Abstract
Background:
Muscle flap reconstruction has become a mainstay of therapy following treatment of sternal wound complications; however, success depends on removing wound exudate and infectious material from the wound before reconstruction and closure. Importantly, time to closure is a key factor affecting morbidity/mortality and cost-to-treat for this wound type.
Methods:
A retrospective analysis of 30 patients who were treated for sternal wound complications between June 2015 and October 2017 was performed. After surgical debridement, group 1 patients (n = 15) received negative pressure wound therapy (NPWT) with instillation and dwell time (NPWTi-d), instilling 1/8-strength Dakin’s solution with a 20-minute dwell time followed by 2 hours of NPWT (-125 mm Hg); group 2 patients (n = 15) were treated with wet-to-moist dressings soaked in 1/8-strength Dakin’s solution. After muscle flap reconstruction and closure with sutures, group 1 patients received closed incision negative pressure therapy, and group 2 patients received Benzoin and wound closure strips. Data collected included time to closure, therapy duration, number of debridements/dressing changes, drain duration, and complications.
Results:
There was a significantly shorter time to closure (P < 0.0001) for group 1 when compared with group 2. In addition, there were fewer therapy days (P = 0.0041), fewer debridements/dressing changes (P = 0.0011), and shorter drain duration (P = 0.0001) for group 1 when compared with group 2.
Conclusions:
We describe a novel regimen consisting of adjunctive NPWTi-d, along with debridement and systemic antibiotics, followed by closed incision negative pressure therapy after muscle flap reconstruction and closure, to help manage preexisting sternal wounds that had failed to close following a previous cardiac procedure.
INTRODUCTION
Median sternotomy was first proposed over a century ago and replaced bilateral anterior thoracotomy after 1957 as the standard incision type utilized for cardiac surgeries.1,2 Recent reports estimate that over 500,000 median sternotomies are performed in the United States each year as part of coronary artery bypass grafting (CABG) procedures, heart transplants, and other cardiothoracic procedures.3 The vast majority of patients undergoing median sternotomy require coronary revascularization procedures and share comorbid conditions leading to coronary artery disease (eg, advanced age, obesity, and insulin-dependent diabetes), placing the patient at a higher risk for sternal wound infection (SWI).4 Sternal wounds resulting from SWIs, which include superficial, deep, and organ/space infections,4–6 can be classified into 4 types based on sternal stability and bone viability.7,8 SWIs can be an extremely lethal condition due to the proximity to multiple critical organs in the mediastinal space, with mortality rates of greater than 50% reported in some cases.9–16 Importantly, wound depth, sternal instability, and the infection type are all known to impact the treatment time and mortality rates associated with these wounds.
The foundation for managing SWIs is controlling the source of infection with antibiotics and debriding the wound; however, filling sternal defects with soft-tissue flaps (eg, omental or muscle flaps) and sternal stabilization (ie, surgical wire fixation or fixation of transverse plates) are also important treatment modalities before reconstruction, especially for wounds with deep infections.8,17–19 Following debridement(s), traditional treatment of infected sternal wounds has involved systemic antibiotics and intensive dressing care with antimicrobial wet-to-moist dressings or closed irrigation of the wound with antibiotic solutions.8,20 Negative pressure wound therapy (NPWT) has also been used as adjunctive therapy in assisting with sternal wound management, in part, by promoting granulation of the wound bed.21,22 Importantly, the early placement of muscle flaps has been reported to significantly reduce the length of stay in the hospital, length of stay in intensive care unit, recurrent infection rate, and mortality associated with deep SWIs.8,23,24 In addition, the application of closed incision negative pressure therapy (ciNPT) after closure following pectoralis muscle flap procedures has been reported to reduce the number of intensive care unit days and the number of necessary revision surgeries when compared with conventional incision dressings only.25
NPWT with instillation and dwell time (NPWTi-d) is a recent technological advancement to NPWT that includes the periodic instillation of a topical solution onto the wound bed, thereby utilizing both negative pressure and the instillation of topical wound solutions for wound cleansing and the removal of infectious materials.26–29 NPWTi-d has been reported as an effective adjunctive therapy for contaminated or infected wounds in open fractures, breast reconstruction, necrotizing fasciitis, upper and lower extremity wounds, ulcers, and other complex wounds.30–36 However, there are no studies comparing NPWTi-d with other wound management modalities for sternal wounds.
In this study, NPWTi-d was retrospectively compared with standard wet-to-moist dressing changes as an adjunctive modality for managing sternal wounds resulting from sternal incision complications. The data compared between groups included demographic information, days to wound closure, total therapy days, the number of debridements, the number of dressing changes, drain duration, and complications.
PATIENTS AND METHODS
A retrospective assessment of 30 patients who were treated and underwent reconstructive surgery by a single surgeon for sternal wound complications between June 2015 and October 2017 at a large medical center in the Chicago Metropolitan Area was performed. Data were collected for the 30 most recent patients (15 patients who received NPWTi-d and 15 patients who received wet-to-moist dressings) during the aforementioned time period, excluding those with the presence of vascular grafts. The data collected for each patient included demographic information, days to wound closure, total therapy days, number of debridements, number of dressing changes, drain duration, and complications.
