Abstract
Background:
Chronic wounds represent a significant financial burden to the healthcare system and a quality-of-life burden to patients. Many chronic wounds have elevated bioburden in the form of biofilm, which has been associated with delayed wound healing. This study examined the use of a native type I collagen matrix with the antimicrobial polyhexamethylene biguanide (PCMP) in the management of bioburden and treatment of chronic, nonhealing wounds over 12 weeks.
Methods:
A prospective case series of PCMP enrolled adults ≥18 years old with a nonhealing wound. At week 0, the wound was prepared by sharp or mechanical debridement. Patients received standard wound care plus PCMP applications at week 0 and then weekly up to week 12 at the investigator’s discretion. Dressings were applied over PCMP to fix it in place. At each visit, wounds were assessed for the extent of healing and signs of wound infection.
Results:
Of the 41 wounds studied, 44% were pressure ulcers, 22% were surgical wounds, 12% were venous ulcers, 10% were diabetic ulcers, and 12% were another type. The median (interquartile range) baseline wound area was 7.2 (14.9) cm2, and the mean wound duration was 103 weeks. Of the 41 wounds, 73% demonstrated a reduction in wound area at 12 weeks, and 37% achieved complete wound closure, with a mean time of 6.7 weeks to complete closure.
Conclusion:
PCMP treatment appeared to positively impact the course of wound healing in a variety of complex, chronic wounds that were unresponsive to prior treatment.
INTRODUCTION
Chronic wounds are characterized by repeated tissue injury, ischemia, and elevated bacterial burden, all of which combine to result in continued inflammation and an imbalance in tissue proteases and inhibitors.1–3 Chronic wounds, such as diabetic foot ulcers, venous leg ulcers, and pressure ulcers, lead to a significant burden on patient quality of life and healthcare cost.4–9 An administrative claims study showed that 15% of Medicare beneficiaries are affected by chronic wounds, with an estimated annual cost of $28 billion.10
The elevated bioburden in chronic wounds is often in the form of biofilm.11 Biofilm occurs when planktonic (free-floating) bacteria adhere to a solid surface, proliferate, and exude substances to form a polymeric matrix enclosing a monomicrobial or polymicrobial community.12,13 Approximately 10–20% of the biofilm is composed of microorganisms and 80–90% is composed of extracellular polymeric matrix.14 In 2010, there were an estimated 17 million biofilm-associated disease cases in the United States, with direct costs of approximately $94 billion.15 Chronic wounds with a large quantity of slough may be at an increased risk of developing pathogenic and antimicrobial-resistant biofilms.14 Emerging evidence indicates that the presence of biofilm is associated with delayed wound healing.16–18 Compared with planktonic bacteria, bacteria in biofilms are more difficult to eradicate using topical or systemic antibiotics.13,19,20
Debridement of a chronic wound is the first step to disrupting a pathogenic biofilm and removing necrotic tissue, both of which contribute to delayed wound healing.14,21 However, debridement is not sufficient by itself. Recent research has shown that clinic-based debridement of chronic ulcers has minimal effect on surface bacterial counts.22 Topical antiseptics and antimicrobials can be applied after debridement to reduce the bacterial count and assist in wound healing.23,24 Historical options for wound antimicrobial treatment include silver, honey, iodine, or chlorhexidine. However, questions remain about the efficacy and safety of these agents when they are applied in dressings.24,25
Polyhexamethylene biguanide hydrochloride (PHMB) is a cationic topical antimicrobial that strongly binds to bacterial cell walls and membranes, disrupting the transport, biosynthesis, and catabolic functions of the bacterium.26,27 PHMB possesses broad antimicrobial activity against Gram-positive and Gram-negative bacteria, plaque- and biofilm-forming bacteria, and intracellular bacteria such as Chlamydiae and Mycoplasma.27 In addition, PHMB binds to biofilm matrix components and increases in concentration during application, thereby creating an increasingly toxic environment for bacteria.28 PHMB does not seem to be affected by multidrug efflux pumps or acquired resistance developed by bacteria.26,27 It is regarded as a very biocompatible antiseptic that shows little-to-no toxicity or systemic uptake when applied on intact skin or wounds.27,29 A recent systematic review demonstrated that use of PHMB dressings was associated with faster and more substantial reductions in bacterial counts, including multidrug-resistant species, than control dressings.30 A study of PHMB dressings used in wounds with biofilm showed positive outcomes for wound disinfection and patient-reported pain.31
