Abstract
Background:
Poly Implant Prothèse (PIP) silicone breast implants were removed from the market between 2010 and 2012 because of the use of nonmedical grade silicone filler. The chemical and physico-chemical properties of PIP implants have been analyzed by several groups. In addition, our previous study illustrated that PIP implant shells were more permeable. Therefore, we analyzed the chemical composition of the envelope and gel of PIP silicone breast explants. Also, the composition of absorbed material into the implant was analyzed.
Methods:
This study was conducted on 3 PIP implants explanted from 2 patients. The envelope was analyzed using Raman microscopy, whereas the gel was analyzed using near-infrared spectra, nuclear magnetic resonance spectroscopy, and gas chromatography coupled to mass spectrometry. Absorbed material was investigated with Fourier-transform infrared spectroscopy and sodium dodecyl sulfate polyacrylamide gel electrophoresis.
Results:
The 3 implants appeared to be Rofil implants, and all implants displayed a yellow color. None of the envelope showed a barrier layer. Amounts of D4, D5, and D6 were found to be below 100 ppm. Water was found in all 3 implants and also proteins were absorbed into the implants.
Conclusions:
The current study shows that the analyzed implants originate from the manufacturer Rofil but have PIP1 hallmarks. Apparently, these are own brand labeling implants. The presence of water and proteins in the explants indicate exchange of small and large molecules into the explants, even in the implant with a visually intact envelope. Because of the PIP1 hallmarks of the Rofil implants, patients with such implants are advised to be counseled by their physicians.
BACKGROUND
Silicone breast implants manufactured by Poly Implant Prothèse (PIP) were suspected to be of inferior quality compared with other implants used by physicians in 2007, because of an increase in reports of implant ruptures.1,2 After chemical investigations, the French Medical Regulatory Authority (ANSM) concluded in 2010 that PIP silicone breast implants were fraudulently manufactured.3 This led to many European countries banning the medical use of the PIP implants. Other organizations may have marketed PIP implants using another brand name, a process known as own brand labeling or virtual manufacturing, common to medical devices. In the Netherlands, the Dutch Care Inspectorate (IGZ) also recommended local medical institutions to recall their patients with PIP implants to have these removed from their patients.
The chemical and physiochemical properties of PIP implants have been analyzed by several groups.3–6 The ANSM reported a high percentage of low-grade silicone gel and shell, so that patients using PIP implants were at higher risk of an implant shell rupture.3 In 2012, a British NHS Expert Group concluded that the PIP implants contain high levels of low molecular weight cyclic siloxanes (D4-6) in the silicone gel; however, they determined that such levels of these siloxanes did not have cytotoxic or genotoxic effects in the body.5 Nevertheless, cyclic siloxanes D4 and D5 are on the European Chemicals Agency (ECHA) candidate list of substances of very high concern. Subsequent investigations showed that the implants lacked the shell barrier that prevents silicone gel to leak out, a phenomenon called gel bleed.4,6
The effect of this altered chemical composition from a medical grade composition of the PIP implants on implant dynamics such as shell permeability, gel bleed, and rupture is not completely known. However, our previous study illustrated that PIP implant shells were more permeable as intact implants showed postoperative volume increase as well as decrease.7 Also, we found a correlation between gel bleed and an increase in the postoperative implant volume. We concluded that this was most probably because of the inferior quality of the implant shell. This led to the following questions as to why the implant shells were too permeable; what was the composition of the silicone in the shells and the silicone in the gel, and what was the composition of the material absorbed into the implant? Also why breast implants develop a yellow or brown color with time, and what relation this could have with patient symptoms.
The purpose of this study was to answer these questions by analyzing the chemical composition of 3 PIP implants extracted from patients from the Jan van Goyen and OLVG location West, explanted after being carried for several years by 2 women.4
METHODS AND MATERIALS
Patient Characteristics
Three implants were derived from 2 patients. The mean age during primary implantation was 60 years for patient A and 63 years for patient B. The primary indication for patient A was an augmentation. There was an implantation duration of 10 years. The patient complained of pain and a changed aspect of the breasts leading to asymmetry. A preoperative ultrasound showed siliconomas in both axillas. One of the breast implants was documented as a PIP implant. For patient B, the primary indication for breast implants was augmentation. There was an implantation duration of 17 years. Reoperation was performed due to leakage of the right implant. Both implants were explanted. All 3 implants were noted as PIP implants by the operating surgeon.