After collecting demographic information for each patient, the initial evaluation by Plastic Surgery included a thorough history and physical, which was directed to (1) the purpose and nature of the previous sternotomy; (2) the wound duration before the examination; and (3) graft harvest sites. The overall patient condition was evaluated, including assessing patient comorbidities that included obesity and dyslipidemia, glycemic status and diabetes, heart and vascular diseases, pulmonary disease, and smoking status. In addition, a careful assessment of antiplatelet and anticoagulant medications was performed to assess the bleeding risk and potential need for transfusion in a patient population that would be sensitive to changes in circulating oxygen levels. These medications were then discontinued if it was deemed safe by the cardiovascular team; otherwise, appropriate steps were taken to risk stratify these patients. Lastly, complete blood counts and metabolic panels were analyzed for each patient to assess surgical fitness and to aid in assessing complication risk.
Physical exploration of the wound, which was performed under general anesthesia for patients in both groups, included evaluation of wound characteristics: drainage, purulence, exposed foreign material (sutures, wires, fixation hardware), and sternal stability. When necessary, imaging was performed to determine the extent of the fluid collection and infectious bony involvement. In addition, wound cultures were obtained, and targeted antibiotic therapy based on culture and sensitivity was managed by Infectious Disease specialists. Patients in both groups received systemic antibiotics throughout the treatment to control infection, and intravenous (IV) antibiotics were continued for 6 weeks postreconstruction.
After the assessment, the wounds of patients in both groups were surgically debrided of all nonviable tissue including skin, fat, fascia, muscle, bone, and cartilage to healthy, punctate bleeding tissue. Electrocautery was judiciously used during debridement to help achieve hemostasis. Twenty-four hours after the initial debridement, patients in group 1 received NPWTi-d (V.A.C. VERAFLO Therapy; KCI, An Acelity Company, San Antonio, Tex.) using a reticulated open-cell foam dressing with through holes (ROCF-CC; V.A.C. VERAFLO CLEANSE CHOICE Dressing; KCI, An Acelity Company) following the initial debridement (Fig. 1). Wounds were instilled with 1/8-strength Dakin’s solution every 2 hours with a 20-minute dwell time, followed by negative pressure at −125 mm Hg. The pericardium was intact for all patients in this study, and there was no free flow of instillation solution into the thoracic cavity. NPWTi-d was also not employed if vascular grafts were present, even though the vascular grafts were not directly exposed. Wounds of patients in the group 1 were evaluated for the presence of nonviable tissue and the development of granulation tissue every 72 hours when changing the ROCF-CC dressing. Patients in group 2 received wet-to-moist dressings soaked in 1/8-strength Dakin’s solution, which were changed every 6 hours. The wounds of patients in group 2 were evaluated daily at the bedside for the presence of nonviable tissue and the development of granulation tissue. For patients in both groups, wounds that failed to progress at evaluation or had persistent culture growth underwent subsequent debridement until the wound was free from infection.
Fig. 1.
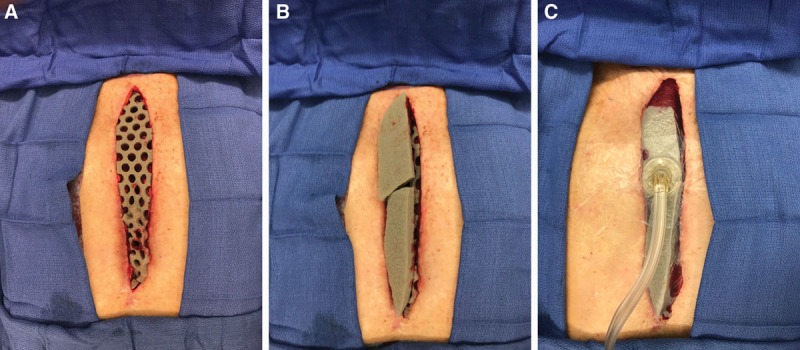
Application of NPWTi-d using ROCF-CC dressing. A contact layer of the ROCF-CC dressing containing 10-mm circular holes was placed onto the wounds of patients in group 1 immediately following surgical debridement (A). Cover layers of the ROCF-CC dressing were then placed over the contact layer (B), and NPWTi-d was applied (C).
Once wounds were deemed appropriate for closure, a pectoralis major muscle flap reconstruction was performed for patients in both groups. The criteria for closure were the same in both groups: (1) negative for infection, as assessed by clinical and laboratory evaluation; (2) presence of granulation tissue formation; and (3) absence of nonviable tissue. Briefly, bilateral advancement or transposition muscle flaps were raised in an avascular plane off the anterior chest wall (Fig. 2A). The muscle was released from its costal attachments as dissection was continued laterally only as far as necessary to advance the muscle medially to cover the sternal wound. When necessary, a Doppler probe was used to assess the viability of the thoracoacromial vascular pedicle. Once the muscle flap was adequately elevated, a minimal subcutaneous release was performed (Fig. 2B). Care was taken to preserve the anterior pectoralis muscle fascia since the fascial layer proved critical at the time of repairing the flaps—the fascia tissue was thicker and held sutures more reliably than muscle alone. Subcutaneous dissection was performed only so much as to advance the muscle medially to cover the wound. Once bilateral muscle flaps were elevated and advanced toward each other, 2 closed suction (19 Fr) channel drains were placed in the subpectoral plane bilaterally of each patient in both groups, and the muscle flaps were repaired to each other at the midline using sturdy interrupted monofilament sutures fastened in an inverted figure of 8 fashion (Fig. 2C). Skin and subcutaneous tissue were freshened and repaired centrally with absorbable monofilament sutures.
Fig. 2.
