Abstract
Background
Although use of nonsteroidal antiinflammatory drugs and low-dose irradiation has demonstrated efficacy in preventing heterotopic ossification (HO) after THA and surgical treatment of acetabular fractures, these modalities have not been assessed after traumatic blast amputations where HO is a common complication that can arise in the residual limb.
Questions/purposes
The purpose of this study was to investigate the effectiveness of indomethacin and irradiation in preventing HO induced by high-energy blast trauma in a rat model.
Methods
Thirty-six Sprague-Dawley rats underwent hind limb blast amputation with a submerged explosive under water followed by irrigation and primary wound closure. One group (n = 12) received oral indomethacin for 10 days starting on postoperative Day 1. Another group (n = 12) received a single dose of 8 Gy irradiation to the residual limb on postoperative Day 3. A control group (n = 12) did not receive either. Wound healing and clinical course were monitored in all animals until euthanasia at 24 weeks. Serial radiographs were taken immediately postoperatively, at 10 days, and every 4 weeks thereafter to monitor the time course of ectopic bone formation until euthanasia. Five independent graders evaluated the 24-week radiographs to quantitatively assess severity and qualitatively assess the pattern of HO using a modified Potter scale from 0 to 3. Assessment of grading reproducibility yielded a Fleiss statistic of 0.41 and 0.37 for severity and type, respectively. By extrapolation from human clinical trials, a minimum clinically important difference in HO severity was empirically determined to be two full grades or progression of absolute grade to the most severe.
Results
We found no differences in mean HO severity scores among the three study groups (indomethacin 0.90 ± 0.46 [95% confidence interval {CI}, 0.60-1.19]; radiation 1.34 ± 0.59 [95% CI, 0.95-1.74]; control 0.95 ± 0.55 [95% CI, 0.60-1.30]; p = 0.100). For qualitative HO type scores, the radiation group had a higher HO type than both indomethacin and controls, but indomethacin was no different than controls (indomethacin 1.08 ± 0.66 [95% CI, 0.67-1.50]; radiation 1.89 ± 0.76 [95% CI, 1.38-2.40]; control 1.10 ± 0.62 [95% CI, 0.70-1.50]; p = 0.013). The lower bound of the 95% CI on mean severity in the indomethacin group and the upper bound of the radiation group barely spanned a full grade and involved only numeric grades < 2, suggesting that even if a small difference in severity could be detected, it would be less than our a priori-defined minimum clinically important difference and any differences that might be present are unlikely to be clinically meaningful.
Conclusions
This work unexpectedly demonstrated that, compared with controls, indomethacin and irradiation provide no effective prophylaxis against HO in the residual limb after high-energy blast amputation in a rat model. Such an observation is contrary to the civilian experience and may be potentially explained by either a different pathogenesis for blast-induced HO or a stimulus that overwhelms conventional regimens used to prevent HO in the civilian population.
Clinical Relevance
HO in the residual limb after high-energy traumatic blast amputation will likely require novel approaches for prevention and management.
Introduction
Heterotopic ossification (HO) is a type of ectopic bone formation in soft tissues characterized by lamellar organization and the presence of marrow that is often associated with traumatic injuries and hip arthroplasty [10]. Likewise, HO in the residual limb after blast amputation is a known complication in soldiers who survive high-energy trauma. With the advent of more advanced weaponry and explosives producing increased blast forces, more extensive soft tissue injuries have been observed in combatants of recent military conflicts [9, 12]. At the same time, substantial improvements in armor and emergency care on the battlefield have been responsible for the increased survival of soldiers after these severe injuries, which were previously most often fatal [24, 35]. In recent conflicts, blast injuries from improvised explosive devices have become the most common cause of injury requiring in-theater trauma center care, even as in-theater facilities such as the Role III Hospital at Kandahar Airfield now see relatively low overall mortality rates (4.45% from 2009 to 2010) [3]. This suggests that military personnel are more likely to sustain and survive blast injuries than in previous conflicts.
In their analysis of > 350 traumatic and combat-related amputations treated at military medical centers between 2001 and 2005, Potter et al. [36] reported a 63% prevalence of HO in residual limbs after extremity blast amputations. Of note, they found that 80% of patients with blast injuries and a final level of amputation within the zone of injury were associated with formation of HO [35, 36]. HO formation is both challenging and clinically important given that it may cause considerable pain and loss of function in addition to muscle and skin breakdown [10]. Each of these problems may result in multiple revision surgical procedures and compromise prosthetic fitting and functional use [1, 2, 9, 12, 17, 30, 35, 39].
Several Level I clinical studies have demonstrated the efficacy of preemptive administration of nonsteroidal antiinflammatory drugs (NSAIDs) or low-dose external beam irradiation to prevent HO after THA and surgical treatment of acetabular fractures [5, 15, 21, 25, 31, 32]. These prophylactic treatments have comparable efficacy in civilian practice [5, 25, 31], but neither has been studied in the setting of high-energy blast trauma on the battlefield.
Our previous work has led to the establishment of a blast amputation rat model, which reliably produces heterotopic bone without the addition of any exogenous osteogenic agents [43]. From this model, we aimed to investigate the effect of NSAIDs and external beam radiation on the mitigation of ectopic bone formation in the blast-amputated extremity. We hypothesized that one or both prophylactic regimens would limit or prevent HO in the residual limb.
