Abstract
Background:
Prepectoral prosthetic breast reconstruction is gaining popularity, offering muscle preservation and outcomes similar to subpectoral implant placement in small or moderate size breasts. This study compares the complications of prepectoral and subpectoral immediate prosthetic breast reconstructions following skin reduction mastectomy in large and ptotic breasts.
Methods:
All consecutive patients who underwent immediate tissue expander-based reconstruction following Wise pattern skin reduction mastectomy from November 2011 through August 2017 by a single surgeon were reviewed. The collected data included patient demographics and complications (eg, skin necrosis, hematoma, infection, seroma, implant loss, capsular contracture).
Results:
A total of 54 patients underwent 87 immediate breast reconstructions including 45 subpectoral and 42 prepectoral tissue expander placements. The subpectoral patients had greater body mass indexes (32.5 ± 6.6 versus 29.9 ± 5.4, P = 0.026), higher initial (518 ± 168 ml versus 288 ± 140 ml, P < 0.001) and final (694 ± 123 ml versus 585 ± 122 ml, P = 0.014) implant volumes, more skin flap necrosis (40.0% versus 16.7%, P = 0.044) and infections (37.8% versus 11.9%, P = 0.01) than their prepectoral counterparts, whereas seromas were more common in the prepectoral group (4.4% versus 26.2%, P = 0.015). The overall complication rate, although higher in the subpectoral group compared with the prepectoral group, was not significantly different (62.2% versus 40.5%, P = 0.072).
Conclusions:
Prepectoral tissue expander placement after skin reduction mastectomy is an appealing reconstructive option in patients with large and ptotic breasts. Prosthetic reconstruction following Wise-pattern skin reduction mastectomy is invariably associated with high complication rates irrespective of the plane of implant placement. Greater emphasis should be placed on patient counseling and complication prevention in this challenging patient population.
INTRODUCTION
Prepectoral immediate reconstruction is gaining popularity by offering superior breast shape, no animation, improved implant pocket control, faster expansion, and less pain.1 In the recent years, multiple studies have emphasized the advantages of prepectoral direct or 2-stage prosthetic reconstruction displaying low complications in selected patients, small to medium size breast size, and none to moderate breast ptosis.2–5 In the published reports, a breast prosthesis is wrapped entirely or partly in a sheet of acellular dermal matrix or mesh and placed in the subcutaneous plane substituting the removed breast tissue.2,3 Although such breast “replacement” approach presents an attractive strategy in moderate breast size, it is not applicable in large and/or severely ptotic breasts, where skin reduction is required.
Wise-pattern skin reduction has been employed in immediate implant-based breast reconstruction offering reshaping of the large breast skin envelope.6 The original technique of immediate breast reconstruction following skin reduction mastectomy was associated with a substantial risk of implant exposure at the inframammary fold. The reason for this complication stemmed from the fact that the inferior portion of the implant was located directly under the inverted “T” incision line, which frequently experiences malperfusion and break down.7 The technique was further modified by Bostwick who utilized the deepithelialized inferior breast skin to enhance the coverage of the implant under the troublesome skin juncture.8 In this approach, the superior edge of the deepithelialized skin flap was sutured to the inferior edge of the raised pectoralis major muscle, creating thus a separate well-vascularized implant pocket.8,9 Although the risk of implant exposure decreased with the use of the inferior mastectomy skin flap, the drawbacks associated with subpectoral implant placement persisted. Moreover, although enhanced tissue padding over an implant is frequently desired, additional soft-tissue boost consisting of superiorly located pectoralis muscle does not protect against ischemic complications occurring mostly in the inferior portions of the reconstructed breast. In our study, we hypothesized that immediate prepectoral tissue expander breast reconstruction following skin reduction mastectomy will have similar outcomes to its subpectoral equivalent.
METHODS
All consecutive patients who underwent immediate tissue expander-based reconstruction following Wise pattern skin reduction mastectomy from November 2011 through August 2017 were included in the study. The study as approved by the Institutional Review Board at Spectrum Health Hospitals (IRB Protocol number: 2017-108-SH/HDVCH). All the reconstructions were performed by a single surgeon.
The Surgical Technique
Each patient was marked preoperatively in standing position. A periareolar opening was outlined with a marking pen and continued from the lowest portion of the areola medially and laterally parallel to the bottom contour of the breast to resemble the Wise pattern skin reduction (Fig. 1). The inframammary fold was also marked. No full-thickness incision was performed through the inframammary fold.
Fig. 1.