Implant Characteristics
The 3 implants have been registered in project RP2017-001, in folders A1106 and A1136 under order numbers A110601, A113601, and A113602. The results of the analyses are reported here.
Chemical Analysis
The breast implants were visually examined upon receipt. Nuclear magnetic resonance spectroscopy (NMR spectroscopy) was performed using a Spinsolve 60 MHz benchtop spectrometer (Magritek) and an UltraShielded AV-500 spectrometer (Bruker), equipped with a 5 mm Triple TXI-Z-Gradient probe. The benchtop spectrometer was operated by Spinsolve 1.6.3 software for data acquisition and MestReNova 11.0.0 for data analysis. Topspin 3.0 was used for data acquisition and analysis on the AV-500.
Gas chromatography coupled to mass spectrometry was performed using A Varian CP-3800 was coupled to an Agilent Technologies 240 ion trap MS equipped with a GL Sciences InertCap Aquatic-2 60 m × 0.25 mm column. A temperature gradient from 40 to 250° C was used, with an injector temperature of 180° C and helium as carrier gas. Varian Workstation software was used for operation and data analysis.
Fourier-transform infrared spectroscopy was performed using a Bruker Alpha Fourier-transform infrared spectrometer, operating with Opus 7.5 software. At least 32 scans were acquired in attenuated total reflection mode with a resolution of 2 cm-1 and a range of 400–4000 cm-1.
Near-infrared spectra spectroscopy (NIR spectra) measurements were performed using an Antaris II FT-NIR spectrometer and Result software versus 3.0 (Thermo Scientific, Madison, Wis.). An auxiliary transflection piece with 1.2 mm spacer was used to create films of equal size of the gels. Spectra were collected in the transflection mode, resolution 8 cm-1, spectral range 12,000–3,000 cm-1. Principal component analysis was carried out on the first derivative of the spectra in the range of 8,000–4,000 cm-1 without additional spectral pretreatments using TQ-Analyst software versus 8.4 (Thermo Scientific, Madison, Wis.).
A DXR Raman microscope (Thermo Scientific, Madison, Wis.) was employed to record Raman spectra in area maps of a cross-section of the envelope of an implant. Measurements were carried out using a 10× objective, a 780 nm laser with a laser power of 14 mW, a collection time of 3 × 15 seconds and a slit width of 25 µm. Spectral range: 3,400–50 cm-1, estimated resolution 4.7–8.7 cm-1.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS PAGE) was performed using a 4–20% wedgewells precast gel (Thermo Scientific) and a Mark 12 unstained standard (Thermo Scientific). The gel was stained using Coomassie brilliant blue, and masses and intensities were analyzed using a GS900 densitometer (BioRad).
RESULTS
Visual Inspection of Implants
Examination of the implants revealed these were not PIP, but Rofil implants, as indicated by the company name on the implant patch. One of the implants was ruptured, one was bleeding, and one was intact, see Table 1. The bleeding implant contained air bubbles. It is unclear whether these were present when the implant was removed from the patient or whether these appeared during storage after removal. All implants had obtained a yellow color (Fig. 1). The ones with a damaged envelope (A110601 and A113602) displayed a more intense yellow color and contained brown colored particles as well.
Table 1.
Implant Characteristics
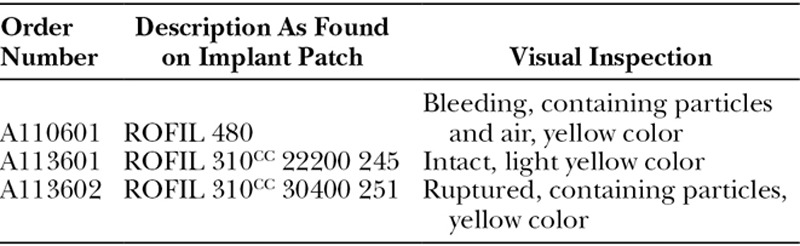
Fig. 1.
Photographs of the removed silicone breast implants with order number A110601 (A), A113601 (B), and A113602 (C). The implants are oriented with their sealing dots up.
