Abstract
Background:
Orofacial clefting (OFC) is the most common developmental craniofacial malformation, and causal etiologies largely remain unknown. The opioid crisis has led to a large proportion of infants recovering from neonatal abstinence syndrome (NAS) due to in-utero narcotics exposure. We sought to characterize the prevalence of OFC in infants with NAS.
Methods:
This cohort study analyzed live births at our institution from 2013 to 2017 to identify any association between OFC and NAS.
Results:
Prevalence of OFC was 6.79 and 1.63 (per 1,000 live births) in the NAS and general population, respectively. Odds ratios for NAS patients having developed OFC, isolated cleft palate, isolated cleft lip, and combined cleft lip and palate compared with the general population were found to be 4.18 (P = 0.001), 5.92 (P = 0.001), 3.79 (P = 0.05), and 2.94 (P = 0.35), respectively. Analyses performed comparing the NAS and general populations to control for potential confounding variables influencing the NAS population yielded no significant differences with exception of in-utero exposure to physician prescribed opioids.
Conclusions:
Prevalence of OFC in infants with NAS was higher than the general live birth population. Isolated cleft palate and isolated cleft lip, specifically, were significantly more prevalent in NAS patients compared with the general population and were associated with in-utero opioid exposure.
INTRODUCTION
Orofacial clefting (OFC) is the most common congenital craniofacial malformation in newborns and includes both cleft lip and cleft palate.1,2 OFC prevalence is reported at a rate of 1 in 500–700 live births.1,2 OFC is most commonly nonsyndromic but can be a component of a described syndrome. Comparison between OFC groups show combined cleft lip and palate to be most common, followed by isolated cleft lip (CL), and cleft palate without cleft lip (CP).1 Causal etiologies, specifically environmental risk factors, of both syndromic and nonsyndromic OFC are not fully understood.
A more comprehensive understanding of environmental risk factors contributing to the development of OFC could lend well to more appropriate interventions and maternal patient counseling to minimize risks of congenital anomalies and neonatal complications. Exposures that have been described as contributing to risk of OFC include lack of prenatal care, high rates of smoking during the first trimester of pregnancy, gestational alcohol consumption, maternal obesity, antifolate pharmacologic agents, anticonvulsant drugs, and exposure to air pollutants.3,4 Fetal exposure to opioids has been anecdotally described as a potential contributor to OFC in small cohort reviews5,6 and case control studies,7–9 but literature reviews and meta-analyses have yielded uncertain conclusions.10
Neonatal abstinence syndrome (NAS) is described as a group of symptoms associated with newborns withdrawing from intrauterine exposure to addictive agents.11 Agents associated with NAS include opioids, alcohol, cocaine, amphetamines, benzodiazepines, barbiturates, and some antidepressant medications. Clinical symptoms of NAS include irritability, gastrointestinal distress, autonomic nervous system dysfunction, and respiratory symptoms.11 As the opioid epidemic has escalated in severity throughout the United States, so too has the incidence of NAS.12,13 West Virginia is particularly burdened by the crisis and leads the nation in NAS incidence at a diagnosis rate of 50.6 cases for every 1,000 live births.14,15
Based upon clinical observation at our institution, an association between NAS and OFC was suggested. The aim of this retrospective cohort study was to identify and quantify associations between OFC and NAS. We hypothesized that infants diagnosed with NAS exhibited higher rates of OFC than the general population.
METHODS
After West Virginia University Institutional Review Board approval, we interrogated our tertiary care center’s electronic health record database for all live births at our institution between the defined 4-year study period of January 1, 2013, through December 31, 2017 (Fig. 1). Inclusion criteria captured all neonates born at our institution within the study period and had diagnoses of an OFC variant or NAS.
Fig. 1.