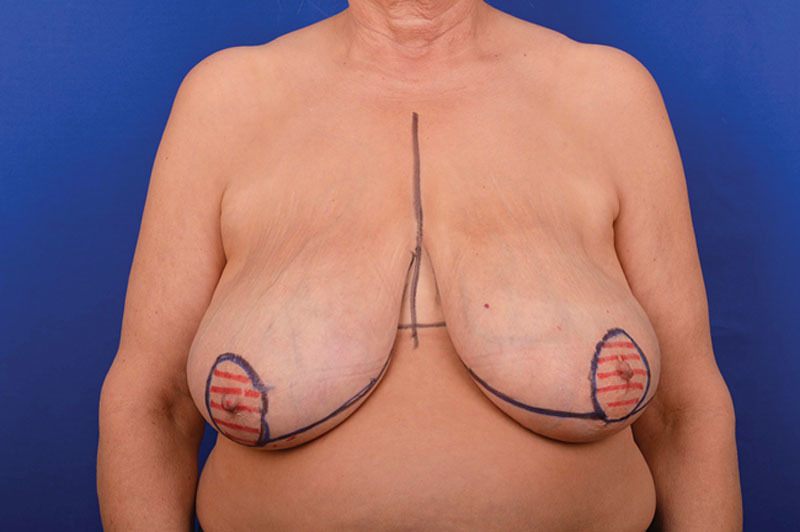
Preoperative markings following the Wise-pattern skin reduction concept.
After a skin-sparing mastectomy through the marked incisions was performed, the previously marked inferiorly based mastectomy skin flap was de-epithelialized and transposed over the tissue expander (Dermaspan smooth, low pole plus, Sientra, Inc, Santa Barbara, Calif.). The superior edge of the de-epithelialized flap was sutured either to the inferior edge of the raised pectoralis major flap (subpectoral placement, Fig. 2) or to the undersurface of the overlaying mastectomy skin flaps (prepectoral placement, Fig. 3) using 2-0 polydioxanone suture. The redundant skin at the top of the vertical incision and “corners” of the superior mastectomy flaps were deepithelialized or removed to achieve the most optimal contour. The fill of each tissue expander was determined intraoperatively assuring that no tension of the overlying mastectomy skin flaps occurred. Two drains were placed in the implant space before closure. The patients chest was wrapped with gauze and an elastic bandage (FLEX-MASTER non-sterile clip closure bandage, Vista, California).
Fig. 2.
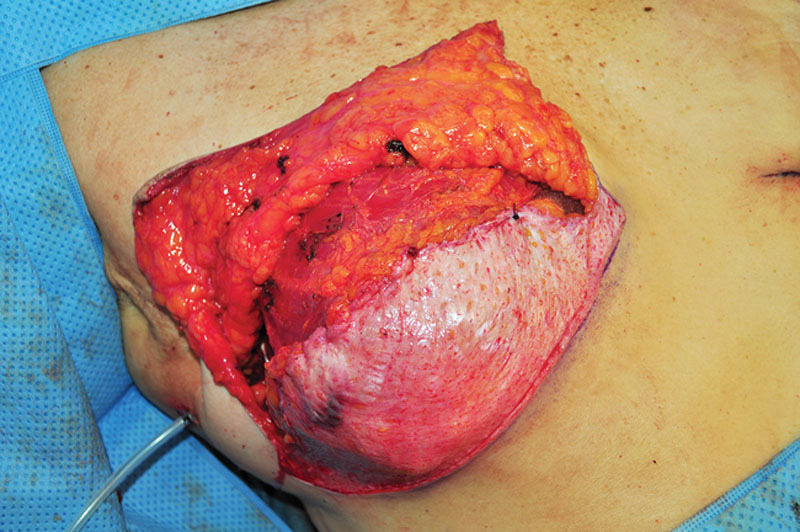
Intraoperative view of the de-epithelialized inferiorly based mastectomy skin flap secured to the inferior edge of the raised pectoralis major muscle. The tissue expander is in subpectoral position.
Fig. 3.

Intraoperative view of the de-epithelialized inferiorly based mastectomy skin flap secured to the undersurface of the superiorly based mastectomy skin flap. The tissue expander is in the prepectoral position.
Follow-up
At 1-week follow-up, the compression dressing was taken down, and the patient was transitioned to a supportive brassiere. The drains were removed once the output fell below 30 ml per day for 2 consecutive days. For later reconstructions, one of the drains was always discontinued after 1 week and the remaining drain was withdrawn before postoperative week 3 irrespective of output.