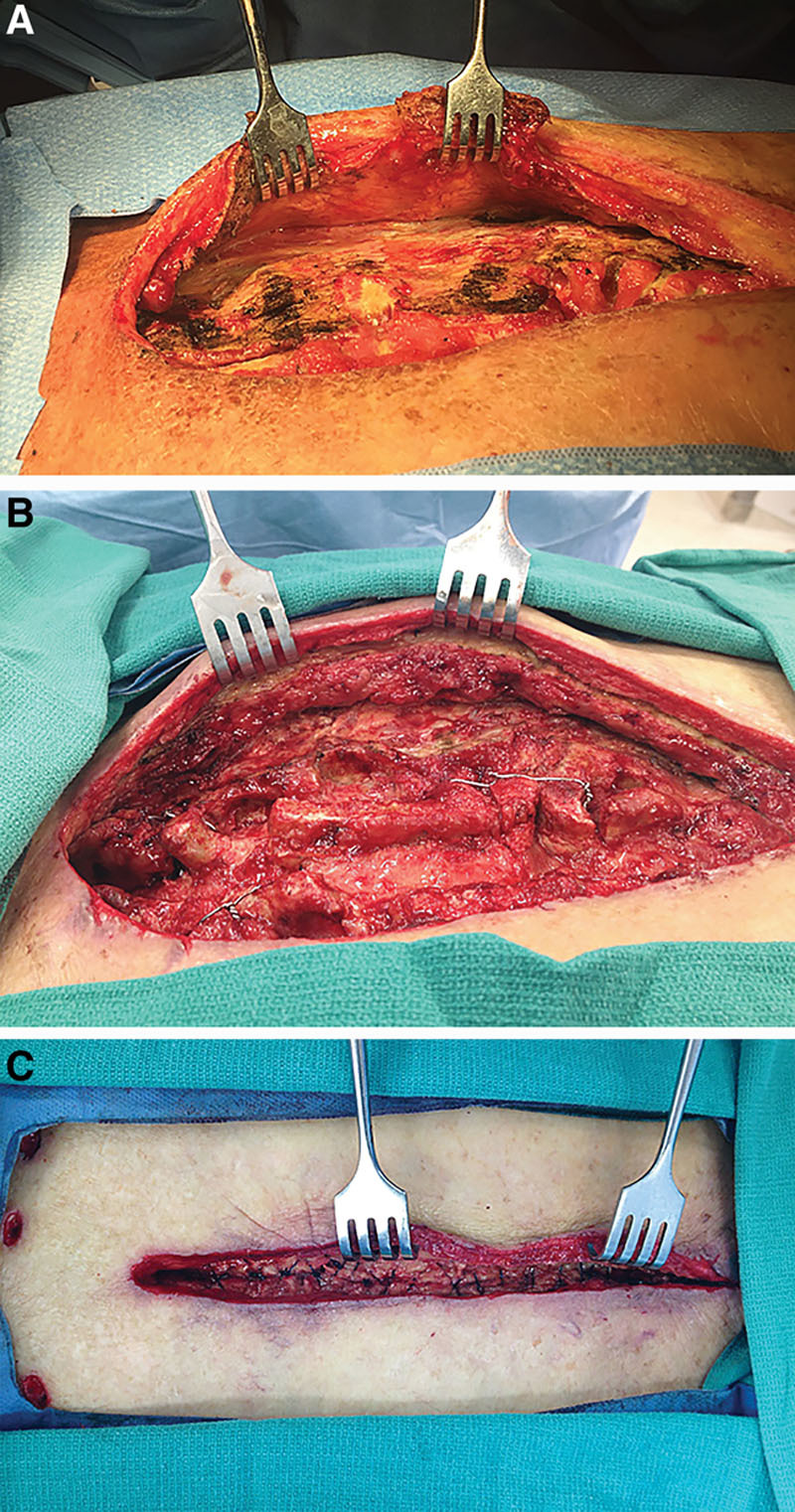
Muscle flap reconstruction of SWIs. Pectoralis major muscle flaps were raised in an avascular plane from the anterior chest wall (A). A minimal subcutaneous release was performed on the raised muscle flap (B). Muscle flaps were repaired to each other at the midline in an inverted figure of 8 fashion (C).
For patients in group 2, Benzoin and wound closure strips were applied over closed incisions, and the incisions were evaluated after 1 and 2 weeks. Dressings were discontinued after 2 weeks if they had not been discontinued before that point. Patients in group 1 received ciNPT (PREVENA Incision Management System using CUSTOMIZABLE Dressing; KCI, An Acelity Company) for a duration of 5–7 days (Fig. 3). Drains placed in patients from both groups remained for a minimum of 5 days and were removed when less than 30 mL per 24-hour period for 2 consecutive days was being collected.
Fig. 3.