Analysis of the Envelope
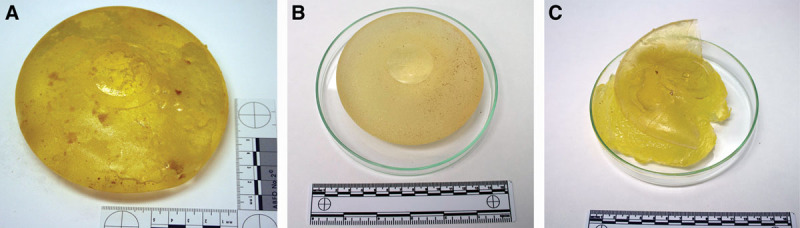
The envelopes of the implants were analyzed by Raman microscopy (Fig. 2). Spectra were acquired of cross-sections to determine their molecular composition in spatial resolution. As a result, all envelopes consisted mostly of dimethylsilicone, with some aromatic contribution similar to methylphenylsiloxane. In none of the implants a barrier layer (such as fluoro-silicone or diphenyl-silicone) was found present in their envelopes. The analyzed spots should be representative for the envelope as a whole as in the production process these are cured layer by layer on a mold.
Fig. 2.
Raman microscopy analysis of the envelopes of the removed breast implants with order number A110601 (A), A113601 (B), and A113602 (C). On the left, a microscopic photograph of a cross-section of the shell is shown, displaying the area of the Raman spectroscopic analysis in a red square. On the right are Raman chemigrams at 834 cm-1 and 3,050 cm-1, wavenumbers at which there is an absorbance maximum of fluorosilicone and diphenyl-silicone, respectively. The intensity of the signal increases from blue to red.
Analysis of the Gel
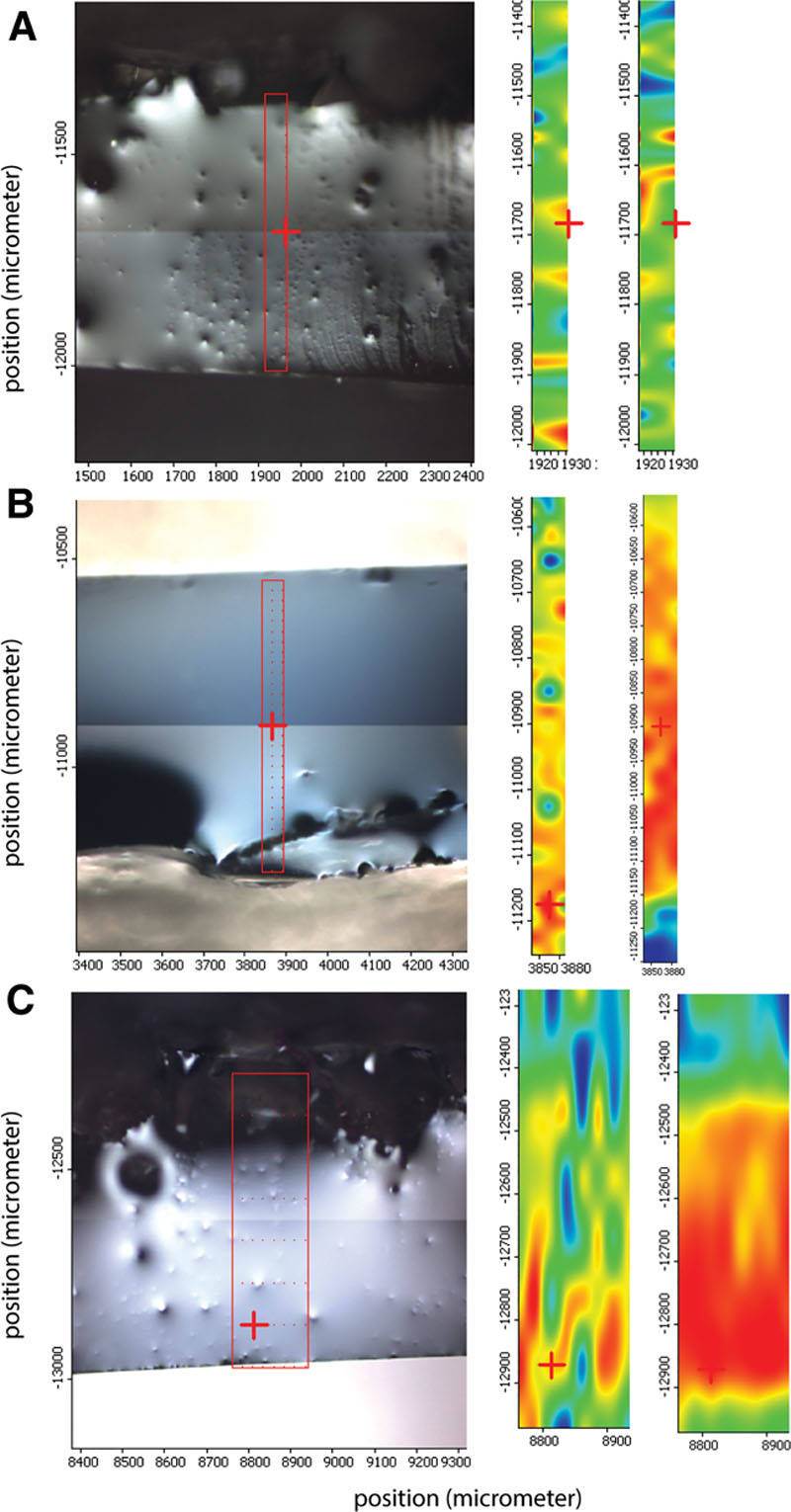
NIR spectra were acquired of the gels of the implants and compared with previous spectra obtained from reference materials and silicone breast implants gels (Fig. 3). NIR spectroscopy is used to determine the type of silicone gel.4 The NIR spectra of order numbers A113601 and A113602 could not be distinguished, and these matched with the spectrum obtained from order number A110601. All 3 spectra cluster together and overlap with the cluster of spectra obtained from gels of new implants containing terminal vinyl groups, such as the reference material Applied Silicone PN 40135.