Materials and Methods
All procedures in this study were performed under a protocol reviewed and approved by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine as well as the Animal Care and Use Review Office of the US Army Medical Research and Materiel Command. This work was funded by the Congressionally Directed Medical Research Program of the Department of Defense under contract # WB1XWH-10-1-0975.
A total of 36 male Sprague-Dawley rats (Harlan Laboratories Inc, Indianapolis, IN, USA) aged 16 to 18 weeks old and weighing approximately 400 g were used in this study. Male rats were chosen because our previous work has been with male rats and their long bones reliably have adequate bone quality [43]. Each animal underwent a left hind limb blast amputation under a well-established experimental protocol. Three groups of 12 animals allowed for systematic study of the interventions; Group 1 (N = 12) served as controls, Group 2 (N = 12) received oral indomethacin, and Group 3 (N = 12) underwent radiation treatment after the blast procedure.
General Animal Preparation
Anesthesia was induced with isoflurane at 3.5% to 4.5% and maintained with 1.5% to 2.5% inhaled isoflurane through a nose cone. Once sedated, an ocular protective lubricant was applied bilaterally. The left hind limb and ipsilateral side of the lower back and abdomen were cleared of hair with an electric clipper and cleansed three times with alternating solutions of Betadine scrub (Purdue Products, Stamford, CT, USA) and 70% isopropyl alcohol in an effort to reduce the likelihood of infection that might potentiate formation of HO. Buprenorphine (0.05 mg/kg) and enrofloxacin (5 mg/kg) were administered subcutaneously for preemptive analgesia and prophylactic antibacterial coverage, respectively. Preemptive administration of 6 to 10 mL of warmed 0.9% normal saline was provided subcutaneously for volume resuscitation in anticipation of hemorrhage related to the blast amputation trauma.
Extremity Blast Amputation
Maintaining deep anesthesia through a nose cone, the rat was positioned prone and tightly secured with industrial strength Velcro® (Velcro USA, Inc, Manchester, NH, USA) on a 2-inch-thick aluminum platform that features a 2.5-inch hole in its center (Fig. 1). The left hind limb was held across the hole, centered at the desired amputation level through the midtibia, with the use of a silk suture and duct tape. The platform was located above a 2 foot x 2 foot x 2-foot steel tank filled with tap water. An explosive (0.75 g of pentaerythritol tetranitrate) was submerged 0.5 inch below the surface of the water, directly beneath the center of the hole in the platform. The explosive was detonated using a commercially available detonation box. The resulting chemical reaction created a large volume of hot, high-pressure gases, which acted against the surrounding water, and accelerated it upward at velocities approaching four times the speed of sound in air (Mach 4). These very high velocities were converted into pressure when the propelled water impacted both the plate and the animal’s exposed extremity. Pressures on the order of tens of thousands of pounds per square inch were absorbed by the limb, which was cleanly amputated.
Fig. 1.
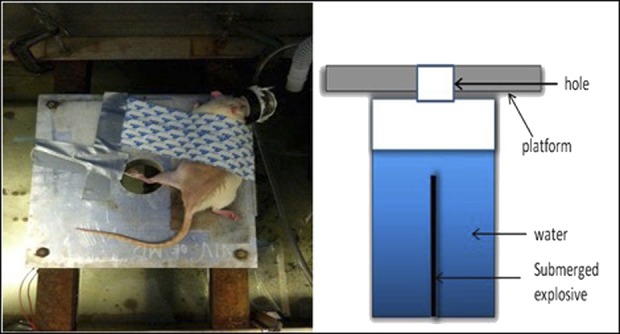
The standard setup for blast amputation of the left hind limb of a Sprague-Dawley rat is pictured.
Postblast Wound Care
After amputation, the animals were immediately transferred to an adjacent sterile operating table over an insulated heating pad while maintained on inhaled isoflurane anesthesia through the nose cone. Minimal blood loss was observed, presumably as a result of cauterization of the vessels from the high temperature and pressure of the blast. The traumatic wounds were manually irrigated with 250 mL of a 40:1 normal saline:2% chlorhexidine solution with a 50-mL bulb syringe. The amputated bone was examined and sharp edges were minimally filed and beveled or trimmed with a rongeur, as necessary, to facilitate closure. No muscle was débrided. Rather, the muscle in the residual limb was inverted over the edge of the bone and closed with a running 4-0 Vicryl® suture (Ethicon, Inc, Somerville, NJ, USA) in the fascia to ensure adequate soft tissue coverage. The charred skin edges were conservatively but sharply débrided to facilitate primary wound healing. The skin was closed with staples and sealed with tissue adhesive (Histoacryl®; B. Braun Corporation, Bethlehem, PA, USA).
Postprocedural Care
Each animal was placed in a separate clean recovery cage with fresh bedding and an isothermal heating pad under the bedding and closely monitored for signs of distress during emergence from anesthesia. All animals received antibiotics (5 mg/kg enrofloxacin subcutaneously twice a day) for a total of 3 days postoperatively. Likewise, analgesia with buprenorphine (0.05 mg/kg subcutaneously three times daily) was provided for 5 days for all animals. Staples were removed at postoperative Week 2. Animals were monitored twice daily for the first 3 days, then daily for 5 days, and then at least twice a week until euthanasia for weight changes, general appearance, activity level, breathing patterns and rates, feeding and excretion, ambulation, and incision site changes. Additional analgesia was provided as dictated by physiological signs of animal discomfort.