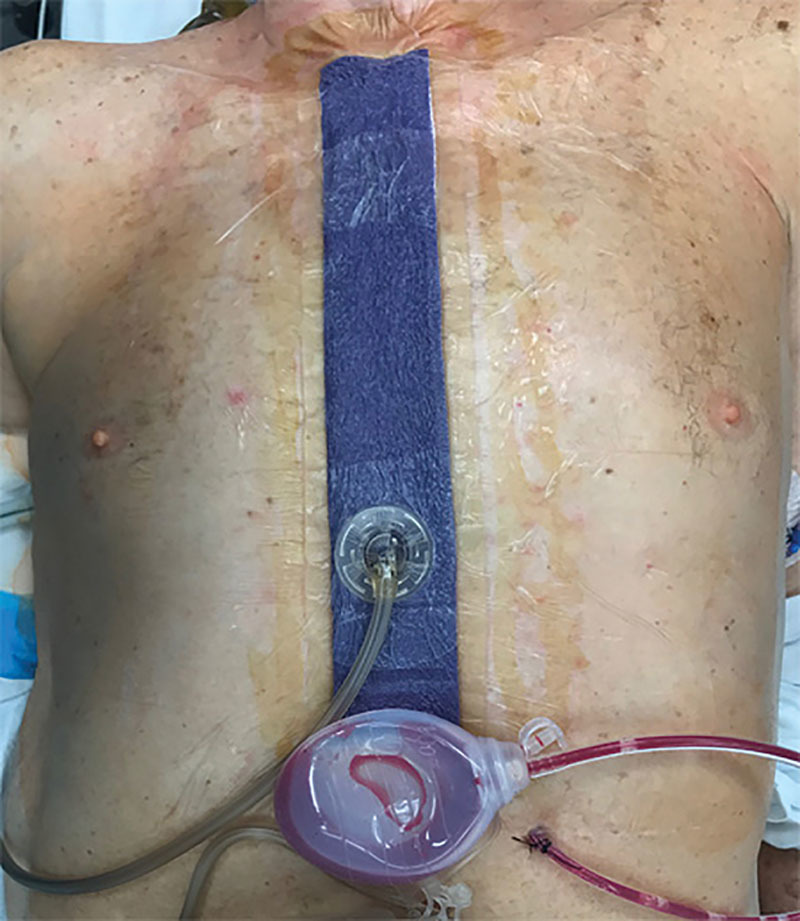
Application of ciNPT dressing. ciNPT was applied over a closed sternal incision of a patient from group 1. ciNPT was applied for 5–7 days before removal.
Statistical Analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, N.C.) software. Continuous variables were presented as the mean and SD, and categorical data were presented as number and percent. t Tests or Wilcoxon tests were used for comparing continuous measures between groups, whereas chi-square or Fisher’s exact tests were used for comparisons of categorical measures between groups. Statistical significance was determined at an alpha of 0.05.
RESULTS
During the study period, a total of 30 patients underwent muscle flap reconstruction for preexisting sternal wounds that had failed to close following a previous cardiac procedure. Each group comprised 15 patients, all of which had undergone a previous CABG procedure. In addition, all patients in both groups had the left internal mammary artery harvested. Overall, the mean age of the patients in this study was 70.0 ± 7.4 years, and the mean body mass index (BMI) was 31.5 ± 4.7 kg/m2. There were no significant differences in patient age, BMI, or sex when comparing the 2 groups (Table 1). There was also no statistical difference between groups in the duration of the sternal wounds before reconstruction, with the overall duration of the wound being 7.9 ± 4.5 weeks (Table 1), and all wounds from both groups were positive for bacterial cultures. Lastly, patients in both groups displayed various comorbidities, including hypertension, coronary artery disease, hyperlipidemia, and diabetes mellitus; however, there were no significant differences in either the number of overall comorbidities or the number of specific comorbidities when comparing the groups (Table 1).
Table 1.
Demographics and Baseline Characteristics
When comparing the 2 groups, there was a significantly shorter time to primary wound closure for group 1 when compared with group 2 (P < 0.0001; Fig. 4A). The mean time to primary wound closure for the group 1 was 7.9 ± 2.3 days with a median of 8 days, whereas patients in group 2 required 13.9 ± 3.2 days to primary wound closure with a median of 15 days. Furthermore, 75% of the patients in group 1 could be closed within 9 days, and the remaining NPWTi-d-treated patients were closed within 12 days (Fig. 4B). In contrast, at least 16 days were required until primary closure of 75% of patients in group 2, and 20 days were required until all patients in group 2 underwent primary closure (Fig. 4B).
Fig. 4.
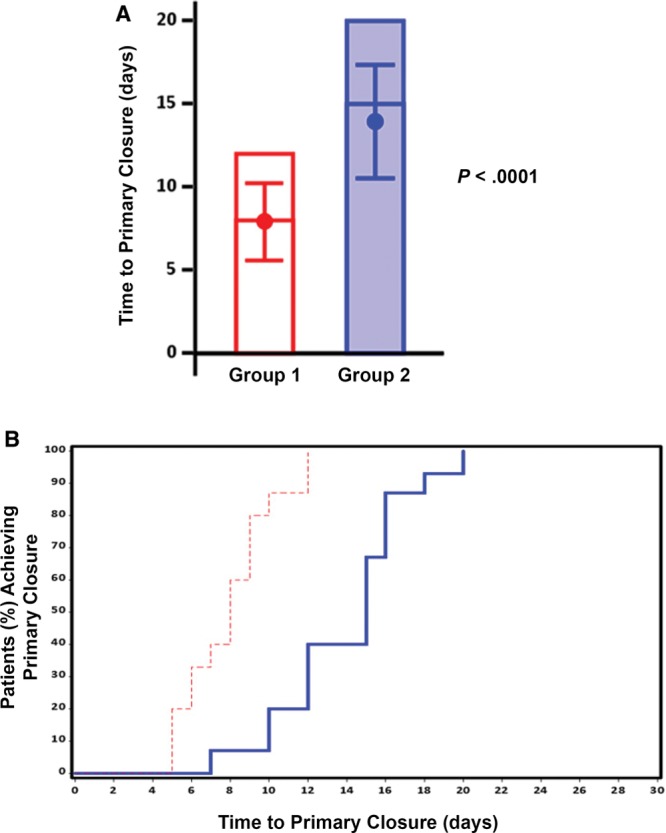
Effect of NPWTi-d and ciNPT on time to primary closure. A, Bar graph indicating the maximum number of days before sternal wound closure. The mean (± SD) and the median time to primary closure are represented by the dot inside the bar and the horizontal line inside the bar, respectively. B, Kaplan–Meier curve showing the time to primary closure for the percentage of patients in group 1 (red dashed line) and group 2 (solid blue line).