Data Collection and Analysis
The collected data included patient demographics, comorbidities, complications, details of surgery, presence of pre- or postoperative radiation, and length of follow-up. The complications consisted of skin necrosis, hematoma, infection, seroma, exposure of tissue expander or final implant, and implant loss. Operative and nonoperative cases of skin necrosis were included. The details of surgery including the weight of mastectomy specimen, plane of tissue expander placement, intraoperative and final tissue expander volume, final implant volume, and the area of deepithelialized flap were recorded and statistically compared. Capsular contracture was assessed after at least 3 months following the exchange of tissue expander for permanent prosthesis and statistically analyzed.
Summary statistics were calculated. Quantitative data are expressed as the mean + SD, with the exception of years of follow-up, which are expressed as the median, with the minimum and maximum in parentheses. Due to the non-normal distribution of follow-up time, this variable was transformed using the inverse hyperbolic sine before comparative data analysis. Nominal data are expressed as a percentage. Comparisons between groups for unique individuals for age, height, weight, and body mass index (BMI) were performed using the t test. All other comparisons involved the number of procedures. Comparisons between the subpectoral and prepectoral groups involving quantitative outcome variables were performed using linear regression, while those comparisons with dichotomous outcome variables were performed using logistic regression. Multivariate logistic regression analysis was performed, with complication as the dependent variable, while the independent variables were treatment (prepectoral versus subpectoral, the latter was the reference variable), age, BMI, follow-up time, and presence of comorbidity. For all of the regression analyses, clustering on the subjects was performed to obtain robust standard errors to adjust for nonindependent observations. Significance was assessed at P < 0.05. All analyses were performed using Stata v.15.0 (StataCorp, College Station, Tex.).
RESULTS
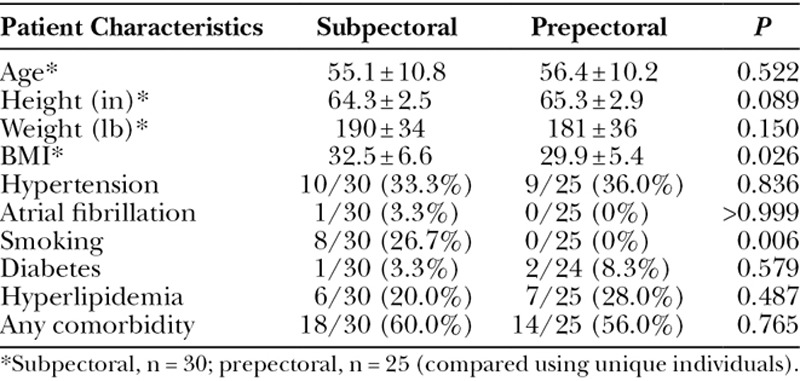
A total of 54 patients underwent 87 immediate breast reconstructions using the tissue expander and skin reduction method as described above. In 30 patients, the implant was placed in the submuscular and in 24 patients in subcutaneous plane. Eight out of 30 patients in the subpectoral group were smokers, and there were no smokers in the prepectoral group (Table 1). The patients in the subpectoral group had higher BMI. Otherwise, both groups had similar demographics and comorbidities (Table 1).
Table 1.
Patients’ Demographics and Comorbidities
The reconstructions were performed bilaterally in 33 patients and on one side in 21 patients. The tissue expander was placed in the subpectoral pocket in 45 breasts and in the prepectoral pocket in 42 reconstructions. Figures 4, 5 depict 12-month result of a subpectoral and prepectoral 2-stage breast reconstruction of the patients presented in Figures 2, 3, respectively. The weights of 23 mastectomy specimens in the submuscular and 41 mastectomy specimens in the prepectoral groups were available for analysis (Table 2) and ranged from 403 to 1,435 g for the subpectoral and 289 to 1,529 g for the prepectoral groups. Despite no statistical difference in the weight of the removed breasts (P = 0.079) or transposed inferior mastectomy flap area (P = 0.054), the reconstructions in the submuscular group had greater initial fill volume, higher final tissue expander volume, and larger size of the permanent implant (Table 2). There was no difference in the laterality of the reconstruction in either group.
Fig. 4.