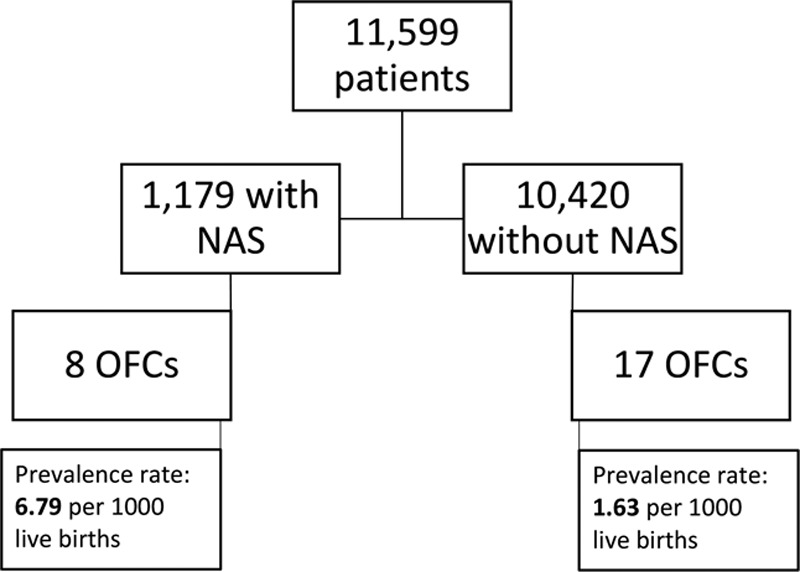
Consort diagram of study population regarding NAS diagnoses and OFC.
NAS and OFC diagnoses among the live birth population were identified based on corresponding ICD-10 codes. Unilateral cleft lip, bilateral cleft lip, unilateral cleft lip and palate, and bilateral cleft lip and palate were identified using the codes Q35.9, Q36.9, Q36.0, Q37.9, and Q37.8, respectively. P96.1 was the ICD-10 diagnosis code used to identify NAS.
Associations between OFC and NAS were quantified by calculating prevalence rates and odds ratios among the OFC group in the general live birth population compared with the OFC group in the NAS population. Demographic information comparing the general clefting population and the clefting with concomitant NAS was collected and compared. Additionally, variables potentially contributing independently to both NAS and OFC between the 2 groups were assessed. These variables included maternal prenatal care, folate supplementation during the first trimester, gestational alcohol exposure, gestational marijuana exposure, maternal smoking status during pregnancy, and whether or not the cleft was syndromically associated.
Univariate analyses were performed to assess associations between OFC and NAS. Fisher’s exact tests and unpaired t tests were performed where indicated using STATA statistical software (STATACorp LLC, College Station, Tex.). P values were considered to be significant at P ≤ 0.05.
RESULTS
There were 11,599 live births during the 4-year study period. Among the population, 1,179 neonates were diagnosed with NAS. A total of 25 patients were identified with OFC, 8 of whom were recovering from NAS. The prevalence of OFC was 6.79 and 1.63 (per 1,000 live births) in the NAS and general population, respectively (Fig. 1). Odds ratios were calculated to probe for any associations between OFC and NAS. Odds ratios for NAS patients having developed OFC, CP, CL, and CL/P compared with the general population were found to be 4.18 (P = 0.001), 5.92 (P = 0.001), 3.79 (P = 0.05), and 2.94 (P = 0.35), respectively (Table 1). Demographic and birth-related information were compared between the 2 distinct OFC populations (Table 2). None of the OFC patients in either group had a documented family history of any OFC variants. Potentially confounding variables (prenatal care status, folate supplementation status, gestational alcohol, marijuana, or tobacco, exposure, and the classification of the cleft) were also compared between the 2 groups (Table 3).
Table 1.
Odds Ratios Comparing OFC in NAS and Non-NAS Populations
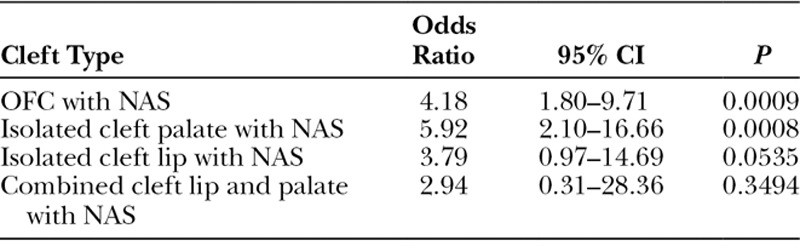
Table 2.