HO Prophylaxis Interventions
NSAIDs
Starting on the first postoperative day, Group 2 animals received a 10-day course of orally administered indomethacin suspension at a dose of 3 mg/kg once daily through syringe feeding.
External Beam Irradiation
On postoperative Day 3, all animals assigned to Group 3 received external beam radiation. Anesthesia was induced as previously described with isoflurane at 3.5% to 4.5% in an induction chamber. Thereafter the animals were transferred and secured with tape on a custom-fabricated platform, where maintenance anesthesia was administered through a nose cone at 1.5% to 2.5% isoflurane. The platform served as a customized irradiation apparatus and was fitted with a quarter-inch thick lead and a 1.5 x 1.5-inch aperture to restrict irradiation to only the amputated residual limb. A PANTAK X-ray unit (Elimpex-Medizintechnik, Moedling, Austria) operated at 250 kVP delivered a single dose of 8 Gy, calculated to be biologically equivalent to the human dose used for HO prophylaxis, from below the protective metallic shield through the aperture.
Outcome Assessment
Clinical
Of the 36 animals that underwent left hind limb blast amputation, all survived the procedure without untoward events. The animals typically ambulated in a tripod configuration almost immediately on recovery from anesthesia and returned to their preinjury baseline activities within 1 week after the procedure. One animal assigned to the irradiation group died 2 weeks after the procedure of unknown causes and was not replaced. Wound healing in the residual limb was monitored on a regular basis, and skin staples were routinely removed 2 weeks after the blast procedure. Late wound complications were likewise monitored and surgical revision with secondary closure was performed for delayed wound breakdown.
Radiographic
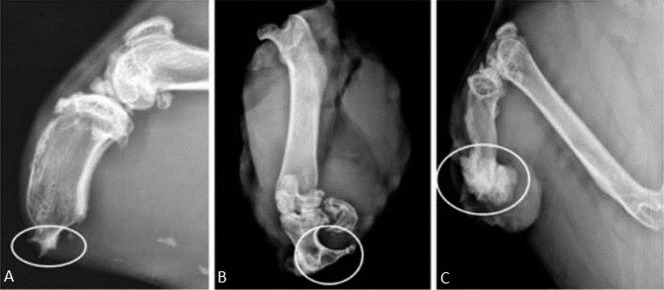
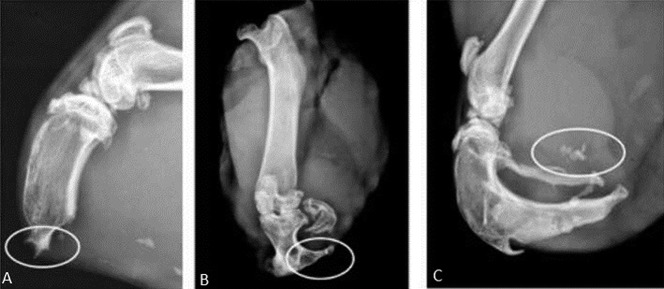
Modeled after a prior pilot study [43], the radiographic presence of heterotopic bone in the residual limb was the primary outcome measure. The animals were imaged with orthogonal views on a small animal digital radiography machine (Faxitron X-Ray LLC, Lincolnshire, IL, USA) immediately postoperatively, at 10 days, and every 4 weeks thereafter until euthanasia at 6 months. Assessment of HO was modified based on proportionality with the residual tibia from the method originally described in humans by Potter et al. (Table 1) [36]. Severity of ectopic bone was characterized as absent (0), mild (1), moderate (2), or severe (3) based on the amount of HO formed in relation to tibial shaft thickness (Fig. 2). Orthogonal digital radiographic views of the residual limb were utilized for severity assessment and the highest grade of bone formation noted on the two views determined the final grade. Ectopic bone that spanned < 25% of the width of the tibial plateau was classified as mild (Fig. 2A); between 25% and 50% of the width of the tibial plateau was classified as moderate (Fig. 2B); and ectopic bone that spanned > 50% of the width of the tibial plateau was considered to be severe (Fig. 2C). Type of ectopic bone was qualitatively assessed from the same pair of orthogonal digital radiographs at each time point as used for severity determination (Fig. 3). The absence of any ectopic bone was considered Type 0; ectopic bone that grew contiguously and remained within the normal shape of the tibia was considered Type 1 (Fig. 3A); ectopic bone that remained contiguous with the residual osseous skeleton but extended beyond the normal bony envelope of the tibia was considered Type 2 (Fig. 3B); and ectopic bone that appeared as distinct islands in the soft tissues and not contiguous with the tibia was considered Type 3 (Fig. 3C). Each radiograph was assessed by five independent graders (ADR, TPN, DEJ, CG, OT) on three separate occasions in random sequence. The median score from each individual reviewer’s three readings of each radiograph was then averaged for all five reviewers to determine the final grade for each radiograph.
Table 1.
Grading scale of heterotopic ossification severity and type modified from that originally devised by Potter et al. [36]
Fig. 2 A-C.