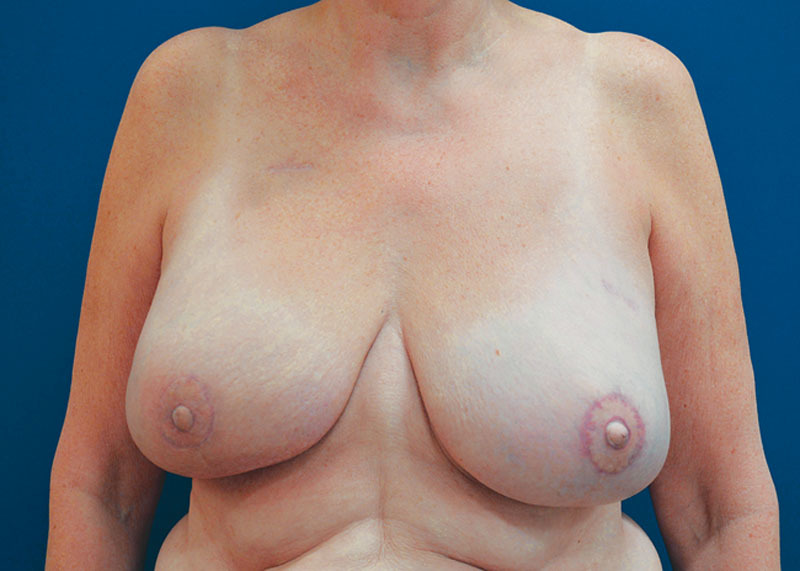
One-year result of a subpectoral 2-stage reconstruction of the patient depicted in Figure 2.
Fig. 5.
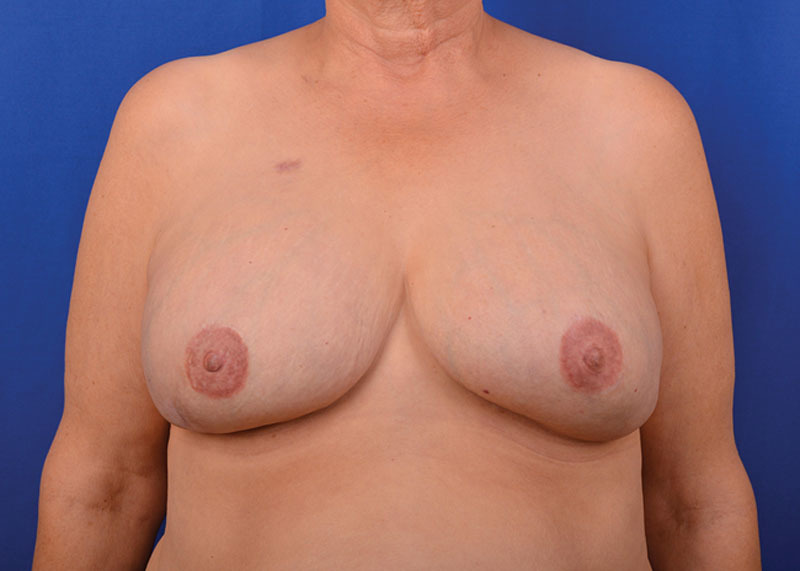
One-year result of a prepectoral 2-stage reconstruction of the patient depicted in Figure 3.
Table 2.
Characteristics of Mastectomy Specimens and Reconstructed Breasts

Similarly, the number of breasts with history of preexisting or adjuvant radiation treatment was uniform among the submuscular and prepectoral reconstructions. The follow-up was significantly shorter in the prepectoral group and ranged 0.8–66 months with the shortest follow-up of 23 days in a 76-year-old patient with multiple comorbidities and extensive mastectomy flap necrosis who decided to abandon reconstruction after tissue expander removal and excision of necrosis. The length of the follow-up in the subpectoral group ranged 6–71 months. The difference in follow-up length stems from the preference in reconstructive technique, which primarily consisted of the prepectoral approach after March of 2015.
Complications
The subpectoral breasts displayed a higher incidence of necrosis and infection than the prepectoral counterparts (Table 3). However, the incidence of implant exposure and loss were identical in both groups. Interestingly, the number of seromas requiring aspiration was significantly higher in the prepectoral breasts. This could be attributed to different protocols of drain management in the subpectoral versus prepectoral reconstructions. In fact, the number of seromas increased with time from 4.4% (2/45) in the first part of the study to 26.2% (11/42) later in the observation period. There was no statistically significant difference in the number of complications overall between the groups despite the highly discrepant percentages in the subpectoral versus prepectoral breasts (62.2% versus 40.5%, P = 0.072). Additionally, when comparing breasts with or without necrosis, neither initial fill volume (422 ± 226 ml versus 401 ± 179 ml, respectively; P = 0.698) nor final implant fill volume (436 ± 150 ml versus 493 ± 172 ml, respectively; P = 0.181) were significantly different.