Comparison of Demographic and Birth-related Information between NAS and Non-NAS OFC Populations
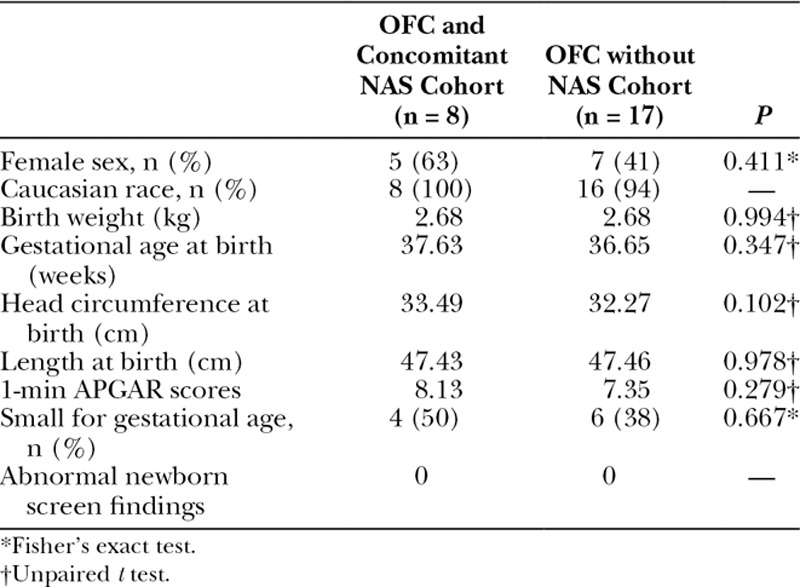
Table 3.
Comparison of Potentially Confounding Variables between the NAS and Non-NAS OFC Populations
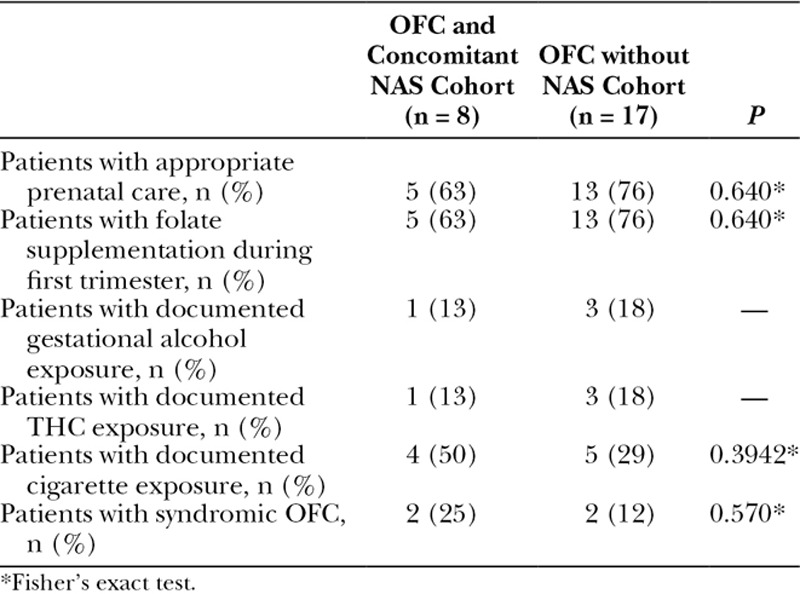
The only identifiable difference between the NAS and non-NAS population was fetal exposure to physician-prescribed opioids during gestation. Seven of the NAS infants were exposed to buprenorphine and the eighth was exposed to methadone. There was only 1 exposure to addictive agents in the general non-NAS cleft population, which was a case of amphetamine exposure.
DISCUSSION
Elevated prevalence rates and odds ratios suggest an association between NAS and OFC (CP and CL subtypes specifically). The odds ratio for NAS and CL/P was not statistically significant, perhaps because the study was underpowered due to the low prevalence of OFC and small number of patients with CL/P in our population.
