Supplemental Digital Content is available in the text.
Abstract
Background:
Identification and localization of functional lymphatic vessels are important for lymphaticovenular anastomosis. Conventional high-frequency ultrasound (CHFUS) has been reported to be useful for them, but it has some disadvantages. In this article, we present new capabilities of ultra high-frequency ultrasound (UHFUS) for imaging of the lymphatic vessels, which may overcome the weakness of CHFUS.
Methods:
Thirty unaffected extremities in 30 unilateral secondary lymphedema patients (13 upper limbs and 17 lower limbs) were examined. Identification of the lymphatic vessels using UHFUS and CHFUS were performed at 3 sites in each unaffected extremity. Number and diameter of the detected lymphatic vessels were compared between UHFUS and CHFUS groups. At the same time, new characteristics of the lymphatic vessels seen with UHFUS were investigated.
Results:
One hundred sixty-nine lymphatic vessels were detected with UHFUS, and 118 lymphatic vessels with CHFUS. The number of lymphatic vessels found in upper and lower extremities was significantly larger with UHFUS than with CHFUS. The diameter of lymphatic vessels found in upper and lower extremities was significantly smaller with UHFUS than with CHFUS. All lymphatic vessels that were detected in UFHUS were less likely to collapse when the transducer was against the skin of the examined sites.
Conclusions:
Detection rate of the lymphatic vessels in nonlymphedematous extremities with UHFUS was higher than that with CHFUS. UHFUS provides images with extremely high resolution, demonstrating new characteristics of the lymphatic vessels.
INTRODUCTION
Lymphedema of the extremities is a chronic, debilitating disease. Lymphedema can be treated conservatively or surgically, including tissue resection and lymph drainage (direct or indirect).1–9 Among the surgical procedures, lymphaticovenular anastomosis (LVA) is an effective and minimally invasive surgical treatment for refractory lymphedema.10–17 A positive correlation between the number of LVA performed and therapeutic effectiveness has been reported.18 Preoperative identification of functional lymphatic vessels and veins can contribute to shorter operative time.19–21
Indocyanine green (ICG) lymphography has been reported to be a minimally invasive imaging modality that can not only evaluate the severity of lymphedema but also determine the location of the lymphatic vessels as linear patterns.16,22–24 However, ICG lymphography cannot visualize lymphatic flow that is masked beneath dermal backflow patterns, particularly in stardust and diffuse patterns, in the extremities affected with severe lymphedema.25 ICG lymphography also requires ICG injection before the examination, which cannot be performed on patients who are allergic to iodine. For detection of lymphatic vessels in a region masked by dermal backflow pattern or in patients with allergic reactions to ICG, conventional high-frequency ultrasound (CHFUS) has been reported to be useful as a substitute for ICG lymphography even in the limbs severely affected by lymphedema.19,20 Ultrasound-guided detection of lymphatic vessels for lymphedema results in more effective LVA surgery.20
Disadvantages of this CHFUS system with an upper frequency of 15–18 MHz are that it is highly operator dependent and that it is difficult to distinguish the lymphatic vessels with the subcutaneous veins or the nerves when the lymphatic vessels are smaller than 0.3 mm.20 Precise imaging of small anatomical structures is often difficult with conventional ultrasound. Recent developments in ultra high-resolution ultrasound systems provide frequencies as high as 70 MHz and capability resolution as fine as 30 μm, which could allow more precise imaging of small anatomical structures. Ultra high-frequency ultrasound (UHFUS) may allow for more accurate imaging of the lymphatic vessels and provide valuable and new information in detection of the lymphatic vessels.
In this study, we investigated the performance of UHFUS in detecting the lymphatic vessels compared with CHFUS.