Table 3.
Postoperative Complications

Although the rate of capsular contracture was almost 6-fold higher in the subpectoral group compared with the prepectoral group, this difference was not statistically significant (Table 3). Ignoring the surgical grouping, those with capsular contracture (n = 8) had a significantly longer median follow-up than the 74 breasts without capsular contracture 1.7 (1.3–3.9) years versus 1.2 (0.06–5.9 years, respectively; P = 0.012). Additionally, only 1/16 (6.3%) of subjects who received radiation treatment had a capsular contracture, compared with 7/66 (10.6%) subjects who did not receive radiation, which was not a significant difference (P = 0.620).
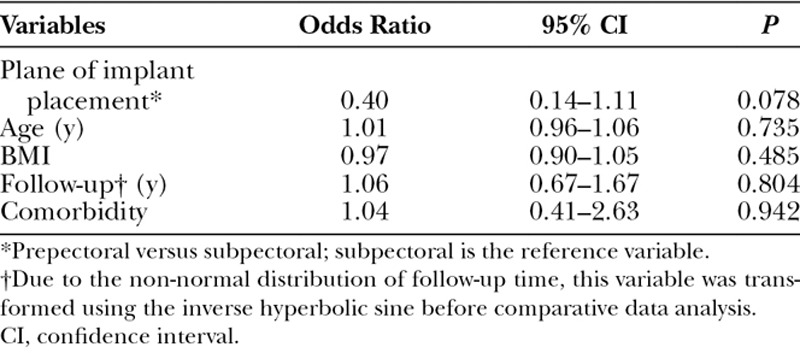
Univariate analysis failed to identify BMI, weight of the mastectomy specimen, area of the inferior mastectomy flap, initial, or final implant volumes as risk factors for complications (Table 4). In the multivariate analysis, age, BMI, the length of follow-up, and comorbidity were not found to be predictors of complications (Table 5). Placement under the muscle was not a predictor of complication; however, the statistical analysis showed a trend toward significance (P = 0.078). Smoking could not be included in the multivariate analysis of risk factors as there were no smokers in the prepectoral group.
Table 4.
Univariate Analysis of Complications
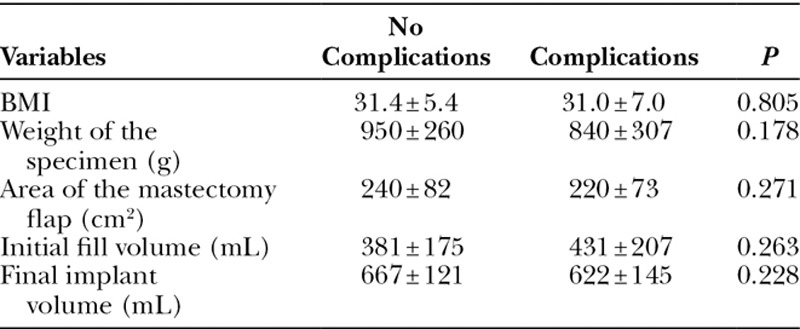
Table 5.
Multivariate Analysis of Postoperative Complications
DISCUSSION
Immediate reconstruction of large breasts following Wise pattern skin reduction has been associated with high complication rates.6,8,10 As frequently stated in the literature, obesity and smoking are the main factors contributing to high complications rates in this patient population.11,12 In fact, for every point increase in BMI, the odds of complications increase by 5.9% and reconstructive failure by 7.9%.11 Obese patients tend to have larger and more ptotic breasts than their thinner counterparts.
In a study examining 59 immediate prosthetic-based reconstructions following Wise pattern skin reduction, mastectomy specimens with weight exceeding 700 g resulted in complication rates of 53%, which was in distinct contrast to breast specimens below 700 g associated with complication rate of 14.3%.6 The authors calculated that for every 100 g increase in the weight of mastectomy specimen, the risk of major complications raises by 1.18-fold.6 The results of our investigation corroborate the previously published outcomes. In the present study, immediate reconstruction using Wise pattern skin reduction was associated with high complication rates irrespective of the plane of implant placement. It is possible, however, that with a larger clinical sample, statistically notable differences favoring the prepectoral group could be observed.
The main complications in both groups consisted of mastectomy skin necrosis, seroma, and infection, with the latter likely being the consequence of the first 2 adverse outcomes.6,11 As previously described, the greater number of mastectomy flap necroses observed in the subpectoral group can be attributed to higher BMI and presence of smokers in this group.8,10 Furthermore, in our study, the weights of mastectomy specimens and initial or final implant volumes were higher in the subpectoral group and intuitively could contribute to mastectomy flap ischemia. However, these factors were not found to be related to mastectomy flap necrosis overall.