The radiographic key used for grading HO severity includes: (A) mild: ectopic bone spanning 1%-25% of the width of the tibial plateau; (B) moderate: ectopic bone spanning 25%-50% of the width of the tibial plateau; and (C) severe: ectopic bone spanning > 50% of the width of the tibial plateau.
Fig. 3 A-C.
The radiographic key used for grading HO type includes: A) Type 1: ectopic bone appearing within the normal contour of the bone; (B) Type 2: ectopic bone contiguous with the bone and extending beyond its normal contour; and (C) ectopic bone appearing as islands in the soft tissues.
Based on extrapolation of experience with human clinical trials utilizing radiation and indomethacin for HO prophylaxis after THA, it was determined a priori that a difference of two radiographic grades and/or an increase to the most severe grade would be necessary to constitute a minimum clinically important difference in our model. This assumption was based on the observation that a two-grade difference in Brooker scale or the difference between Grade III and Grade IV HO was necessary to result in a clinically meaningful difference in patient outcomes after hip surgery.
Statistics
Fleiss’ κ statistic was used to evaluate the consistency and reliability of agreement among the five raters’ gradings of radiographs for ectopic bone severity and type. The κ statistics for the percent of overall interobserver agreement of HO scores for severity and type were calculated as 0.41 and 0.37, respectively. This correlates with a fair to moderate strength of agreement between observers. Kruskal-Wallis one-way analysis of variance was used to assess the statistical significance of the observed differences in HO formation in the control and two treatment groups with p < 0.05 taken as significant. This analysis revealed no difference in HO severity scores between control and treatment groups and a higher HO type in the radiation group compared with the indomethacin and control groups (Table 2).
Table 2.
Mean radiographic (severity and type) and clinical HO assessments by group
Clinical Outcome
Five animals, two in each of the treatment intervention groups and one control animal, required at least one surgical wound revision for what clinically appeared to be either wound dehiscence or protrusion of the bony skeleton at approximately 4 weeks postoperatively (Fig. 4). In each case, the revision procedure included minimal shortening of the tibia with a rongeur, copious manual irrigation with sterile saline by syringe to reduce infection risk, and reclosure of the fascial layer with suture and the skin with staples. Appropriate analgesia and prophylactic antibiotic coverage were provided; wound cultures were not suggestive of infection in any animal. Two of the five animals, one each in the indomethacin and radiation groups, exhibited evidence of severe HO (severity score = 3). Four additional animals, one each in the control and indomethacin groups (Fig. 5) and two in the irradiation group (Fig. 6), developed late persistent fungating granuloma-like lesions in the residual limb with each having radiographic evidence of severe underlying HO (severity score = 3). None of these animals had surgical stump revision.
Fig. 4 A-B.
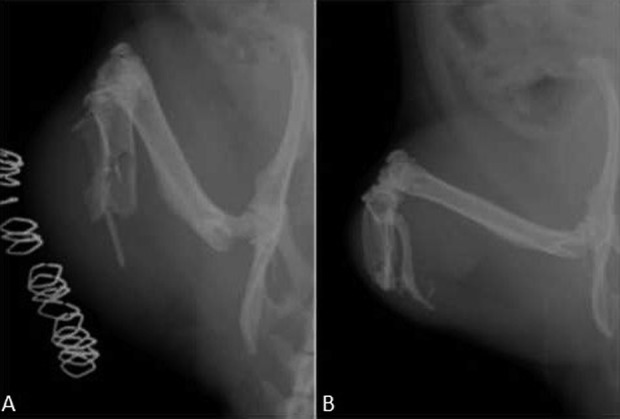
An example of a control group animal is illustrated: the radiographic evolution of a control animal with severe HO and ectopic bony islands is shown 6 months postblast amputation. Plain radiographs demonstrate ectopic bone formation at two time intervals: (A) immediately postblast and (B) 6 months postblast.
Fig. 5 A-B.
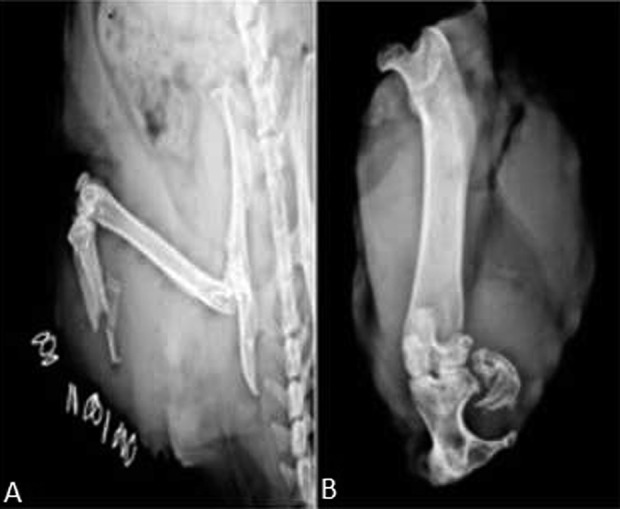
An example of an indomethacin group animal is illustrated: the radiographic evolution of HO in one animal who exhibited severe HO in the residual tibia in conjunction with a chronic granuloma-like exophytic lesion is shown. Plain radiographs demonstrate ectopic bone formation at two time intervals: (A) immediately postblast and (B) 6 months postblast.