Comparison of NAS and non-NAS populations revealed no significant differences between sex, race/ethnicity, birth weight, gestational age at birth, length at birth, head circumference at birth, APGAR scores, or newborn screen findings. There were no significant differences between the 2 groups related to prenatal care, folate supplementation during the first trimester, gestational alcohol, cigarette, or marijuana exposures. All 8 newborns recovering from NAS with OFC had documented exposure to physician-prescribed opioids during the first trimester. Seven patients were exposed to buprenorphine, and 1 patient was exposed to methadone. These findings implicate physician prescribed opioids as contributing toward OFC development in this population.
An understanding of the relative influence of environmental risk factors in OFC is not yet established. Associations that have been described in the literature include multivitamin and folate supplementation during pregnancy, which are thought to modestly decrease rates of OFC.3 Conversely, high levels of maternal smoking, first trimester alcohol consumption, pre-pregnancy diagnosis of diabetes mellitus, maternal obesity, prescription drugs with antifolate mechanisms, and anticonvulsant prescription drugs are recognized environmental risk factors associated with increased rates of OFC.3 Maternal exposure to air pollutants has also been described as affecting risk of development of OFC as well.4
The role of opioids in OFC is unknown.16 Reports to date include small case control studies and cohort studies. In a cohort reported by Meyer et al.6 comparing buprenorphine and methadone for treatment of maternal opioid dependence during pregnancy, one infant whose mother was prescribed buprenorphine in the study was anecdotally noted to have developed CP. In a 1995 cohort study by Thomas analyzing pediatric congenital malformations over a 10-year experience, 7 OFC were analyzed for potential contributory exposures. In all 7, combinations of substances were described. Of these, methadone was included in 3 instances of OFC but was not a solitary agent.5 These are the only studies where the potential association between physician prescribed opioids and OFC were specifically described.
A systematic review and meta-analysis by Lind et al.10 was performed in 2017 of existing cohort and case control studies and analyzed associations between congenital malformations and maternal opioid use during pregnancy. The implications of opioid exposure on the development of OFC were inconclusive. Zero out of the 17 cohort studies analyzed revealed any significant associations between opioid exposure and OFC. Of the 10 case control studies, three7–9 identified statistically significant associations between in utero opioid exposure and development of some OFC variant. However, all of these studies mainly emphasized opioids such as morphine and heroin as well as other narcotics such as codeine.
A literature review was also performed by Yazdy et al.17 studying birth outcomes among women who received prescription narcotics and opioids during pregnancy. Associations between buprenorphine and methadone medical therapy for prenatal opioid-dependent women and NAS were described. An accumulating body of evidence suggests that NAS severity is greater among patients who receive buprenorphine therapy as compared with methadone therapy. In fact, NAS has been found to be more severe in neonates exposed to methadone compared with heroin.18 Both buprenorphine and methadone medical therapy have been associated with low birth weight, small head circumference, and preterm delivery rates, but distinct correlations with specific congenital anomalies such as OFC were not as clear.19,20
Unlike these former reports, our cohort does suggest an association between in utero physician prescribed opioid exposure, OFC, and NAS. However, this study is not without inherent limitations. The study is retrospective in nature and relies on accurate patient data in the electronic health record. Additionally, more specific details related to the exposures such as dosing regimens and time points during gestation that the fetus was exposed to different substances were unavailable. Finally, given the relatively rare prevalence of OFC, this study is limited in cohort size. Future efforts will include development of a statewide congenital malformation database, which will permit an evaluation in a much larger patient cohort.
CONCLUSIONS
There appears to be a correlation between NAS and OFC. This may imply that in utero exposure, including prescribed opioids, are a contributory environmental risk factor in the development of OFC.
ACKNOWLEDGMENTS
The authors acknowledge the West Virginia Clinical and Translational Science Institute (WVCTSI) for their assistance in biostatistics review and validation of our results in this study.
Footnotes
Published online 11 January 2019.