Fig. 3.
Near infrared spectroscopy of the removed breast implant gels and the reference silicone gels Nusil MED3-6300; olive, Applied Silicone PN 40135.
No vinyl-containing components were found present in gel extracts of the 3 implants, as determined using NMR spectroscopy (data not shown). In a similar way, no large amounts of cyclosiloxanes were found present. This was confirmed by analyzing gel extracts by gas chromatography coupled to mass spectrometry, where amounts below 100 ppm of D4, D5, and D6 were determined (data not shown).
A considerable amount of water was present in the gel of the 3 implants, as became apparent in the Dimethyl sulfoxide (DMSO) extracts examined by NMR spectroscopy.
Identity of Particle Material
Some of the particles seen in order number A110601 were isolated and subjected to further analysis to identify the origin of the material. Fourier-transform infrared spectroscopy and SDS-PAGE analysis revealed a proteinaceous nature of the material. Various proteins were found to be present, of which the most dominant one had an apparent mass of about 62 kDa (Fig. 4).
Fig. 4.
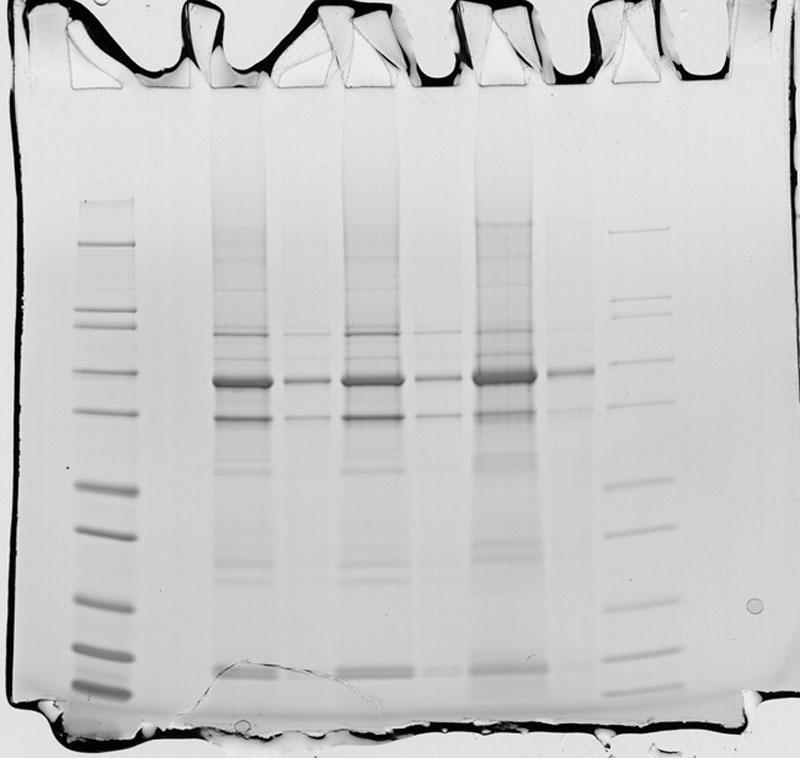
Analysis of some dark colored particles present in the gel of the removed breast implant with order number A110601. AL SDS PAGE analysis of 3 particles. Lane 1 contains a reference marker, lane 2 contains a blank, lanes 3, 5, and 7 contain a sample buffer extract of 3 different particles, lanes 4, 6, and 8 are 10-fold dilutions of the prior samples, lane 9 is a 3-fold diluted reference marker.