Collagen matrices can serve as a sacrificial surface for matrix metalloproteinases and elastase that are prevalent in chronic wounds, thus protecting tissue collagen deposition.32 Retaining the native collagen matrix structure supports healing and has been shown to effectively address the protease imbalance seen in chronic wounds, and support granulation tissue formation and epithelialization.33 Collagen matrices can sequester proteolytic enzymes and act as a biocompatible scaffold to support healing.34
Here, we propose using native type I porcine-derived collagen matrix coated with 0.1% PHMB (porcine collagen matrix with PHMB, further referenced as PCMP). PCMP is a Food and Drug Administration Class II Medical Device 510(k) cleared #K051647 and intended for the management of wounds, as an effective barrier to resist microbial colonization within the dressing and reduce microbes penetrating through the dressing. PCMP is the only dressing available in the United States with the combination of native collagen matrix and PHMB. It is supplied dry in sheet form and packaged in sterile, sealed single patches.35
The aim of this study was to assess the ability of PCMP to meet wound-specific treatment goals including management of bioburden, support of granulation tissue formation, and support of wound closure over a 12-week period in chronic, nonhealing wounds of various etiologies.
METHODS
Study Design
A single-center, prospective, case series of PCMP (PuraPly Antimicrobial, Organogenesis Inc., Canton, Mass.) was conducted (NCT03070925). The study was approved by Biomedical Research Alliance of New York, IRB #16-08-209-03, and was conducted in accordance with current International Council for Harmonisation and Good Clinical Practice guidelines. All patients provided their written informed consent to participate in the study. Eligible wounds were evaluated weekly for up to 12 weeks for size, healing, and improvement in granulation tissue.
Participants
Adults ≥18 years of age with at least 1 appropriate wound (including partial- and full-thickness wounds, pressure ulcers, venous leg ulcers, diabetic foot ulcers, chronic vascular ulcers, surgical wounds, or trauma wounds) were eligible for inclusion. Individuals were excluded from participating if they were receiving concurrent treatment with other topical antimicrobials or skin substitute products, or if they had a third-degree burn or a known sensitivity to any of the PCMP materials.
Procedures
Eligible patients with an identified target wound underwent clinical assessments and received standard of care as determined by the treating physician. The treating physician determined the frequency and type of assessments performed for each patient, according to standard of care for their target wound and the patient’s individualized needs for treatment and follow-up. Data were collected at every clinical visit in which PCMP was applied to the target wound and at postapplication follow-up visits as appropriate.
Wounds were cleaned and prepared with sharp debridement or mechanical debridement at the initial visit. After preparation, PCMP was applied at week 0 to the study wound. Each PCMP sheet was on the wound for a minimum of 1 week and a maximum of 3 weeks. In moist wounds, when PCMP was applied; no saline was needed to moisten the product. It was fixated with nonadherent dressing (CONFORMANT 2; Smith & Nephew, Largo, Fla.) and secured at the edges with steri-strips or strips of retention dressing (Hypafix; Smith & Nephew). The nonadherent dressing allowed the wound to drain. Foam dressing (Allevyn; Smith & Nephew) was placed over the nonadherent dressing. Gauze and stretch bandage were applied to fix all the dressings in place. For drier wounds, saline-moistened PCMP was covered with nonadherent dressing, and secured at the edges with steri-strips or strips of retention dressing. Gauze and stretch bandage were applied to fix the dressings and PCMP in place. The outer dressings could be changed within 1 week, if needed, without disturbing the PCMP and the nonadherent dressing.
Assessments
At each clinic visit, wounds were assessed for the extent of healing and any signs of wound infection. Wound measurements included wound area and depth, and achievement of complete wound closure (Yes/No). Photographs were taken before PCMP application, before removal of the PCMP, after debridement, after placement of a new PCMP, and, if it occurred, upon healing.