Fig. 6 A-B.
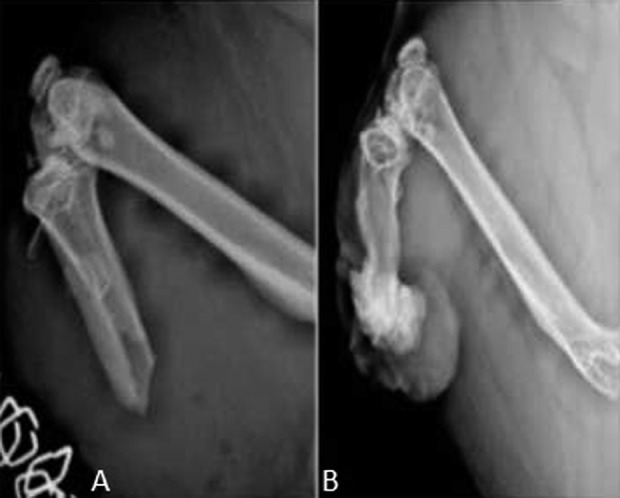
An example of an irradiation group animal is illustrated: the radiographic evolution of HO in an animal exhibiting severe HO on the distal aspect of the residual tibia in conjunction with a nonhealing granuloma-like lesion is shown. Plain radiographs demonstrate ectopic bone formation at two time intervals: (A) immediately postblast and (B) 6 months postblast.
In total, nine of 35 (25.7%) animals exhibited late wound complications: two in the control group, three in the indomethacin group, and four in the radiation group (Table 2). In all animals the wound complications developed > 4 weeks after the blast procedure, after removal of the incisional staples at 2 weeks when primary wound healing was clinically apparent. Six of those nine animals demonstrated severe (Grade 3) underlying HO in the residual limb on radiographs.
Results
HO Severity
With the numbers available, we found no differences among the three study groups in terms of HO severity scores (indomethacin 0.90 ± 0.46 [95% confidence interval {CI}, 0.60-1.19]; radiation 1.34 ± 0.59 [95% CI, 0.95-1.74]; control 0.95 ± 0.55 [95% CI, 0.60-1.30]; p = 0.100) (Table 2). The lower bound of the 95% CI on mean severity in the indomethacin group and the upper bound of the radiation group barely spanned a full grade and involved only numeric grades < 2 reflecting mild to moderate HO, suggesting that even if a small difference in severity could be detected, it would be less than our a priori-defined minimum clinically important difference of two HO grades, suggesting that any differences that might be present between these treatment groups are unlikely to be clinically important.
HO Type
Likewise, with the numbers available among the three study groups in terms of qualitative HO type scores, the radiation group had a higher HO type profile than either control or indomethacin groups, but indomethacin was no different than controls (indomethacin 1.08 ± 0.66 [95% CI, 0.67-1.50]; radiation 1.89 ± 0.76 [95% CI, 1.38-2.40]; control 1.10 ± 0.62 [95% CI, 0.70-1.50]; p = 0.013) (Table 2). Qualitative scoring of HO type resulted in a lower bound of the 95% CI in the indomethacin group and the upper bound of the radiation group that spanned 1.73 grade, but the importance of qualitative HO type is not established as a reliable surrogate for HO severity in any clinical trials or animal models.
Discussion
HO complicating high-energy blast amputation threatens the speed and completeness of functional recovery after these injuries through compromised healing of the residual limb and delayed prosthetic fitting and use [1]. Once symptomatic, treatment options are limited to surgical excision of the offending bone from the residual limb rather than prevention [1, 26, 37]. Primary prophylaxis of HO with external beam irradiation or NSAIDs, administered within 4 days of operation, has been well studied and reliably prevents HO in the civilian setting [32]. However, little is known about the efficacy of either radiation or NSAID prophylaxis of HO in the blast-injured limb despite its profound compromise of limb function after military injury. The objective of this work was to test the reliability of these established civilian measures in an animal model of military blast amputation. Surprisingly, neither indomethacin nor external beam irradiation reduced HO formation after high-energy traumatic blast amputation. Both intervention groups developed radiographic HO as well as severe clinical HO associated with fungating masses protruding through the residual limb at rates equal to or greater than controls.
The primary limitations of this study involve the subjective grading system used to assess severity and type of HO in the residual limbs and the sample size necessary to avoid a type II statistical error with the animal model. The Fleiss’ κ statistic for the percent of overall interobserver agreement for assessment of HO severity and type, 0.41 and 0.37, respectively, is indicative of only fair to moderate agreement and the low interobserver agreement is likely a reflection of the lack of quantitative specificity of the grading scale based on plain radiographs. The use of two-dimensional radiographs was affordable and readily available but provided less precision than more sophisticated imaging studies. A quantitative measurement of three-dimensional bone volume for assessing HO based on CT scans and a much larger sample size would have been needed to discern small differences in our animals with greater precision, but we posit that the magnitude of these small observed differences would likely have limited clinical importance [13]. Although a more objective and precise grading system may have potentially eliminated discordant interobserver grading and revealed a quantifiable difference in HO severity between study groups, 95% CIs demonstrated no clinically meaningful differences between treatment groups or controls. A minimum clinically meaningful difference was defined a priori based on radiographic severity grading of HO extrapolated from human clinical studies; a difference of two radiographic grades or attainment of the absolute maximum severity grade was felt to represent the lower bounds of clinically important differences. Neither of these conditions was met. More importantly, although there was a trend that indomethacin provided better prophylaxis for blast-induced HO than radiation, even the indomethacin group developed HO with comparable frequency as did the untreated controls. Likewise, observed wound complications were always associated with severe underlying HO and occurred with equal or greater frequency in the two intervention groups compared with controls. Similarly, the qualitative assessment of HO patterns has not been shown to be associated with clinical severity or relevance in human clinical trials, but rather serves as a more general biologic marker of the propensity to form ectopic bone after trauma.