DISCUSSION
Poly Implant Prothèse produced inferior silicone breast implants, which were taken from the market between 2010 and 2012 after physicians reported higher implant rupture rates in their patients with these implants.1,2 In our previous study, there was a significant increase as well as decrease of postoperative implant volume in intact implants.7 In cases with postoperative implant volume increase there was also a correlation with gel bleed.
The primary aim of this study was to analyze the chemical composition of the 3 explanted PIP implants, to learn about the chemical composition of these implants and the changes that may occur to implants in time in the human body. The present study found that all of the explanted implants had a yellow color. They also lacked a barrier layer in their envelopes. The implants also lacked vinyl-containing components and did not contain high levels of cyclosiloxanes. They did, however, contain a high amount of water.
The 3 implants were lacking a barrier layer in their envelopes, as well as vinyl groups in their gel extracts, similar to what was previously found for PIP implants of the type PIP1.4 So the 3 implants do have the hallmarks of PIP1 implants, although these have been marketed by Rofil. It is known that Rofil marketed PIP implants under the brand name M-implant, but apparently also as own brand labeling.
The limitations of this study are the small number of breast implants examined and the fact that these implants have been banned from use as the dynamics of currently used implants may be different. However, the chemical analyses of PIP breast implants provide us with insight into dynamics of breast implants since there is more published knowledge available due to high explantation rates and more detailed analysis. This way, we can identify different aspects of the breast implant and relate them to factors that influence shell rupture as well as capsular contracture, both of which are complications regularly occurring in patients with new models of breast implants. If we can compare the identified factors in the PIP implants, and compare these with the new implants, we can get a step closer to understanding the dynamics leading to these complications.
All 3 implants had a yellow color. The one with a damaged envelope, displayed a more intense color. This is a known phenomenon in clinical practice, yet it is still unknown what the cause is. Chummun and McLean8 analyzed 78 PIP implants and found a third of the implants without rupture to be yellow. They theorize this to be due to influx of serum substances by the implants. This could explain our finding of damaged envelopes allowing for more ingression of material. Godwin et al.9 had comparable findings with their gel bleed and ruptured PIP implants developing a darker color. This was thought to be caused by influx of iodine into the implant. Kaali et al.10 hypothesize that the microorganisms in the biofilm could be responsible for the discoloration of the polymer surface by causing lipophilic pigments to diffuse into the silicone envelope. Other studies report implants changing color and becoming yellow with time.11 It is not yet clear what is the mechanism behind this.
Previous tests done by British and Australian expert groups confirm our finding that these silicone breast implants manufactured by PIP lacked a barrier layer.4,6 This was a major concern for patient safety as this can lead to higher bleeding rates.3,12
Chemical tests performed by Beretta and Malacco13 in 2013 on 2 PIP breast implants demonstrated that silicone PIP implants significantly adsorbed cholesterol molecules while implanted inside the body. Our study confirmed the finding of endogenous compounds adsorbed by the implants as well as a high level of water absorption.
In the time after implantation, the composition of a silicone breast implant may change, in comparison to new implants.4 The visual inspection and previous research indicate silicone breast implants as a whole not to be inert.7 The 3 implants had become yellow, absorbed water and 2 out of 3 contain particles consisting of proteins. The extent of the change in composition seems to be related to the magnitude of porosity of the implant envelope; the more damaged the explant was, the more changes in the appearance of the implant.