Statistical Analysis
All analyses were performed on patients who received PCMP treatment (intent-to-treat population). Descriptive statistics (eg, mean, SD, median) were calculated with Microsoft Excel 2016 (version 16.0.9330.2124) for demographic and baseline characteristics, the time to wound closure, the change in wound surface area from baseline to week 12, and the percent wound closure from baseline to week 4 or 12. The Wilcoxon signed rank test was performed (Microsoft Excel 2016) to examine whether the wound area at week 12 was significantly different (at α = 0.05) from the baseline area. No patient showed signs of complications or infection at the conclusion of the study.
RESULTS
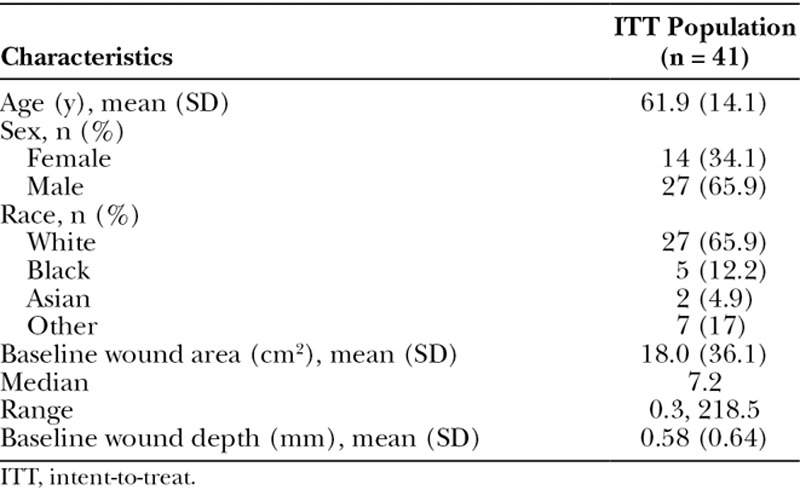
A total of 41 wounds (1 wound per participant) were included in the analysis. The mean (SD) age of participants was 61.9 (14.1) years and 65.9% (27/41) were male (Table 1). At baseline, 18 of 41 (43.9%) of the wounds were pressure ulcers, 9/41 (22.0%) were surgical wounds, 5/41 (12.2%) were venous leg ulcers, 4/41 (9.8%) were diabetic foot ulcers, and 5/41 (12.2%) were another type of wound. The median (interquartile range) baseline wound area was 7.2 (14.9) cm2, and the mean (SD) depth was 0.58 (0.64) mm. The mean wound duration before PCMP application was 103.1 weeks (n = 41).
Table 1.
Demographic and Baseline Characteristics
The anatomical locations of patient wounds varied. Wounds were located on the foot (n = 19), ankle (n = 5), anterior leg (n = 5), toe (n = 3), heel (n = 2), sacrum (n = 2), ischium (n = 2), and 1 each on the thigh, breast, knee, and posterior leg.
There was a vast array of comorbidities present within the cohort; however, certain ailments were represented heavily. Hypertension was present in 28 patients, type 2 diabetes mellitus in 22, hyperlipidemia in 15, peripheral vascular disease in 13, coronary artery disease in 11, and chronic kidney disease in 9 patients.
Before participating in the study, 16 (38.1%) patients underwent compression therapy, 27 (64.3%) had attempted off-loading, 42 (97.7%) patients had the target wound debrided at least once before starting the study, 10 (23.8%) patients used negative pressure therapy, 10 (23.8%) underwent hyperbaric oxygen therapy, 7 (17.1%) patients had an amputation performed due their wound, 7 (17.1%) attempted surgical closure of their wounds, 12 (29.3%) patients were treated with skin substitutes, and 13 (34.2%) used topical antimicrobials on their wounds.
PCMP is typically applied on a weekly basis according to standard of care and the patient’s individualized needs for treatment and follow-up. For a few patients in this study, PCMP was left in place for 2–3 weeks. At the week 1 follow-up visit after PCMP application, the usual course of action is to remove the PCMP and reapply. However, in these few patients, the PCMP was found to be dry and stuck to the wound at the follow-up. Rather than remove the PCMP and potentially open the wound, the PCMP was left in place until a subsequent visit, at which point it had either integrated into the wound or could lift off easily, revealing a healed wound.