PATIENTS AND METHODS
We examined 30 patients with unilateral lymphedema of the extremities (13 unilateral upper limb lymphedema and 17 unilateral lower limb lymphedema) at the Department of Lymphatic Surgery, AZ Sint-Maarten Hospital. In each patient, we investigated the healthy side using 2 types of ultrasonography. There were 25 women and 5 men whose average age was 55.7 years (range, 36–74 years) and the average body mass index 25.4 (range, 21.2–32.8). No patient had potential allergic reaction to ICG. This study was conducted under the institutional ethical review board. All patients provided written informed consent for participation in this retrospective observational study.
Detection of the Lymphatic Vessel Using Ultrasound
Identification and marking of the lymphatic vessels using UHFUS and CHFUS were performed at 3 fixed sites in each unaffected extremity. The lymphatic vessels of the upper extremity were identified in distal one-third of the volar aspect of forearm, proximal one-third of the dorsal aspect of forearm, and distal one-third of the volar aspect of upper arm. The lymphatic vessels of the lower extremity were identified in middle one-third of the medial aspect of the lower leg, distal one-third of the posterior aspect of the lower leg, and distal one-third of the medial aspect of the thigh. The following ultrasonographic findings from previous studies were used for identification of the lymphatic vessels: (1) intermittent homogeneous, hypoechoic, and specular misshapen images in sagittal B-mode; (2) no colors seen with color Doppler mode; and (3) no convergence seen with the artery, the vein, or the nerve.19,20 The lymphatic vessels identified using UHFUS were marked with a cross with a black pen, and the lymphatic vessels found using CHFUS were marked with a circle with a black pen. The marked points were checked with ICG lymphography (Fig. 1). Number, diameter, and depth of the lymphatic vessels, which were detected with each ultrasound were recorded. For measuring the vessels, the major axis of lumen of the lymphatic vessel in short axis was measured using UHFUS with the partially image enlargement function. New characteristics and imaging findings of the lymphatic vessels seen with UHFUS that could not be seen with CHFUS were investigated. At the same time, the locations of each site where lymphatic vessels were not identified by each ultrasound were recorded. Then, the recorded locations were also checked with ICG lymphography.
Fig. 1.
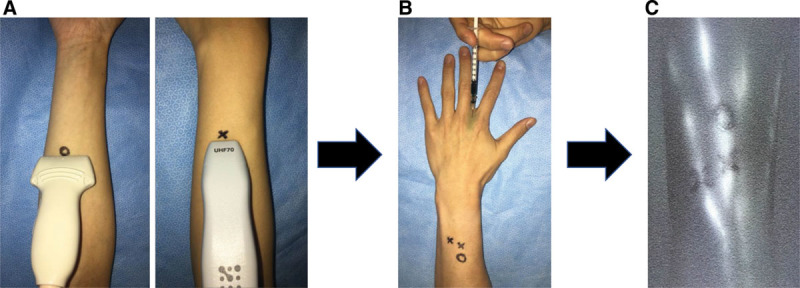
A, The identified lymphatic vessels using UHFUS were marked as cross with a black pen, and ones using CHFUS were marked as circle with a black pen. B, After the identification of lymphatic vessels using ultrasound, ICG was injected subcutaneously into the extremity. C, Then, the marked points were checked with ICG lymphography.
UHFUS was performed with Vevo MD ultrasound device (FUJIFILM VisualSonics, Amsterdam, the Netherlands) using a 70 MHz linear array transducer. CHFUS was performed with ProSound F75 (Hitachi Medical Corporation, Tokyo, Japan) using a 18 MHz linear array transducer. All ultrasound detections were performed by one of the study authors (A.H.). ICG lymphography was performed as follows: 0.2 mL of ICG (VERDYE 0.125%; Diagnostic Green GmbH, Deutschland, Germany) was injected subcutaneously into the upper extremities at the second web space of the hand and the ulnar border of the palmaris longus tendon at the level of the wrist, and the lower extremities at the first web space of the foot and the lateral border of the Achilles tendon.22–24
Statistical Analysis
The accuracy of ultrasonography in detecting the lymphatic vessels was estimated by calculating and comparing the sensitivity and specificity of UHFUS and CHFUS with ICG lymphography findings. Comparisons were made between UHFUS and CHFUS on the number and diameter of the lymphatic vessels in the upper and lower extremities, which could be detected in each ultrasound system. Plus-minus values represented mean ± SD. Differences in the means between groups were analyzed by Mann-Whitney U test, and difference in sensitivity between UHFUS and CHFUS was analyzed by chi-square test. All P values were 2-sided, and statistical significance was accepted at P < 0.05.