Although 30% necrosis at the “T-juncture” following skin reduction mastectomy has been reported previously,7 the extent of flap necrosis observed in our study was rather overwhelming and emphasizes the need for preventative measures. As on-table clinical assessment of mastectomy flap perfusion is not reliable, fluorescein and Wood’s lamp, replaced recently by the far more accurate intraoperative fluorescent angiography utilizing indocyanine green, can be employed to outline and eliminate the area of future tissue necrosis, reducing both complications and the need for additional surgery.13 Wise-pattern skin reduction predisposes to tissue ischemia specifically within the “triangles” of the superiorly based mastectomy flap, with greater malperfusion associated with longer flaps.14 Although skin reduction design predetermines placement of the surgical incisions, it is still possible to eliminate the ischemic area and achieve immediate reconstruction through design modification (Figs. 6, 7). Clearly, in the instance of global mastectomy flap ischemia, immediate reconstruction should be aborted. Unfortunately, indocyanine green angiography was not available to us during reconstructions in the presented series.
Fig. 6.
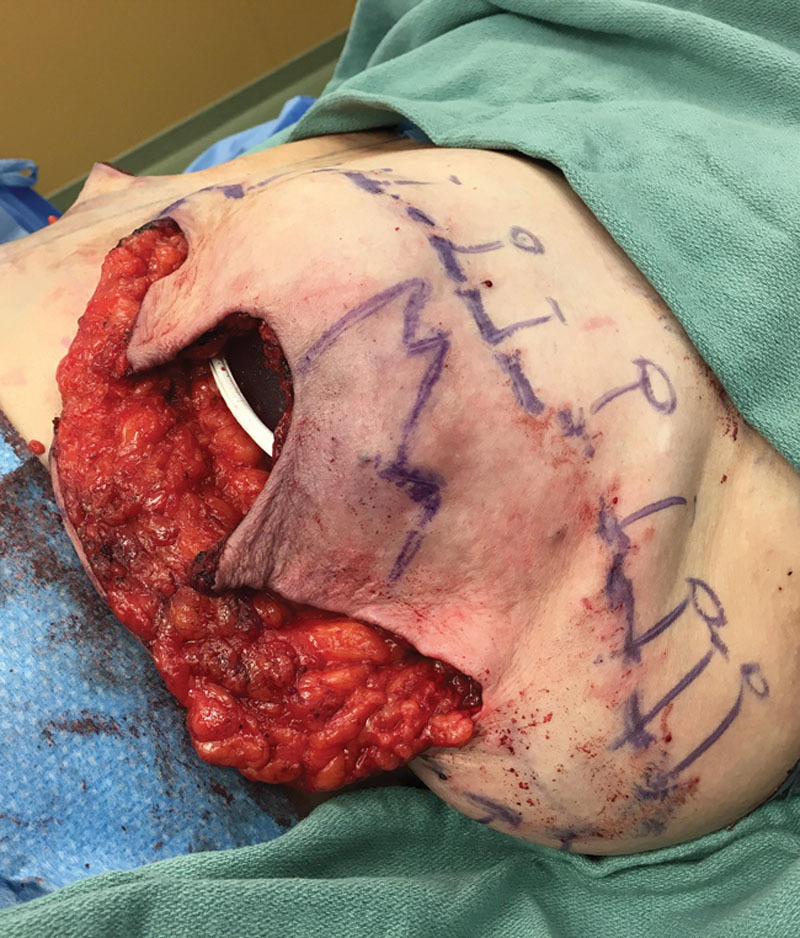
Clinically suspicious ischemia of the superiorly based “triangular” mastectomy flaps during immediate reconstruction. Marked area indicates a zone of malperfusion as detected by intraoperative angiography (solid line). After excision of the ischemic areas, the reconstructive design was changed to use the inferiorly based flap to overlay the deepithelialized well-perfused edge of the superiorly based mastectomy flap along the dashed line.
Fig. 7.

Postoperative result of a prepectoral 2-stage prosthetic reconstruction in the same patient as depicted in Figure 6 after on-table elimination of the ischemic areas and design change.