In addition to time to primary closure, the number of therapy days was also compared between groups. There were significantly fewer therapy days for patients in group 1 when compared with patients in group 2 (P = 0.0041; Table 2). The mean number of therapy days for group 1 was 5.4 ± 2.1 with a median of 6 days, whereas group 2 required 8.4 ± 2.9 days of therapy with a median of 8 days (Table 2).
Table 2.
Therapy Days

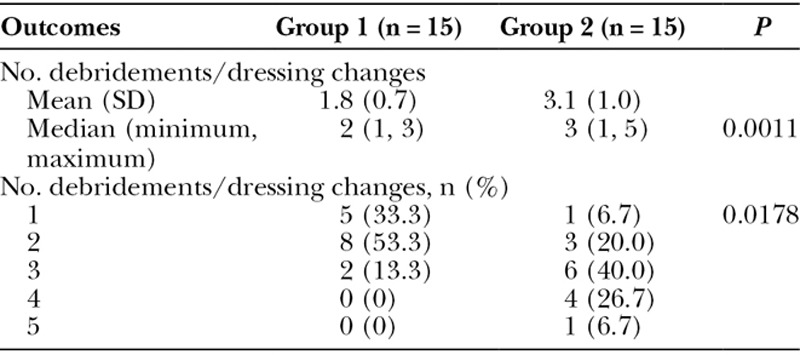
The total number of excisional debridements and dressing changes were also compared between the 2 groups—dressing changes were performed at the time of surgical debridements. There were significantly fewer surgical debridements and dressing changes for patients in group 1 when compared with patients in group 2 (P = 0.0011; Table 3). The mean number of debridements for patients in group 1 was 1.8 ± 0.7 with a median of 2, whereas patients in group 2 required 3.1 ± 1.0 debridements with a median of 3 (Table 3). Furthermore, most patients in group 2 required 3–4 debridements, whereas only 2 patients in group 1 received 3 surgical debridements and none received more than 3 (Table 3).
Table 3.
Number of Debridements/Dressing Changes
Lastly, drain duration was compared between group 1, which received ciNPT over the closed incision, and group 2, which had wound closure strips placed over the closed incision. There was a significantly shorter drain duration for patients in group 1 when compared with patients in group 2 (P = 0.0001; Fig. 5A). The mean drain duration for group 1 was 15.0 ± 2.0 days with a median of 14 days, whereas the mean drain duration for group 2 was 21.7 ± 3.9 days with a median of 22 days (Fig. 5A). In group 1, 75% of patients underwent drain removal by day 16, and the remaining patients in group 1 had drains removed by day 21 (Fig. 5B). In group 2, 75% of patients underwent drain removal by day 24 and all patients had drains removed by day 28 (Fig. 5B).
Fig. 5.
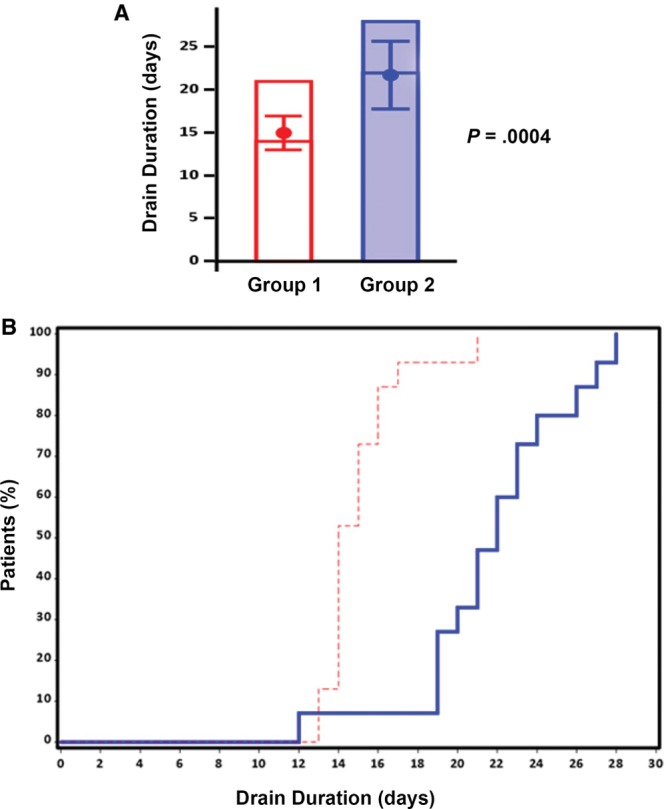
Effect of ciNPT on drain duration. A, Bar graph indicating the maximum number of days before drain removal. The mean (± SD) and the median time to drain removal are represented by the dot inside the bar and the horizontal line inside the bar, respectively. B, Kaplan–Meier curve showing the time to drain removal for the percentage of patients in group 1 (red dashed line) and group 2 (solid blue line).
There were 3 patients with complications (ie, seromas) in group 2 and no complications in the group 1 patients; however, the difference in complications between groups was not statistically significant (P = 0.2241). For patients in both groups, all incisions remained healed at the 90-day follow-up visit.
DISCUSSION
Although muscle flap reconstruction of sternal wounds has become a mainstay of therapy, it is critical for wounds to be rendered free of infection as expeditiously as possible before flap reconstruction. The results from this study indicate that NPWTi-d favorably affects time to closure when compared with wet-to-moist dressings. Our results also indicate that NPWTi-d positively affects the number of debridements/dressing changes and the number of therapy days when compared with wet-to-moist dressings. Lastly, the results of this study indicate that NPWTi-d followed by ciNPT favorably affects drain duration for sternal wound reconstructions when compared with wet-to-moist dressings followed by application of wound closure strips. This novel approach to adjunctively manage preexisting sternal wounds that had failed to close following a previous cardiac procedure, in conjunction with debridement, ultimately reduced the time to provide a clean wound bed and allowed for expedited flap reconstruction.