RESULTS
In total, 178 lymphatic vessels were detected using UHFUS and CHFUS at 90 sites in 30 unaffected extremities. ICG lymphography showed linear pattern in all extremities, and all linear lines passed over the one of or both marked points, which were detected as the lymphatic vessel on ultrasound.
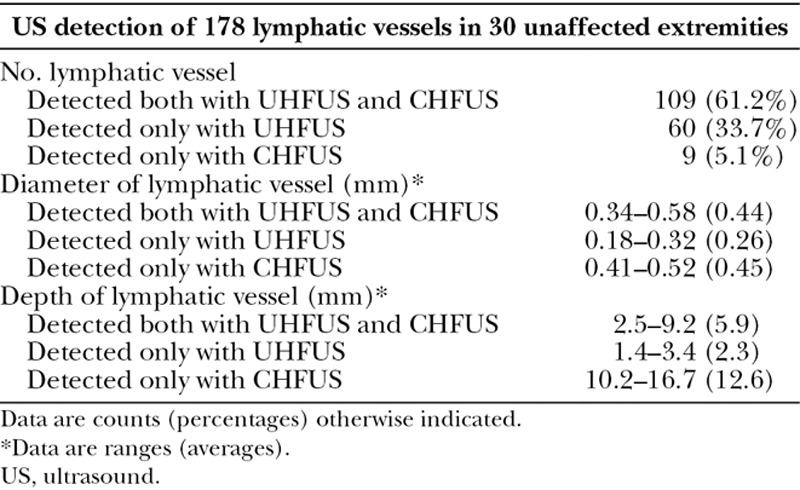
One hundred sixty-nine lymphatic vessels were detected with UHFUS, whereas 118 lymphatic vessels with CHFUS. The average diameter of lymphatic vessel detected with UHFUS was 0.38 mm, and that detected with CHFUS was 0.44 mm. The average depth of lymphatic vessel detected with UHFUS was 4.6 mm, and that detected with CHFUS was 6.4 mm; Table 1).
Table 1.
Summary of Lymphatic Vessels in US
Comparison of Ultra High-frequency Ultrasound and Conventional High-frequency Ultrasound
The sensitivity and specificity of UHFUS for detection of lymphatic vessel were 94.9% and 98.3%, respectively, and those of CHFUS were 66.3% and 91.3%, respectively.
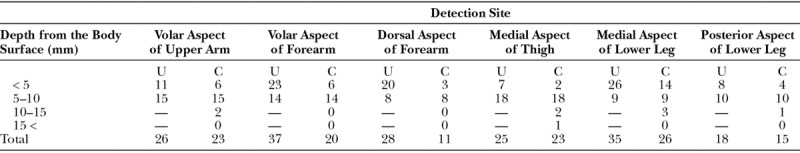
UHFUS detected more lymphatic vessels in superficial layer, which is within 5 mm than CHFUS in all sites of extremities (Table 2).
Table 2.