The 41 wounds in this study received an average of 8 PCMP applications each. During this study, 2/41 patients received adjunctive therapy of hyperbaric oxygen treatments; both patients had pressure ulcers that had been present for over 1 year before study entry and had prior hyperbaric oxygen treatments for the study wound. Of the 41 wounds, 30 (73.2%) demonstrated an overall reduction in wound area over the 12-week study and 15/41 (36.6%) achieved complete wound closure (Fig. 1). Of the wounds achieving complete closure, the mean (SD) time to closure was 6.7 (3.0) weeks. By wound type, 7/18 (38.9%) of pressure ulcers, 5/9 (55.6%) of surgical wounds, 1/1 (100%) of trauma wounds, 1/4 (25%) of diabetic foot ulcers, and 1/5 (20%) of venous leg ulcers achieved complete closure. Mean wound closure by wound type is shown in Table 2. Of the 30 wounds that showed a decrease in area, 26 had decreased by ≥70% from baseline to week 12. After 12 weeks, the median decrease in wound area was 3.1 cm2 and the mean (SD) was 7.5 (11.2) cm2 (Fig. 2). When a paired Wilcoxon signed rank test is performed, the wound area at week 12 was significantly different from baseline (P < 0.001). Wounds also visibly had an increase in granulation tissue with healing. Five wounds increased in size from baseline to week 12 due to patient noncompliance.
Fig. 1.
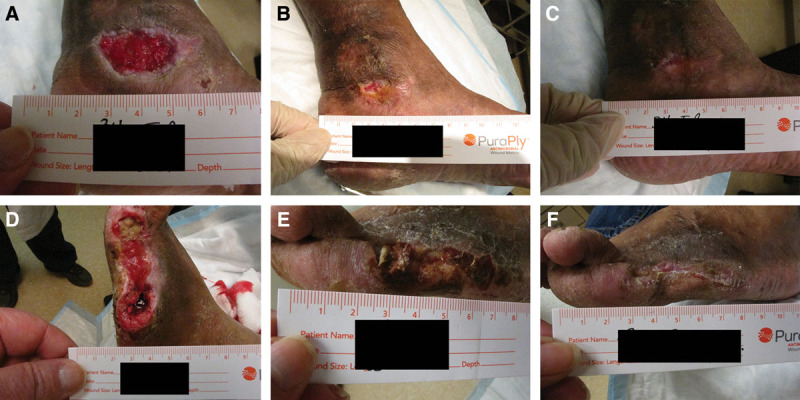
Photographs show wound healing progress after PCMP application. A–C, A venous leg ulcer with a baseline wound area of 19.5 cm2 had been present for 4 months. Previously, the patient underwent compression, debridement, and collagenase treatments. The patient received 11 applications of PCMP. D–F, A surgical wound with baseline wound area of 21.4 cm2 had been present for 1 month. The patient underwent off-loading, debridement, and negative pressure therapy before study participation. The patient received 9 applications of PCMP.
Table 2.
Mean Percent Wound Closure from Baseline, by Wound Type
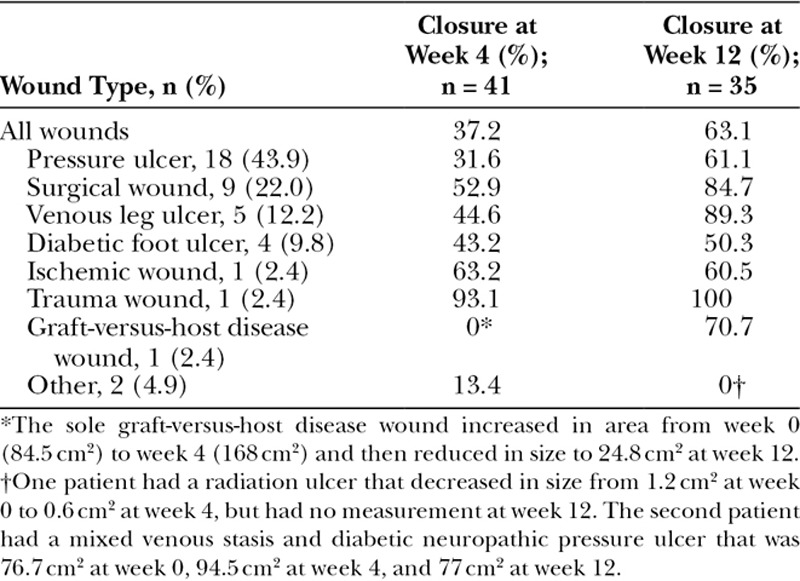
Fig. 2.