Early plastic surgery referral and effective flap reconstruction for deep SWIs have shown the benefit of reduced ventilator dependence, chronic infection, tracheostomy, development of pressure sores, wound dehiscence, mortality, and hospital length of stay.24,37,38 In fact, the odds of chronic wound infections have been reported to increase 1.2 times per day for each day of delay from the diagnosis of infection to flap closure.38 A previous study has demonstrated good results with radical wound excision and immediate flap reconstruction of deep SWIs; however, flap closure complications and recurrent wound infection remained at 18.8% and 6.5%, respectively.23 In the current study, patients in group 1 (ie, NPWTi-d) had a reduced time to closure of 7 days on average when compared with group 2 (ie, wet-to-moist dressings), and there were no infections observed in this study. These results suggest that additional studies assessing the benefits of NPWTi-d on early flap closure for patients with recurrent sternal wounds are warranted.
This retrospective study was the first to compare the effectiveness of NPWTi-d with a conventional therapeutic regimen for helping to manage sternal wounds, although there is a previous case report describing NPWTi-d as a temporizing technique before sternal closure to help manage mediastinitis caused by Mycoplasma hominis.39 There are several differences between our NPWTi-d protocol and the protocol from the previous case report, including differences in topical antiseptic solutions, foam dressings, dwell times, and cycle times. Nonetheless, patients in group 1 (ie, NPWTi-d) of our study had a 1.75-fold shorter time-to-closure and significantly fewer debridements/dressing changes when compared with conventional dressings. These results are similar to results from 2 previous studies assessing other types of nonhealing surgical wounds. First, Gabriel et al.30 reported a reduced time to closure for various nonhealing surgical wound types when managed with NPWTi-d versus standard moist wound-care therapy. Second, Kim et al.27 reported a reduced number of debridements and a reduced time to closure for various types of wounds when managed with NPWTi-d versus NPWT.
Clinical studies have shown that NPWTi-d can help remove bioburden through repeated cleansing cycles.40,41 This clinical benefit is supported by studies in a porcine wound model, which demonstrated that NPWTi-d may provide more effective wound cleansing and causes less edema when compared with lavage cleansing.42 NPWT itself provides mechanical forces that cause tissue strain, draw the wound edges together, and reduce the overall wound area,43 which have been shown to promote perfusion, reduce infectious materials, reduce edema, and lead to granulation tissue formation.44,45 Preclinical studies have also shown that NPWT using ROCF enhances cell viability, cell migration, and intracellular metabolism when compared with NPWT using gauze.46,47 Thus, the selection of the underlying dressing used with NPWT can also potentially impact wound outcomes. In this study, patients treated with NPWTi-d had a significantly lower number of surgical debridements, suggesting that this therapeutic regimen may have helped with wound cleansing or removal of debris. In addition, we speculate that the ROCF-CC dressing and intermittent instillation may have allowed for better contact between the topical antimicrobial solution and the wound surface than could be achieved with the dressings soaked in antimicrobial solution.
The pectoralis major muscle advancement technique in this study was selected primarily because the technique takes advantage of the dominant vascular supply from the thoracoacromial system to the pedicle, which provides reliable circulation in most cases. In addition, the advancement technique also maintains the pectoralis muscle humeral insertion and, thereby, reduces arm and shoulder donor morbidity. An alternative muscle turnover technique involves a complete subcutaneous dissection laterally and release of the humeral insertion of the pectoralis major muscle. However, this technique requires a division of the thoracoacromial pedicle and basing the flap on its medial segmental perforating vessels. Therefore, care must be taken to maintain intercostal vascular supply to ensure flap viability. In addition, the majority of sternal wound reconstructions occur in the setting of CABGs,3 in which the internal mammary arteries have been harvested for grafting. Harvest of the internal mammary arteries may make a turnover flap considerably less reliable as the segmental vessels will not have a robust originating vascular pedicle.
Another important finding from this study was that patients treated with NPWTi-d followed by ciNPT (group 1) had a significantly shorter drain duration than patients in the group 2. These data support a previous study by Gabriel et al.48 in patients treated with ciNPT on closed incisions after postmastectomy breast reconstructions, whereby the estimated drain duration was similar to those reported for group 1 in our study.48 Based on the results from our study, we cannot distinguish the individual effect that either NPWTi-d or ciNPT had on drain duration from this study; however, it is possible that a cleaner wound with potentially less edema may lead to less serous fluid formation, thereby reducing drain duration and seroma potential.
In summary, optimal wound preparation allows for rigid sternal fixation and early flap reconstruction. This study was limited in that it included a relatively small sample size and was a retrospective study with the potential for selection bias and other known disadvantages of this study type. In addition, the exclusion of patients with vascular grafts from both groups could have introduced selection bias into the study. Nonetheless, NPWTi-d or NPWTi-d+ciNPT could become a more effective alternative for the management of sternal wound reconstructions. Considering that time to closure is a key factor affecting morbidity, the cost-to-treat, and mortality rate from SWIs, the data from this study suggest that prospective, randomized, controlled studies are needed to determine whether NPWTi-d expedites the time to closure and improves clinical outcomes for this wound type, especially in patients with an elevated risk for recurrent deep SWIs.