Depth of Lymphatic Vessels That Could Be Detected Using UHFUS and CHFUS
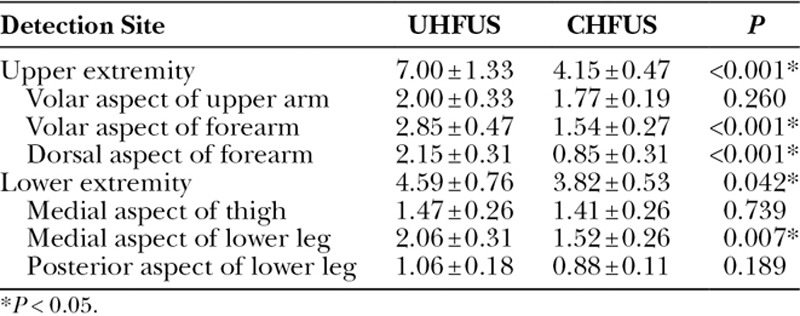
Significant differences were seen between UHFUS and CHFUS in the number of lymphatic vessel of upper and lower extremity (7.00 ± 1.33 versus 4.15 ± 0.47; P < 0.001, 4.59 ± 0.76 versus 3.82 ± 0.53; P = 0.042). The numbers of the lymphatic vessels found at the volar aspect of the forearm, the dorsal aspect of the forearm and the medial aspect of the lower leg were significantly larger with UHFUS than those with CHFUS (2.85 ± 0.47 versus 1.54 ± 0.27; P < 0.001, 2.15 ± 0.31 versus 0.85 ± 0.31; P < 0.001, 2.06 ± 0.31 versus 1.52 ± 0.26; P = 0.007; Table 3).
Table 3.
Comparison between UHFUS and CHFUS in Number of Detected Lymphatic Vessel
Significant differences were seen between UHFUS and CHFUS in the diameter of lymphatic vessel of the upper and lower extremity (0.336 ± 0.008 versus 0.403 ± 0.002 mm; P < 0.001, 0.417 ± 0.001 versus 0.468 ± 0.003 mm; P < 0.001). The diameter of the lymphatic vessels found at the volar aspect of the forearm, the dorsal aspect of the forearm and the medial aspect of the lower leg were significantly smaller with UHFUS than those with CHFUS (0.321 ± 0.005 versus 0.381 ± 0.001 mm; P < 0.001, 0.288 ± 0.005 versus 0.366 ± 0.001 mm; P < 0.001, 0.383 ± 0.008 versus 0.439 ± 0.003 mm; P = 0.007; Table 4).
Table 4.
Comparison between UHFUS and CHFUS in Diameter of Detected Lymphatic Vessel

New Characteristic and Imaging Finding of the Lymphatic Vessel in UHFUS
UHFUS showed clearer images for detecting lymphatic vessels and surrounding tissues than CHFUS (Figs. 2, 3). The veins were collapsed when the transducer was pushed against the skin of the examined sites, while the lymphatic vessels were less likely to collapse under the same setting (Figs. 4, 5; see video, Supplemental Digital Content 1, which demonstrates difference in UHFUS findings between vein and lymphatic vessels in lower leg, http://links.lww.com/PRSGO/A951; see video, Supplemental Digital Content 2, which demonstrates difference in UHFUS findings between vein and lymphatic vessels in forearm, http://links.lww.com/PRSGO/A952). Lymphatic fluids moving inside the lumen, as well as functioning valves were visualized in 36 lymphatic vessels with UHFUS (see video, Supplemental Digital Content 3, which demonstrates lymphatic fluids moving with valve functioning, http://links.lww.com/PRSGO/A953).
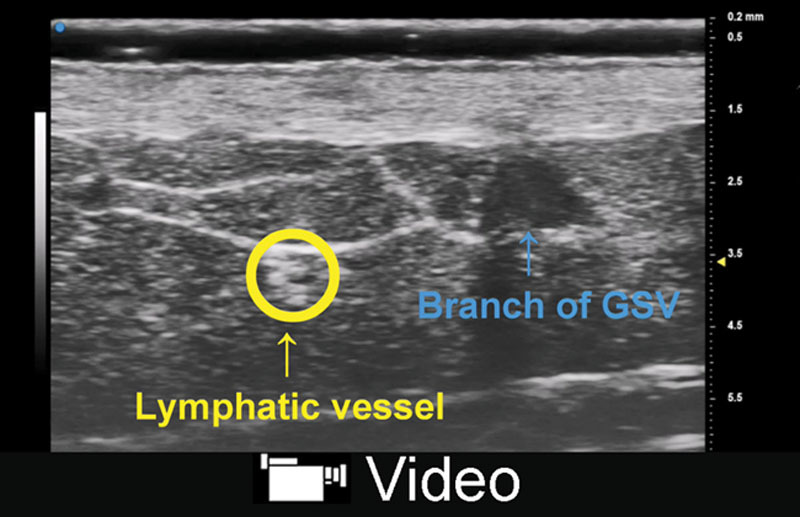
Fig. 2.