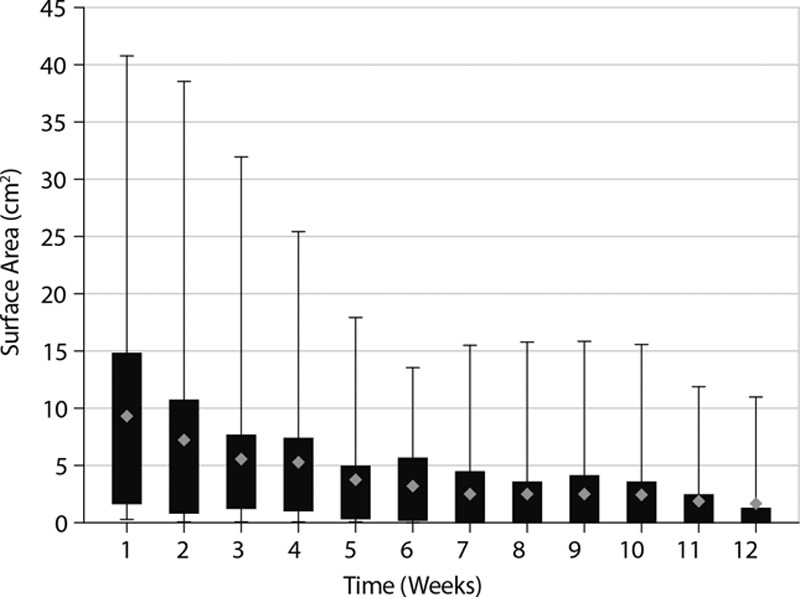
Box and whisker plot of change in wound surface area with PCMP application for wounds of baseline area less than 45 cm2. The boxes represent the interquartile range, the error bars represent the range, and the diamonds represent the mean.
One participant died during the study period due to a comorbid condition. No wounds showed signs of complications or infection after 12 weeks, and there were no reports of adverse events attributable to PCMP.
DISCUSSION
This study of PCMP represents an extensive case series of a native type I collagen matrix with PHMB antimicrobial for the management of chronic, nonhealing skin wounds. The application of PCMP appeared to positively impact the course of wound healing in a variety of complex, chronic wounds that were unresponsive to prior treatment, and in many cases even resulted in wound closure.
At 12 weeks, most (73.2%) wounds reduced in area from baseline and many (63.4%) had reached a ≥70% reduction in area. Complete closure was achieved in many types of wounds, including surgical and trauma wounds, pressure ulcers, diabetic foot ulcers, and venous leg ulcers. The time to complete wound closure was relatively short (6.7 weeks) relative to the duration of the wounds (103 weeks). An earlier case series with PCMP demonstrated an average time to complete wound closure of 6.8 weeks.36 PCMP was well tolerated, and no systemic or localized adverse events were reported that were attributed to PCMP. Another case series of 9 wounds treated with PCMP showed that 6 of the 9 wounds healed in an average of 10 weeks, with the remaining wounds showing improvements in granulation tissue and a reduction in wound area.37
In recent studies of skin substitutes for the treatment of wounds, at baseline, venous leg ulcers had a median area of 6.0–7.5 cm2 and diabetic foot ulcers had a median area of 3.0–3.9 cm2. The median times to wound closure were reported as 19.5 weeks for dehydrated human amnion chorion membrane (dHACM; EpiFix, MiMedx, Marietta, Ga.), 25 weeks for fetal bovine collagen dressing (Primatrix, Integra Life Sciences, Waltham, Mass.), and 36 weeks for acellular porcine small intestinal submucosa collagen dressing (SIS; Oasis, Smith & Nephew, Largo, Fla.).38,39 In this prospective study of a variety of wound types in which the median baseline wound area was 7.2 cm2, time to wound closure with PCMP was 12.9 to 29.4 weeks shorter than that reported for other skin substitutes, representing a 66% to 82% reduction in time to closure. Similarly, wound closure rates of other skin substitutes by or at 12 weeks were 32% for dHACM, 27% for fetal bovine collagen dressing, and 14% for SIS.38,39 In this study, PCMP resulted in a 37% wound closure rate.