Another problem associated with prosthetic reconstruction of large breasts is seroma formation, ranging from 0.2% to 20%, with a higher incidence in obese patients.15 As noted by Jordan et al.,15 for every unit of BMI increase, the seroma rate raises by 7–14%. Seroma in breast reconstruction can be a result of large dead space, presence of foreign object, sheering forces, impaired lymphatic function, and occult infection.15 Presence of seroma is directly associated with major complications and implant loss. In fact, it remains uncertain whether seroma is a result or a marker of infection.15 Notably, submuscular reconstruction has been associated with greater seroma formation.15 This was not confirmed in our study.
The reason for this discrepancy stems from the duration of the drain, which was removed before 3 weeks irrespective of output in all prepectoral but not subpectoral patients. Drain duration has been related to increased infections with 5% rise in complications for each day of prolonged drainage.16 This likely explains why infections were significantly higher in the subpectoral group.
Because considerable fluid production is unavoidable after large breast removal, elimination of dead space appears paramount.17 Quilting stitches obliterating the dead space have been advocated as a more effective means of seroma prevention, as compared with tissue sealants or topical steroids.15,18 Gabriel and Maxwell11 recommended aggressive reduction of the mastectomy pocket with quilting resorbable stitches to eliminate the usually excessive lateral and superior gutters, which we have recently implemented. However, placement of a high-volume implant appropriate for a fuller body habitus demands a substantial pocket to be preserved. The raw tissue remains in dynamic contact with the prosthetic and continues to produce fluid until a capsule is formed. Therefore, additional measures aimed at reduction of sheering forces, such as compressive bandage or negative-pressure wound therapy applied over the reconstructed site, seem appropriate.15,19 All of our patients received a compression bandage around their chest that stayed in place for 1 week following surgery. Negative-pressure wound therapy could not be implemented in the presented reconstructions due to insurance constraints.
Our study demonstrated that prepectoral tissue expander reconstruction following Wise-pattern skin reduction mastectomy leads to similar outcomes as subpectoral placement. Preservation of the pectoralis muscle conserves the functionality of shoulder movements such as anteversion, adduction, and internal rotation of the upper extremity, and contributes to truncal stability, which is frequently impaired in patients with higher BMI.4,11 Subcutaneous implant mimics the anatomic breast location, prevents animation, and appears to be better tolerated than the submuscular placement as shown in augmentation patients.20
One of the conditions invariably associated with prosthetic reconstruction is capsular contracture. Historically, capsular contracture rates were lower after submuscular placement than subcutaneous placement, with 11% versus 33% Baker III capsular contracture rates.21 While analyzing these traditional reports, one must keep in mind that the prosthetic reconstructions were performed in the context of radical or non–skin-sparing mastectomies, which by default created increased tension of tissues overlaying the implant, likely predisposing to capsular contracture. Interestingly, a histological analysis of periprosthetic capsules from breasts reconstructed with tissue expanders placed below and above the pectoralis major muscle revealed that prepectoral capsules were thinner and had lower vascularity than the subpectoral specimens.22 In contrast to the earlier clinical studies emphasizing the beneficial role of submuscular implant position in prevention of capsular contracture, the recent articles analyzing subcutaneous 2-stage reconstruction following skin-sparing mastectomy without the use of acellular dermal matrix have noted a 7.6% Baker III/IV capsular contracture rate after at least 55 months of follow-up.23 Our series similarly demonstrated lower capsular contracture rates in the prepectoral group although the difference was not statistically significant.
The main limitations of our study were the small sample size and short follow-up. In the present analysis, the overall early postoperative complications in the prepectoral versus subpectoral groups were similar. However, decisive conclusions about capsular contracture rates in prepectoral implant placement after skin reduction mastectomy cannot be drawn since capsular contracture may occur even after 2–3 years after reconstruction.23
CONCLUSIONS
The results of our study demonstrate that prepectoral tissue expander placement after skin reduction mastectomy is a viable reconstructive option in patients with large and ptotic breasts. Prosthetic reconstruction following Wise-pattern skin reduction mastectomy is invariably associated with high complication rates irrespective of the plane of implant placement. Greater emphasis should be placed on patient counseling and complication prevention in this challenging patient population.
Footnotes
Published online 11 January 2019.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by Advanced Plastic Surgery.