Both clinical outcomes as well as radiographic assessment of HO severity failed to reveal a difference between the control and two intervention groups. Neither external beam irradiation nor indomethacin, both proven interventions for the prevention of HO after hip surgery in the civilian setting, demonstrated any substantial mitigation of this bone-forming process in the high-energy blast setting. By way of contrast, in the typical nonmilitary setting, administration of a single dose of radiation within 24 hours preoperatively or 72 hours postoperatively, or administration of indomethacin for 10 to 14 days immediately after surgery, offers reliable HO prophylaxis [16, 21, 22, 32, 41, 42]. In civilian practice, where HO commonly complicates hip surgery, local stem cells are committed to progress down a bone-forming cell line within 5 days postinjury or surgery. These cells produce ectopic bone within otherwise normal muscle [4, 6, 7, 14, 23, 38]. Radiation therapy is believed to inhibit the proliferation and differentiation of cells that contribute to HO, whereas NSAIDs diminish the inflammatory response and prevent extracellular matrix mineralization [20]. In animals, the minimum effective prophylaxis dose is 700 to 800 cGy of radiation or 2 to 3 mg/kg of indomethacin [8, 28, 29, 33, 34, 40, 44]; however, our scaled doses of indomethacin at 3 mg/kg daily for 10 days and 8 Gy in a single postoperative administration were based on animal models that involved simulated hip surgery or exogenous osteogenic agents to induce HO. It is conceivable that these agents might provide effective prophylaxis at a higher dose, in proportion to the greater energy of blast injury, but at this time, there is no rational scientific basis for such a dose-response speculation because progressively smaller doses have been utilized clinically for civilian HO prevention.
This study, performed in a well-established rat model, provides important but disheartening insight into the clinical management of HO after blast-induced extremity amputation. Despite the acknowledged effectiveness of radiation and NSAIDs in preventing HO in the civilian setting, both agents provided ineffective prophylaxis compared with controls after high-energy traumatic blast amputation. This observation is consistent with clinical reports of aggressive HO appearing in the residual limbs of wounded veterans after high-energy blast amputation [11, 18, 19, 27]. Moreover, we observed the association of late fungating granuloma-like lesions (clinically manifest as nonhealing ulcers) with the aggressive formation of underlying ectopic bone in our model. Of the four animals with such lesions, all had developed severe HO. Moreover, the five other animals that underwent revision amputation surgery all did so > 4 weeks after the blast procedure after primary clinical healing of the wound had been noted. Each of these animals also had associated underlying HO in the residual limb. In aggregate, more than one-fourth of animals in this study experienced wound complications with no differences between groups, and each was associated with severe underlying HO. This lends credence to the clinical observations about the aggressiveness of HO and that delayed wound healing plays an important role in the practical management of combat-related HO in residual limbs after blast amputation.
The severity and high prevalence of combat-related blast-induced HO have important clinical consequences. Alfieri et al. [1] have suggested that the uniqueness of combat-related HO formation is likely explained by a blast mechanism of injury, local and systemic inflammatory system dysregulation, delayed wound healing, and bacterial colonization. The paucity of animal studies or human clinical trials evaluating the effectiveness of known methods of prophylaxis of civilian HO in the military setting further challenges the management of blast amputations with this complication. In our rat animal model, indomethacin and external beam radiation administered soon after traumatic insult did not alter the development of HO in the setting of blast-induced amputation. This may be indicative of either an inciting stimulus that overwhelms conventional prophylactic interventions or the existence of a different pathway of osteogenesis that is refractory to these modalities, or both [11, 18, 19, 27]. More innovative research based on early molecular markers and cell signaling is warranted to understand the specific and unique pathogenesis of blast-induced HO. In the interim, nontraditional methods of treatment such as sacrifice of limb length or primary amputation above the zone of injury may be required to mitigate the process of ectopic bone formation after blast injury to facilitate rapid rehabilitation and optimal restoration of residual limb function.
Acknowledgments
The authors gratefully acknowledge the assistance of Cullen Griffith MD, and Oliver Tannous MD, in the reading and grading of animal radiographs to assess HO severity.
Footnotes
This work was funded by the Department of Defense Peer Reviewed Orthopaedic Research Program (VDP, Principal Investigator; Award Number: W81XWH-10-1-0975).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research® editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research® neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
Experimental procedures were performed at the University of Maryland, Baltimore, MD, USA; and data analysis was performed at the Medical University of South Carolina, Charleston, SC, USA.