Reducing the time to complete wound closure could substantially reduce the costs associated with chronic wound management.10 A registry study of nonhealing wounds showed that the cost of unhealed wounds was $4,000 per patient at 6 months, which increased to $18,000 per patient at 2 years of wound duration.40 PCMP treatment appeared to be beneficial for the wound microbial status as well, as none of the chronic wounds in this study showed signs of infection at week 12.
Native extracellular matrix is a potent inhibitor of proteases, which are implicated in the dysfunctional healing process of chronic wounds.3,34 Native collagen has been found to inhibit elastase, resulting in a positive effect on wound healing.34 In addition, PHMB is a barrier against microbial colonization and biofilm formation.41 The use of PCMP appeared to positively affect the number of wounds with complete closure and the rate of closure compared with the wound history and other literature, which could potentially reduce the total cost of wound care. For example, PCMP may have reduced the need for other, more expensive, wound healing treatments such as other skin substitutes, surgical procedures, skin flaps, or grafts. For those wounds that necessitated further intervention, PCMP assisted in supporting healing and improving granulation tissue. PCMP could also reduce the number of office visits needed to achieve complete wound closure, likely reducing associated healthcare costs.
All skin wounds show bacterial colonization, and some may even progress to active infection of the superficial, deep dermal, or underlying tissues extending through to muscle, tendon, capsule, or bone. Persistent, nonhealing wounds are likely to be deep, large, and poorly vascularized, with significant necrotic tissue, which pose the greatest risk for high bacterial counts and biofilm reformation. The use of PCMP represents a novel approach for managing bioburden, thus controlling biofilm formation, while neutralizing destructive enzymes and providing a biocompatible extracellular matrix to support wound closure.
This study included a relatively small number of wounds, so it is difficult to make conclusive statements about closure rates or comparisons of closure success for different wound types. The study involved a variety of wound types and there were few surgical wounds or pressure wounds examined. PCMP was also not compared with a control standard-of-care group. There was no attempt to randomize patients to different treatment arms and the individuals who were assessing the wound healing were not blinded to the treatment.
CONCLUSIONS
PCMP represents a new class of wound management tools and is comprised of both native type I collagen and the antimicrobial PHMB. PCMP appeared to positively impact the management of wound healing across a variety of complex chronic wounds. Results need to be further explored and validated with randomized, controlled studies in a larger sample of patients.
ACKNOWLEDGMENTS
The author thanks Sally Kaplan, RN, Farisha Baksh, BS, Russell Caprioli, DPM, John Haight, DPM, Michael Pliskin, DPM, and Raymond Ferguson, DPM, for collecting and contributing data to this article; Kristina Shyta, Asad Baig, Michael Sabolinski, MD, and Santina Wendling for statistical assistance; Organogenesis Clinical Affairs (Canton, Mass.) for clinical support; and Agnella Izzo Matic, PhD, CMPP, of The Curry Rockefeller Group, LLC (Tarrytown, N.Y.) for editorial assistance. Funding for editorial support was provided by Organogenesis Inc.
Footnotes
Published online 15 January 2019.
Presented at the following meetings: 1. Oropallo, Alisha; Shyta, Krisiva; Kaplan, Sally; Baksh, Farisha; Nicastro; Coppa, Gene; Caprioli, Russell; Haight, John; Pliskin, Michael; Ferguson, Raymond. Use of a Purified Collagen Matrix Plus Polyhexamethylene Biguanide Antimicrobial in the Management of Non- Healing Pressure Ulcers. Symposium on Advanced Wound Care, April 25–29, 2018, Charlotte, N.C. 2. Oropallo, Alisha; Shyta, Krisiva; Kaplan, Sally; Baksh, Farisha; Nicastro, Jeffrey; Coppa, Gene; Caprioli, Russell; Haight, John; Pliskin, Michael; Ferguson, Raymond. Case Series: A Prospective, Single-Center Controlled Clinical Study of a Purified Collagen Matrix with Polyhexamethylene Biguanide on Non-Healing Pressure Injuries.” American Professional Wound Care Association, Sept 7–9, 2017, Philadelphia, Pa. 3. Oropallo, Alisha; Shyta, Krisiva, Kaplan, Sally; Baksh, Farisha; Nicastro, Jeffrey; Coppa, Gene; Caprioli, Russell; Haight, John; Pliskin, Michael; Ferguson, Raymond, Examining the Use of Purified Collagen Matrix plus Polyhexamethylene Biguanide (PHMB) Antimicrobial in the Management of Chronic Wounds. Symposium on Advanced Wound Care, October 20–22, 2017, Las Vegas, Nev.