REFERENCES
- 1.Zhu L, Mohan AT, Abdelsattar JM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg. 2016;69:e77–e86. [DOI] [PubMed] [Google Scholar]
- 2.Sigalove S, Maxwell GP, Sigalove NM, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg. 2017;139:287–294. [DOI] [PubMed] [Google Scholar]
- 3.Bernini M, Calabrese C, Cecconi L, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open. 2015;3:e574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vidya R, Masià J, Cawthorn S, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017;23:670–676. [DOI] [PubMed] [Google Scholar]
- 5.Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: a new dimension to implant-based breast reconstruction. Clin Breast Cancer. 2017;17:266–271. [DOI] [PubMed] [Google Scholar]
- 6.Inbal A, Gur E, Lemelman BT, et al. Optimizing patient selection for direct-to-implant immediate breast reconstruction using wise-pattern skin-reducing mastectomy in large and ptotic breasts. Aesthetic Plast Surg. 2017;41:1058–1067. [DOI] [PubMed] [Google Scholar]
- 7.Salgarello M, Visconti G, Barone-Adesi L, et al. Inverted-T skin-reducing mastectomy with immediate implant reconstruction using the submuscular-subfascial pocket. Plast Reconstr Surg. 2012;130:31–41. [DOI] [PubMed] [Google Scholar]
- 8.Carlson GW, Bostwick J, 3rd, Styblo TM, et al. Skin-sparing mastectomy. Oncologic and reconstructive considerations. Ann Surg. 1997;225:570–575; discussion 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond DC, Capraro PA, Ozolins EB, et al. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg. 2002;110:206–211. [DOI] [PubMed] [Google Scholar]
- 10.Nava MB, Cortinovis U, Ottolenghi J, et al. Skin-reducing mastectomy. Plast Reconstr Surg. 2006;118:603–610; discussion 611. [DOI] [PubMed] [Google Scholar]
- 11.Gabriel A, Maxwell GP. Prepectoral breast reconstruction in challenging patients. Plast Reconstr Surg. 2017;140:14S–21S. [DOI] [PubMed] [Google Scholar]
- 12.Tasoulis MK, Iqbal FM, Cawthorn S, et al. Subcutaneous implant breast reconstruction: time to reconsider? Eur J Surg Oncol. 2017;43:1636–1646. [DOI] [PubMed] [Google Scholar]
- 13.Komorowska-Timek E, Gurtner GC. Intraoperative perfusion mapping with laser-assisted indocyanine green imaging can predict and prevent complications in immediate breast reconstruction. Plast Reconstr Surg. 2010;125:1065–1073. [DOI] [PubMed] [Google Scholar]
- 14.Caputo GG, Marchetti A, Dalla Pozza E, et al. Skin-reduction breast reconstructions with prepectoral implant. Plast Reconstr Surg. 2016;137:1702–1705. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SW, Khavanin N, Kim JY. Seroma in prosthetic breast reconstruction. Plast Reconstr Surg. 2016;137:1104–1116. [DOI] [PubMed] [Google Scholar]
- 16.Crosby MA, Dong W, Feng L, et al. Effect of intraoperative saline fill volume on perioperative outcomes in tissue expander breast reconstruction. Plast Reconstr Surg. 2011;127:1065–1072. [DOI] [PubMed] [Google Scholar]
- 17.Aho JM, Nickerson TP, Thiels CA, et al. Prevention of postoperative seromas with dead space obliteration: a case-control study. Int J Surg. 2016;29:70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart AM, Duggal C, Pinell-White X, et al. A prospective randomized trial of the efficacy of fibrin glue, triamcinolone acetonide, and quilting sutures in seroma prevention after latissimus dorsi breast reconstruction. Plast Reconstr Surg. 2017;139:854e–863e. [DOI] [PubMed] [Google Scholar]
- 19.Angspatt A, Laopiyasakul T, Pungrasmi P, et al. The role of negative-pressure wound therapy in latissimus dorsi flap donor site seroma prevention: a cohort study. Arch Plast Surg. 2017;44:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallace MS, Wallace AM, Lee J, et al. Pain after breast surgery: a survey of 282 women. Pain. 1996;66:195–205. [DOI] [PubMed] [Google Scholar]
- 21.Gruber RP, Kahn RA, Lash H, et al. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg. 1981;67:312–317. [DOI] [PubMed] [Google Scholar]
- 22.Tomita K, Yano K, Nishibayashi A, et al. Effects of subcutaneous versus submuscular tissue expander placement on breast capsule formation. Plast Reconstr Surg Glob Open. 2015;3:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:30–39. [DOI] [PubMed] [Google Scholar]
- 24.Huo J, Smith BD, Giordano SH, et al. A comparison of patient-centered economic and clinical outcomes of post-mastectomy breast reconstruction between obese and non-obese patients. Breast. 2016;30:118–124. [DOI] [PubMed] [Google Scholar]