References
- 1.Alfieri KA, Forsberg JA, Potter BK. Blast injuries and heterotopic ossification. Bone Joint Res. 2012;1:192-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen RC, Frisch HM, Farber GL, Hayda RA. Definitive treatment of combat casualties at military medical centers. J Am Acad Orthop Surg. 2006;14:S24-31. [DOI] [PubMed] [Google Scholar]
- 3.Beckett A, Pelletier P, Mamczak C, Benfield R, Elster E. Multidisciplinary trauma team care in Kandahar, Afghanistan: current injury patterns and care practices. Injury. 2012;43:2072-2077. [DOI] [PubMed] [Google Scholar]
- 4.Bosch P, Musgrave DS, Lee JY, Cummins J, Shuler T, Ghivizzani TC, Evans T, Robbins TD, Huard Osteoprogenitor cells within skeletal muscle. J Orthop Res. 2000;18:933-944. [DOI] [PubMed] [Google Scholar]
- 5.Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am. 2001;83:1783-1788. [DOI] [PubMed] [Google Scholar]
- 6.Cao B, Huard J. Muscle-derived stem cells. Cell Cycle. 2004;3:104-107. [PubMed] [Google Scholar]
- 7.Chalmers J, Gray DH, Rush J. Observations on the induction of bone in soft tissues. J Bone Joint Surg Br. 1975;57:36-45. [PubMed] [Google Scholar]
- 8.Coventry MB, Scanlon PW. The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am. 1981;63:201-208. [PubMed] [Google Scholar]
- 9.Covey DC. Combat orthopaedics: a view from the trenches. J Am Acad Orthop Surg. 2006;14:S10-17. [DOI] [PubMed] [Google Scholar]
- 10.Davies OG, Grover LM, Eisenstein N, Lewis MP, Liu Y. Identifying the cellular mechanisms leading to heterotopic ossification. Calcif Tissue Int. 2015;97:432-444. [DOI] [PubMed] [Google Scholar]
- 11.Davis TA, O'Brien FP, Anam K, Grijalva S, Potter BK, Elster EA. Heterotopic ossification in complex orthopaedic combat wounds: quantification and characterization of osteogenic precursor cell activity in traumatized muscle. J Bone Joint Surg Am. 2011;93:1122-1131. [DOI] [PubMed] [Google Scholar]
- 12.Edwards DS, Clasper JC. Heterotopic ossification: a systematic review. J R Army Med Corps. 2015;161:315-321. [DOI] [PubMed] [Google Scholar]
- 13.Edwards DS, Kuhn KM, Potter BK, Forsberg JA. Heterotopic ossification: a review of current understanding, treatment, and future. J Orthop Tauma. 2016;30(Suppl 3):S27-S30. [DOI] [PubMed] [Google Scholar]
- 14.Gazzerro E, Canalis E. Bone morphogenetic proteins and their antagonists. Rev Endocr Metab Disord. 2006;7:51-65. [DOI] [PubMed] [Google Scholar]
- 15.Gregoritch SJ, Chadha M, Pelligrini VD, Rubin P, Kantorowitz DA. Randomized trial comparing preoperative versus postoperative irradiation for prevention of heterotopic ossification following prosthetic total hip replacement: preliminary results. Int J Radiat Oncol Biol Phys. 1994;30:55-62. [DOI] [PubMed] [Google Scholar]
- 16.Healy WL, Lo TC, DeSimone AA, Rask B, Pfeifer BA. Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. J Bone Joint Surg Am. 1995;77:590-595. [DOI] [PubMed] [Google Scholar]
- 17.Henrot P, Stines J, Walter F, Martinet N, Paysant J, Blum A. Imaging of the painful lower limb stump. Radiographics. 2000;20(Spec No):S219-235. [DOI] [PubMed] [Google Scholar]
- 18.Jackson WM, Aragon AB, Bulken-Hoover JD, Nesti LJ, Tuan RS. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res. 2009;27:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson WM, Aragon AB, Djouad F, Song Y, Koehler SM, Nesti LJ, Tuan RS. Mesenchymal progenitor cells derived from traumatized human muscle. J Tissue Eng Regen Med. 2009;3:129-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji Y, Christopherson GT, Kluk MW, Amrani O, Jackson WM, Nesti LJ. Heterotopic ossification following musculoskeletal trauma: modeling stem and progenitor cells in their microenvironment. Adv Exp Med Biol. 2011;720:39-50. [DOI] [PubMed] [Google Scholar]
- 21.Kan SL, Yang B, Ning GZ, Chen LX, Li YL, Gao SJ, Chen XY, Sun JC, Feng SQ. Nonsteroidal anti-inflammatory drugs as prophylaxis for heterotopic ossification after total hip arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94:e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjaersgaard-Andersen P, Nafei A, Teichert G, Kristensen O, Schmidt SA, Keller J, Lucht U. Indomethacin for prevention of heterotopic ossification. A randomized controlled study in 41 hip arthroplasties. Acta Orthop Scand. 1993;64:639-642. [DOI] [PubMed] [Google Scholar]
- 23.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment AD, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazurek MT, Ficke JR. The scope of wounds encountered in casualties from the global war on terrorism: from the battlefield to the tertiary treatment facility. J Am Acad Orthop Surg. 2006;14:S18-23. [DOI] [PubMed] [Google Scholar]