Supported by Organogenesis Inc.
Disclosure: This study was sponsored by Organogenesis, Inc., Canton, Mass. The Article Processing Charge was paid for by Organogenesis, Inc., Canton, Mass.
REFERENCES
- 1.Bohn GA, Schultz GS, Liden BA, et al. Proactive and early aggressive wound management: a shift in strategy developed by a consensus panel examining the current science, prevention, and management of acute and chronic wounds. Wounds. 2017;29:S37–S42. [PubMed] [Google Scholar]
- 2.Mast BA, Schultz GS. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. 1996;4:411–420. [DOI] [PubMed] [Google Scholar]
- 3.Martin P, Nunan R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br J Dermatol. 2015;173:370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hicks CW, Selvarajah S, Mathioudakis N, et al. Burden of infected diabetic foot ulcers on hospital admissions and costs. Ann Vasc Surg. 2016;33:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim A. IDF Clinical Practice Recommendation on the Diabetic Foot: A guide for healthcare professionals. Diabetes Res Clin Pract. 2017;127:285–287. [DOI] [PubMed] [Google Scholar]
- 6.Lal BK. Venous ulcers of the lower extremity: definition, epidemiology, and economic and social burdens. Semin Vasc Surg. 2015;28:3–5. [DOI] [PubMed] [Google Scholar]
- 7.Rice JB, Desai U, Cummings AK, et al. Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care. 2014;37:651–658. [DOI] [PubMed] [Google Scholar]
- 8.Rice JB, Desai U, Cummings AK, et al. Burden of venous leg ulcers in the United States. J Med Econ. 2014;17:347–356. [DOI] [PubMed] [Google Scholar]
- 9.van Acker K, Léger P, Hartemann A, et al. Burden of diabetic foot disorders, guidelines for management and disparities in implementation in Europe: a systematic literature review. Diabetes Metab Res Rev. 2014;30:635–645. [DOI] [PubMed] [Google Scholar]
- 10.Nussbaum SR, Carter MJ, Fife CE, et al. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health. 2018;21:27–32. [DOI] [PubMed] [Google Scholar]
- 11.James GA, Swogger E, Wolcott R, et al. Biofilms in chronic wounds. Wound Repair Regen. 2008;16:37–44. [DOI] [PubMed] [Google Scholar]
- 12.Lasa I. Towards the identification of the common features of bacterial biofilm development. Int Microbiol. 2006;9:21–28. [PubMed] [Google Scholar]
- 13.Mihai MM, Holban AM, Giurcaneanu C, et al. Microbial biofilms: impact on the pathogenesis of periodontitis, cystic fibrosis, chronic wounds and medical device-related infections. Curr Top Med Chem. 2015;15:1552–1576. [DOI] [PubMed] [Google Scholar]
- 14.Percival SL, Vuotto C, Donelli G, et al. Biofilms and wounds: an identification algorithm and potential treatment options. Adv Wound Care (New Rochelle). 2015;4:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolcott RD, Rhoads DD, Bennett ME, et al. Chronic wounds and the medical biofilm paradigm. J Wound Care. 2010;19:45–46, 48. [DOI] [PubMed] [Google Scholar]
- 16.Schierle CF, De la Garza M, Mustoe TA, et al. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 2009;17:354–359. [DOI] [PubMed] [Google Scholar]
- 17.Pastar I, Nusbaum AG, Gil J, et al. Interactions of methicillin resistant Staphylococcus aureus USA300 and Pseudomonas aeruginosa in polymicrobial wound infection. PLoS One. 2013;8:e56846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurlow J, Couch K, Laforet K, et al. Clinical biofilms: a challenging frontier in wound care. Adv Wound Care (New Rochelle). 2015;4:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. [DOI] [PubMed] [Google Scholar]
- 20.Walters MC, 3rd, Roe F, Bugnicourt A, et al. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob Agents Chemother. 2003;47:317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wolcott RD, Rumbaugh KP, James G, et al. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J Wound Care. 2010;19:320–328. [DOI] [PubMed] [Google Scholar]