CONCLUSIONS
This retrospective comparative study between NPWTi-d and conventional dressings showed that NPWTi-d reduced the time to wound closure, the number of therapy days, and the number of debridements/dressing changes for muscle flap reconstruction of preexisting sternal wounds that had failed to close following a previous cardiac procedure. In addition, this study showed that NPWTi-d followed by closure and application of ciNPT reduced drain duration in closed sternal wounds when compared with conventional dressings followed by closure and application of wound closure strips.
ACKNOWLEDGMENTS
The authors thank Leah Griffin and Yeni Nieves (Acelity, San Antonio, Tex.) for providing statistical analysis support and Johnny Short (Acelity) for help with article preparation.
Footnotes
Published online 4 January 2019.
Disclosure: Dr. Chowdhry is a consultant for KCI USA, Inc., an Acelity company. Dr. Wilhelmi has no conflicting interests that are related to this study. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Milton H. Mediastinal surgery. Lancet. 1897;149:872–875. [Google Scholar]
- 2.Reser D, Caliskan E, Tolboom H, et al. Median sternotomy. Multimedia Manual Cardiothorac Surg. 2015;2015. [DOI] [PubMed] [Google Scholar]
- 3.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e60. [DOI] [PubMed] [Google Scholar]
- 4.Lepelletier D, Bourigault C, Roussel JC, et al. Epidemiology and prevention of surgical site infections after cardiac surgery. Med Mal Infect. 2013;43:403–409. [DOI] [PubMed] [Google Scholar]
- 5.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. [DOI] [PubMed] [Google Scholar]
- 6.Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13:606–608. [PubMed] [Google Scholar]
- 7.van Wingerden JJ, Ubbink DT, van der Horst CM, et al. Poststernotomy mediastinitis: a classification to initiate and evaluate reconstructive management based on evidence from a structured review. J Cardiothorac Surg. 2014;9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu-Omar Y, Kocher GJ, Bosco P, et al. European Association for Cardio-Thoracic Surgery expert consensus statement on the prevention and management of mediastinitis. Eur J Cardiothorac Surg. 2017;51:10–29. [DOI] [PubMed] [Google Scholar]
- 9.Ridderstolpe L, Gill H, Granfeldt H, et al. Superficial and deep sternal wound complications: incidence, risk factors and mortality. Eur J Cardiothorac Surg. 2001;20:1168–1175. [DOI] [PubMed] [Google Scholar]
- 10.Crabtree TD, Codd JE, Fraser VJ, et al. Multivariate analysis of risk factors for deep and superficial sternal infection after coronary artery bypass grafting at a tertiary care medical center. Semin Thorac Cardiovasc Surg. 2004;16:53–61. [DOI] [PubMed] [Google Scholar]
- 11.Olsen MA, Lock-Buckley P, Hopkins D, et al. The risk factors for deep and superficial chest surgical-site infections after coronary artery bypass graft surgery are different. J Thorac Cardiovasc Surg. 2002;124:136–145. [DOI] [PubMed] [Google Scholar]
- 12.Loop FD, Lytle BW, Cosgrove DM, et al. J. Maxwell Chamberlain memorial paper. Sternal wound complications after isolated coronary artery bypass grafting: early and late mortality, morbidity, and cost of care. Ann Thorac Surg. 1990;49:179–186; discussion 186. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbeak CS, Murphy DM, Koenig S, et al. The clinical and economic impact of deep chest surgical site infections following coronary artery bypass graft surgery. Chest. 2000;118:397–402. [DOI] [PubMed] [Google Scholar]
- 14.Salehi Omran A, Karimi A, Ahmadi SH, et al. Superficial and deep sternal wound infection after more than 9000 coronary artery bypass graft (CABG): incidence, risk factors and mortality. BMC Infect Dis. 2007;7:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg. 1998;65:1050–1056. [DOI] [PubMed] [Google Scholar]
- 16.Mekontso-Dessap A, Kirsch M, Brun-Buisson C, et al. Poststernotomy mediastinitis due to Staphylococcus aureus: comparison of methicillin-resistant and methicillin-susceptible cases. Clin Infect Dis. 2001;32:877–883. [DOI] [PubMed] [Google Scholar]
- 17.Cicilioni OJ, Jr, Stieg FH, 3rd, Papanicolaou G. Sternal wound reconstruction with transverse plate fixation. Plast Reconstr Surg. 2005;115:1297–1303. [DOI] [PubMed] [Google Scholar]
- 18.Robicsek F, Fokin A, Cook J, et al. Sternal instability after midline sternotomy. Thorac Cardiovasc Surg. 2000;48:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Robicsek F, Daugherty HK, Cook JW. The prevention and treatment of sternum separation following open-heart surgery. J Thorac Cardiovasc Surg. 1977;73:267–268. [PubMed] [Google Scholar]
- 20.Bryant LR, Spencer FC, Trinkle JK. Treatment of median sternotomy infection by mediastinal irrigation with an antibiotic solution. Ann Surg. 1969;169:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obdeijn MC, de Lange MY, Lichtendahl DH, et al. Vacuum-assisted closure in the treatment of poststernotomy mediastinitis. Ann Thorac Surg. 1999;68:2358–2360. [DOI] [PubMed] [Google Scholar]
- 22.Gudbjartsson T, Jeppsson A, Sjögren J, et al. Sternal wound infections following open heart surgery—a review. Scand Cardiovasc J. 2016;50:341–348. [DOI] [PubMed] [Google Scholar]
- 23.Jones G, Jurkiewicz MJ, Bostwick J, et al. Management of the infected median sternotomy wound with muscle flaps. The Emory 20-year experience. Ann Surg. 1997;225:766–776; discussion 776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandt C, Alvarez JM. First-line treatment of deep sternal infection by a plastic surgical approach: superior results compared with conventional cardiac surgical orthodoxy. Plast Reconstr Surg. 2002;109:2231–2237. [DOI] [PubMed] [Google Scholar]