- 25.Moore KD, Goss K, Anglen JO. Indomethacin versus radiation therapy for prophylaxis against heterotopic ossification in acetabular fractures: a randomised, prospective study. J Bone Joint Surg Br. 1998;80:259-263. [DOI] [PubMed] [Google Scholar]
- 26.Nauth A, Giles E, Potter BK, Nesti LJ, O'Brien FP, Bosse MJ, Anglen JO, Mehta S, Ahn J, Miclau T, Schemitsch EH. Heterotopic ossification in orthopaedic trauma. J Orthop Trauma. 2012;26:684-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nesti LJ, Jackson WM, Shanti RM, Koehler SM, Aragon AB, Bailey JR, Sracic MK, Freedman BA, Giuliani JR, Tuan RS. Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue. J Bone Joint Surg Am. 2008;90:2390-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilsson OS, Bauer HC, Brosjo O, Tornkvist H. A comparison of indomethacin and diclofenac in the inhibition of experimental heterotopic new bone formation. Int Orthop. 1987;11:283-287. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson OS, Persson PE, Ekelund A. Heterotopic new bone formation causes resorption of the inductive bone matrix. Clin Orthop Relat Res. 1990;257:280-285. [PubMed] [Google Scholar]
- 30.Owens BD, Wenke JC, Svoboda SJ, White DW. Extremity trauma research in the United States Army. J Am Acad Orthop Surg. 2006;14:S37-40. [DOI] [PubMed] [Google Scholar]
- 31.Pakos EE, Ioannidis JP. Radiotherapy vs nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a meta-analysis of randomized trials. Int J Radiat Oncol Biol Phys. 2004;60:888-895. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrini VD, Jr, Konski AA, Gastel JA, Rubin P, Evarts CM. Prevention of heterotopic ossification with irradiation after total hip arthroplasty. Radiation therapy with a single dose of eight hundred centigray administered to a limited field. J Bone Joint Surg Am. 1992;74:186-200. [PubMed] [Google Scholar]
- 33.Persson PE, Sisask G, Nilsson O. Indomethacin inhibits bone formation in inductive allografts but not in autografts: studies in rat. Acta Orthop. 2005;76:465-469. [DOI] [PubMed] [Google Scholar]
- 34.Pohl F, Hassel S, Nohe A, Flentje M, Knaus P, Sebald W, Koelbl O. Radiation-induced suppression of the BMP2 signal transduction pathway in the pluripotent mesenchymal cell line C2C12: an in vitro model for prevention of heterotopic ossification by radiotherapy. Radiat Res. 2003;159:345-350. [DOI] [PubMed] [Google Scholar]
- 35.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski D. Heterotopic ossification in the residual limbs of traumatic and combat-related amputees. J Am Acad Orthop Surg. 2006;14:S191-197. [DOI] [PubMed] [Google Scholar]
- 36.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations. Prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476-486. [DOI] [PubMed] [Google Scholar]
- 37.Potter BK, Forsberg JA, Davis TA, Evans KN, Hawksworth JS, Tadaki D, Brown TS, Crane NJ, Burns TC, O'Brien FP, Elster EA. Heterotopic ossification following combat-related trauma. J Bone Joint Surg Am. 2010;92(Suppl 2):74-89. [DOI] [PubMed] [Google Scholar]
- 38.Reddi AH. Bone morphogenetic proteins, bone marrow stromal cells, and mesenchymal stem cells. Maureen Owen revisited. Clin Orthop Relat Res. 1995;313:115-119. [PubMed] [Google Scholar]
- 39.Salawu A, Middleton C, Gilbertson A, Kodavali K, Neumann V. Stump ulcers and continued prosthetic limb use. Prosthet Orthot Int. 2006;30:279-285. [DOI] [PubMed] [Google Scholar]
- 40.Schneider DJ, Moulton MJ, Singapuri K, Chinchilli V, Deol GS, Krenitsky G, Pellegrini VD., Jr The Frank Stinchfield Award. Inhibition of heterotopic ossification with radiation therapy in an animal model. Clin Orthop Relat Res. 1998;355:35-46. [DOI] [PubMed] [Google Scholar]
- 41.Seegenschmiedt MH, Makoski HB, Micke O; German Cooperative Group on Radiotherapy for Benign Diseases. Radiation prophylaxis for heterotopic ossification about the hip joint--a multicenter study. Int J Radiat Oncol Biol Phys. 2001;51:756-765. [DOI] [PubMed] [Google Scholar]
- 42.Sell S, Phillips O, Handel M. No difference between two doses of diclofenac in prophylaxis of heterotopic ossifications after total hip arthroplasty. Acta Orthop Scand. 2004;75:45-49. [DOI] [PubMed] [Google Scholar]
- 43.Tannous O, Griffith C, O'Toole RV, Pellegrini VD., Jr Heterotopic ossification after extremity blast amputation in a Sprague-Dawley rat animal model. J Orthop Trauma. 2011;25:506-510. [DOI] [PubMed] [Google Scholar]
- 44.Tornkvist H, Bauer FC, Nilsson OS. Influence of indomethacin on experimental bone metabolism in rats. Clin Orthop Relat Res. 1985;193:264-270. [PubMed] [Google Scholar]