- 22.Kim PJ, Attinger CE, Bigham T, et al. Clinic-based debridement of chronic ulcers has minimal impact on bacteria. Wounds. 2018;30(5):138–143. [PubMed] [Google Scholar]
- 23.Kramer A, Dissemond J, Kim S, et al. Consensus on wound antisepsis: update 2018. Skin Pharmacol Physiol. 2018;31:28–58. [DOI] [PubMed] [Google Scholar]
- 24.International Wound Infection Institute (IWII) Wound infection in clinical practice. Wounds International 2016. IWII is the publisher. [Google Scholar]
- 25.Butcher M. PHMB: an effective antimicrobial in wound bioburden management. Br J Nurs. 2012;21:S16, S18–S16, S21. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert P, Moore LE. Cationic antiseptics: diversity of action under a common epithet. J Appl Microbiol. 2005;99:703–715. [DOI] [PubMed] [Google Scholar]
- 27.Hübner NO, Kramer A. Review on the efficacy, safety and clinical applications of polihexanide, a modern wound antiseptic. Skin Pharmacol Physiol. 2010;23:17–27. [DOI] [PubMed] [Google Scholar]
- 28.Kaehn K. Polihexanide: a safe and highly effective biocide. Skin Pharmacol Physiol. 2010;23:7–16. [DOI] [PubMed] [Google Scholar]
- 29.Müller G, Kramer A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J Antimicrob Chemother. 2008;61:1281–1287. [DOI] [PubMed] [Google Scholar]
- 30.To E, Dyck R, Gerber S, et al. The effectiveness of topical polyhexamethylene biguanide (PHMB) agents for the treatment of chronic wounds: a systematic review. Surg Technol Int. 2016;29:45–51. [PubMed] [Google Scholar]
- 31.Lenselink E, Andriessen A. A cohort study on the efficacy of a polyhexanide-containing biocellulose dressing in the treatment of biofilms in wounds. J Wound Care. 2011;20:534, 536–534, 539. [DOI] [PubMed] [Google Scholar]
- 32.Fleck CA, Simman R. Modern collagen wound dressings: function and purpose. J Am Col Certif Wound Spec. 2010;2:50–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chattopadhyay S, Raines RT. Collagen-based biomaterials for wound healing. Biopolymers. 2014;101:821–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negron L, Lun S, May BC. Ovine forestomach matrix biomaterial is a broad spectrum inhibitor of matrix metalloproteinases and neutrophil elastase. Int Wound J. 2014;11:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PuraPly Antimicrobial Wound Matrix [package insert]. 2015Canton, Mass.: Organogenesis Inc. [Google Scholar]
- 36.Brantley J, Park H, Sanchez PJ, et al. The use of a novel antimicrobial and purified native collagen matrix combination to manage bioburden and support healing in challenging wounds: a clinical evaluation. Wounds Int. 2016;7:40–45. [Google Scholar]
- 37.Lintzeris D, Vernon K, Percise H, et al. Effect of a new purified collagen matrix with polyhexamethylene biguanide on recalcitrant wounds of various etiologies: a case series. Wounds. 2018;30:72–78. [PubMed] [Google Scholar]
- 38.Marston WA, Sabolinski ML, Parsons NB, et al. Comparative effectiveness of a bilayered living cellular construct and a porcine collagen wound dressing in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22:334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirsner RS, Sabolinski ML, Parsons NB, et al. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015;23:737–744. [DOI] [PubMed] [Google Scholar]
- 40.Fife CE, Carter MJ. Wound care outcomes and associated cost among patients treated in US outpatient wound centers: data from the US wound registry. Wounds. 2012;24:10–17. [PubMed] [Google Scholar]
- 41.Carpenter S, Davis S, Fitzgerald R, et al. Expert recommendations for optimizing outcomes in the management of biofilm to promote healing of chronic wounds. Wounds. 2016;28:S1–S20.28682298 [Google Scholar]