Presented at the Podium presentation at Ohio Valley Society of Plastic Surgery, June 2018, Cleveland, Ohio (Best Clinical Research Presentation Award). Podium presentation at Plastic Surgery: The Meeting, October 2018, Chicago, Ill.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Watkins SE, Meyer RE, Strauss RP, et al. Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg. 2014;41:149–163. [DOI] [PubMed] [Google Scholar]
- 2.Lakin GE. Plastic Surgery Review: A Study Guide for the In-service, Written Board, and Maintenance of Certification Exams. 2015Thieme. [Google Scholar]
- 3.Mossey PA, Little J, Munger RG, et al. Cleft lip and palate. Lancet. 2009;374:1773–1785. [DOI] [PubMed] [Google Scholar]
- 4.Rao A, Ahmed MK, Taub PJ, et al. The correlation between maternal exposure to air pollution and the risk of orofacial clefts in infants: a systematic review and meta-analysis. J Oral Maxillofac Res. 2016;7:e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DB. Cleft palate, mortality and morbidity in infants of substance abusing mothers. J Paediatr Child Health. 1995;31:457–460. [DOI] [PubMed] [Google Scholar]
- 6.Meyer MC, Johnston AM, Crocker AM, et al. Methadone and buprenorphine for opioid dependence during pregnancy: a retrospective cohort study. J Addict Med. 2015;9:81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bracken MB, Holford TR. Exposure to prescribed drugs in pregnancy and association with congenital malformations. Obstet Gynecol. 1981;58:336–344. [PubMed] [Google Scholar]
- 8.Saxén I. The association between maternal influenza, drug consumption and oral clefts. Acta Odontol Scand. 1975;33:259–267. [DOI] [PubMed] [Google Scholar]
- 9.Saxén I. Associations between oral clefts and drugs taken during pregnancy. Int J Epidemiol. 1975;4:37–44. [DOI] [PubMed] [Google Scholar]
- 10.Lind JN, Interrante JD, Ailes EC, et al. Maternal use of opioids during pregnancy and congenital malformations: a systematic review. Pediatrics. 2017:e20164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kocherlakota P. Neonatal abstinence syndrome. Pediatrics. 2014:2013–3524.peds. [DOI] [PubMed] [Google Scholar]
- 12.Patrick SW, Schumacher RE, Benneyworth BD, et al. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307:1934–1940. [DOI] [PubMed] [Google Scholar]
- 13.Sanlorenzo LA, Stark AR, Patrick SW. Neonatal abstinence syndrome: an update. Curr Opin Pediatr. 2018;30:182–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Services WVDoHaH. [DHHR releases neonatal abstinence syndrome data for 2017]. 2018. Available at https://dhhr.wv.gov/News/2018/Pages/DHHR-Releases-Neonatal-Abstinence-Syndrome-Data-for-2017-.aspx. Accessed October 5, 2018.
- 15.Umer A, Loudin S, Maxwell S, et al. Capturing the statewide incidence of neonatal abstinence syndrome in real time: the West Virginia experience. Pediatr Res. 2018:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behnke M, Smith VC, Abuse CoS. Prenatal substance abuse: short-and long-term effects on the exposed fetus. Pediatr. 2013:2012–3931.peds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazdy MM, Desai RJ, Brogly SB. Prenatal exposures and short and long term developmental outcomes: prescription opioids in pregnancy and birth outcomes: a review of the literature. J Pediatr Genet. 2015;4:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson K, Greenough A, Gerada C. Maternal drug use and length of neonatal unit stay. Addiction. 2003;98:785–789. [DOI] [PubMed] [Google Scholar]
- 19.Lejeune C, Simmat-Durand L, Gourarier L, et al. ; Groupe d’Etudes Grossesse et Addictions (GEGA). Prospective multicenter observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82:250–257. [DOI] [PubMed] [Google Scholar]
- 20.Cleary BJ, Donnelly JM, Strawbridge JD, et al. Methadone and perinatal outcomes: a retrospective cohort study. Am J Obstet Gynecol. 2011;204:139.e1–139.e9. [DOI] [PubMed] [Google Scholar]