- 25.Nickl S, Steindl J, Langthaler D, et al. First experiences with incisional negative pressure wound therapy in a high-risk poststernotomy patient population treated with pectoralis major muscle flap for deep sternal wound infection. J Reconstr Microsurg. 2018;34:1–7. [DOI] [PubMed] [Google Scholar]
- 26.Gupta S, Gabriel A, Lantis J, et al. Clinical recommendations and practical guide for negative pressure wound therapy with instillation. Int Wound J. 2016;13:159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim PJ, Attinger CE, Steinberg JS, et al. The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg. 2014;133:709–716. [DOI] [PubMed] [Google Scholar]
- 28.Wolvos T. The evolution of negative pressure wound therapy: negative pressure wound therapy with instillation. J Wound Care. 2015;24:15–20. [DOI] [PubMed] [Google Scholar]
- 29.Kim PJ, Attinger CE, Steinberg JS, et al. Negative pressure wound therapy with instillation: past, present, and future. Surg Technol Int. 2015;26:51–56. [PubMed] [Google Scholar]
- 30.Gabriel A, Shores J, Heinrich C, et al. Negative pressure wound therapy with instillation: a pilot study describing a new method for treating infected wounds. Int Wound J. 2008;5:399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Timmers MS, Graafland N, Bernards AT, et al. Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen. 2009;17:278–286. [DOI] [PubMed] [Google Scholar]
- 32.Raad W, Lantis JC, 2nd, Tyrie L, et al. Vacuum-assisted closure instill as a method of sterilizing massive venous stasis wounds prior to split thickness skin graft placement. Int Wound J. 2010;7:81–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brinkert D, Ali M, Naud M, et al. Negative pressure wound therapy with saline instillation: 131 patient case series. Int Wound J. 2013;10:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabriel A, Kahn K, Karmy-Jones R. Use of negative pressure wound therapy with automated, volumetric instillation for the treatment of extremity and trunk wounds: clinical outcomes and potential cost-effectiveness. Eplasty. 2014;14:e41. [PMC free article] [PubMed] [Google Scholar]
- 35.Goss SG, Schwartz JA, Facchin F, et al. Negative pressure wound therapy with instillation (NPWTi) better reduces postdebridement bioburden in chronically infected lower extremity wounds than NPWT alone. J Am Coll Clin Wound Spec. 2014;4:74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheong JY, Goltsman D, Warrier S. A new method of salvaging breast reconstruction after breast implant using negative pressure wound therapy and instillation. Aesthet Plast Surg. 2016;40:745–748. [DOI] [PubMed] [Google Scholar]
- 37.Cabbabe EB, Cabbabe SW. Immediate versus delayed one-stage sternal débridement and pectoralis muscle flap reconstruction of deep sternal wound infections. Plast Reconstr Surg. 2009;123:1490–1494. [DOI] [PubMed] [Google Scholar]
- 38.Lo S, Hutson K, Hallam MJ, et al. The importance of early flap coverage in deep sternal wounds. Ann Plast Surg. 2014;73:588–590. [DOI] [PubMed] [Google Scholar]
- 39.Karaca S, Kalangos A. Vacuum-assisted closure (VAC)-Instill(®) with continuous irrigation for the treatment of Mycoplasma hominis mediastinitis. Int Wound J. 2015;12:595–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabriel A. Integrated negative pressure wound therapy system with volumetric automated fluid instillation in wounds at risk for compromised healing. Int Wound J. 2012;9:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolvos TA. Negative pressure wound therapy with instillation: the current state of the art. Surg Technol Int. 2014;24:53–62. [PubMed] [Google Scholar]
- 42.Allen D, LaBarbera LA, Bondre IL, et al. Comparison of tissue damage, cleansing and cross-contamination potential during wound cleansing via two methods: lavage and negative pressure wound therapy with instillation. Int Wound J. 2014;11:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxena V, Hwang CW, Huang S, et al. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg. 2004;114:1086–1096; discussion 1097. [DOI] [PubMed] [Google Scholar]
- 44.Morykwas MJ, Argenta LC, Shelton-Brown EI, et al. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg. 1997;38:553–562. [DOI] [PubMed] [Google Scholar]
- 45.Venturi ML, Attinger CE, Mesbahi AN, et al. Mechanisms and clinical applications of the vacuum-assisted closure (VAC) device: a review. Am J Clin Dermatol. 2005;6:185–194. [DOI] [PubMed] [Google Scholar]
- 46.McNulty AK, Schmidt M, Feeley T, et al. Effects of negative pressure wound therapy on fibroblast viability, chemotactic signaling, and proliferation in a provisional wound (fibrin) matrix. Wound Repair Regen. 2007;15:838–846. [DOI] [PubMed] [Google Scholar]
- 47.McNulty AK, Schmidt M, Feeley T, et al. Effects of negative pressure wound therapy on cellular energetics in fibroblasts grown in a provisional wound (fibrin) matrix. Wound Repair Regen. 2009;17:192–199. [DOI] [PubMed] [Google Scholar]
- 48.Gabriel A, Sigalove SR, Maxwell GP. Initial experience using closed incision negative pressure therapy after immediate postmastectomy breast reconstruction. Plast Reconstr Surg Glob Open. 2016;4:e819. [DOI] [PMC free article] [PubMed] [Google Scholar]


