri N, Hornicek FJ, Kuechle JB, Lindskog D, Mayerson J, McGarry SV, Million L, Morris CD, Movva S, O’Donnell RJ, Randall RL, Rose P, Santana VM, Satcher RL, Schwartz H, Siegel HJ, Thornton K, Villalobos V, Bergman MA, Scavone JL. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J Natl Compr Canc Netw. 2017;15:155-167. [DOI] [PubMed] [Google Scholar]
- 4.Bruns J, Elbracht M, Niggemeyer O. Chondrosarcoma of bone: an oncological and functional follow-up study. Ann Oncol. 2001;12:859-864. [DOI] [PubMed] [Google Scholar]
- 5.Danna EA, Sinha P, Gilbert M, Clements VK, Pulaski BA, Ostrand-Rosenberg S. Surgical removal of primary tumor reverses tumor-induced immunosuppression despite the presence of metastatic disease. Cancer Res. 2004;64:2205-2211. [DOI] [PubMed] [Google Scholar]
- 6.Duchman KR, Lynch CF, Buckwalter JA, Miller BJ. Estimated cause-specific survival continues to improve over time in patients with chondrosarcoma. Clin Orthop Relat Res . 2014;472:2516-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flanigan RC, Salmon SE, Blumenstein BA. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med . 2001;345:1655-1659. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher CDM, Unni KK, Mertens F. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002:247–258. [Google Scholar]
- 9.Gelderblom H, Hogendoorn PC, Dijkstra SD, van Rijswijk CS, Krol AD, Taminiau AH, Bovée JV. The clinical approach towards chondrosarcoma. Oncologist . 2008;13:320-329. [DOI] [PubMed] [Google Scholar]
- 10.Giuffrida AY, Burgueno JE, Koniaris LG, Gutierrez JC, Duncan R, Scully SP. Chondrosarcoma in the United States (1973 to 2003): an analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063-1072. [DOI] [PubMed] [Google Scholar]
- 11.Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, Chaigneau L, Penel N, Duffaud F, Kurtz JE, Collard O, Bertucci F, Bompas E, Le Cesne A, Maki RG, Ray Coquard I, Blay JY. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol . 2013;24:2916-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khan SA. Surgery for the intact primary and stage IV breast cancer...lacking ‘robust evidence.’ Ann Surg Oncol. 2013;20:2803-2805. [DOI] [PubMed] [Google Scholar]
- 13.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132:620-627. [DOI] [PubMed] [Google Scholar]
- 14.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: an analysis of the SEER database. J Bone Joint Surg Am. 2013;95:e89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nota SP, Braun Y, Schwab JH, van Dijk CN, Bramer JA. The identification of prognostic factors and survival statistics of conventional central chondrosarcoma. Sarcoma. 2015;2015:623746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pockaj BA, Wasif N, Dueck AC, Wigle DA, Boughey JC, Degnim AC, Gray RJ, McLaughlin SA, Northfelt DW, Sticca RP, Jakub JW, Perez EA. Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer: time for a second look? Ann Surg Oncol . 2010;17:2419-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi X, Dong F, Wei W, Song K, Huang N, Lu Z, Lei B, Yu P, Liu W, Wang Y, Sun G, Wang Y, Ji Q. Prognostic significance and optimal candidates of primary tumor resection in major salivary gland carcinoma patients with distant metastases at initial presentation: a population-based study. Oral Oncol . 2018;78:87-93. [DOI] [PubMed] [Google Scholar]
- 18.Tarantino I, Warschkow R, Worni M. Prognostic relevance of palliative primary tumor removal in 37,793 metastatic colorectal cancer patients: a population-based, propensity score-adjusted trend analysis. Ann Surg . 2015;262:112-120. [DOI] [PubMed] [Google Scholar]
- 19.Turner N, Tran B, Tran PV. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer. 2015;14:185-191. [DOI] [PubMed] [Google Scholar]
- 20.van Maldegem AM, Gelderblom H, Palmerini E, Dijkstra SD, Gambarotti M, Ruggieri P, Nout RA, van de Sande MA, Ferrari C, Ferrari S, Bovée JV, Picci P. Outcome of advanced, unresectable conventional central chondrosarcoma. Cancer. 2014;120:3159-3164. [DOI] [PubMed] [Google Scholar]