The presence of the water in the implants could be the explanation for the change in density over time. Apparently, with the water also other components such as proteins migrate through (pores in) the envelope. It seems that implants not only bleed silicones into the surrounding tissue, but also absorb components from the surrounding area.
The present study suggests that women with Rofil implants in fact might have PIP implants. Therefore, since PIP implants cause a higher risk for axillary siliconoma’s, we believe there is an ethical duty to inform patients having Rofil implants. Those patients should be recalled and counseled by their physicians. There is also the possibility that the implants which were labeled Rofil are not PIP implants, in which case the patients should be counseled by their physicians as well..
CONCLUSIONS
The 3 implants have the chemical hallmarks of PIP1 implants; no vinyl components, no barrier layer in the envelope, and low amounts of cyclosiloxanes, but these implants have been marketed by the company Rofil. The composition of implants changes during the time of implantation, even when porosity of the envelope is not visually obvious. Water is absorbed by the implants and even large molecules such as proteins are found in implants, indicating signs of a porous envelope and therefore a higher chance for rupture and gel bleed.
This study suggests that women with Rofil implants might in fact have PIP implants and therefore should be recalled and counseled by their physicians.
Finally, further research should assess whether influx and efflux of components occurs through the envelopes of the modern silicone breast implants that are currently on the market. The analysis of the implant composition after explantation could give an indication of the interaction between implant and human body.
Footnotes
Published online 9 January 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Berry RB. Rupture of PIP breast implants. J Plast Reconstr Aesthet Surg. 2007;60:967–968. [DOI] [PubMed] [Google Scholar]
- 2.Lahiri A, Waters R. Locoregional silicone spread after high cohesive gel silicone implant rupture. J Plast Reconstr Aesthet Surg. 2006;59:885–886. [DOI] [PubMed] [Google Scholar]
- 3.AFSSAPS. Topical report PIP silicone gel pre-filled implants. 2018.
- 4.Keizers PH, Vredenbregt MJ, Bakker F, et al. Chemical fingerprinting of silicone-based breast implants. J Pharm Biomed Anal. 2015;102:340–345. [DOI] [PubMed] [Google Scholar]
- 5.Keogh SB. Poly implant prothese (PIP) breast implants: final report of the expert group. 2012.
- 6.Swarts E, Kop AM, Nilasaroya A, et al. Rupture of poly implant prothèse silicone breast implants: an implant retrieval study. Plast Reconstr Surg. 2013;131:480e–489e. [DOI] [PubMed] [Google Scholar]
- 7.Bachour Y, Heinze ZCM, Dormaar TS, et al. Poly implant prothèse silicone breast implants: implant dynamics and capsular contracture. Eur J Plast Surg. 2018;41:563–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chummun S, McLean NR. Poly implant prothèse (PIP) breast implants: our experience. Surgeon. 2013;11:241–245. [DOI] [PubMed] [Google Scholar]
- 9.Godwin Y, Duncan RT, Feig C, et al. Soft, brown rupture: clinical signs and symptoms associated with ruptured PIP breast implants. Plast Reconstr Surg Glob Open. 2014;2:e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaali P., Stromberg E, Karlsson E. Laskovski AN. Prevention of Biofilm Associated Infections and Degradation of Polymeric Materials used in Biomedical Applications. In: Biomedical Engineering, Trends in Material Science, vol Chapter 22. In Tech, Reijeka, pp 513–541. [Google Scholar]
- 11.Bodin F, Jung C, Dieval F, et al. Aging of retrieved gel breast implants: a comparison between two product generations. J Mech Behav Biomed Mater. 2015;46:11–22. [DOI] [PubMed] [Google Scholar]
- 12.Bondurant S, Ernster V, Herdman R. Safety of Silicone Breast Implants; 1999. [PubMed] [Google Scholar]
- 13.Beretta G, Malacco M. Chemical and physicochemical properties of the high cohesive silicone gel from poly implant prothèse (PIP) breast prostheses after explantation: a preliminary, comparative analytical investigation. J Pharm Biomed Anal. 2013;78-79:75–82. [DOI] [PubMed] [Google Scholar]