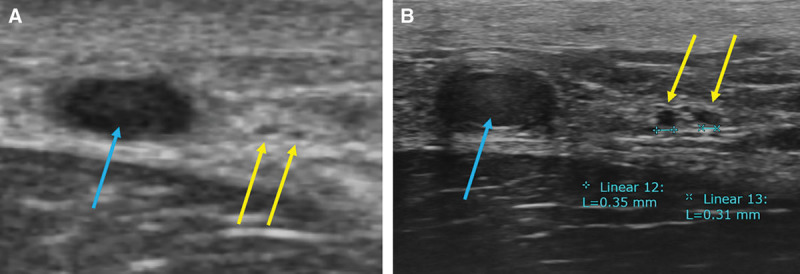
A, CHFUS showed 2 lymphatic vessels (yellow arrow) and great saphenous vein (blue arrow) in the medial aspect of lower leg. B, UHFUS showed clearer image of them. The diameter of lymphatic vessels was around 0.3 mm.
Fig. 3.
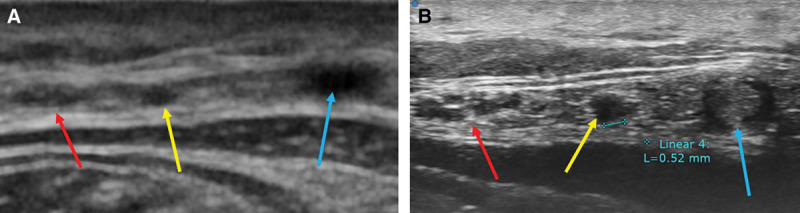
A, CHFUS showed lymphatic vessels (yellow arrow), small saphenous vein (blue arrow), and sural nerve (red arrow) in the posterior aspect of lower leg. B, UHFUS showed clearer image of them. The diameter of lymphatic vessels was around 0.5 mm.
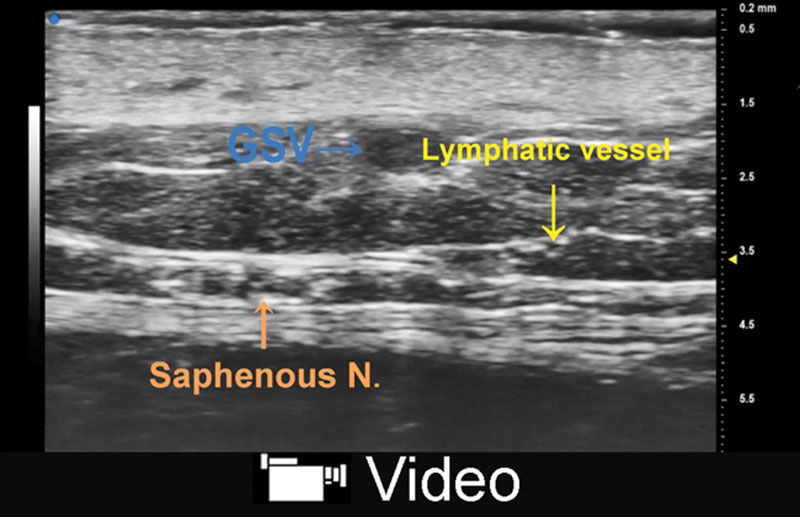
Fig. 4.
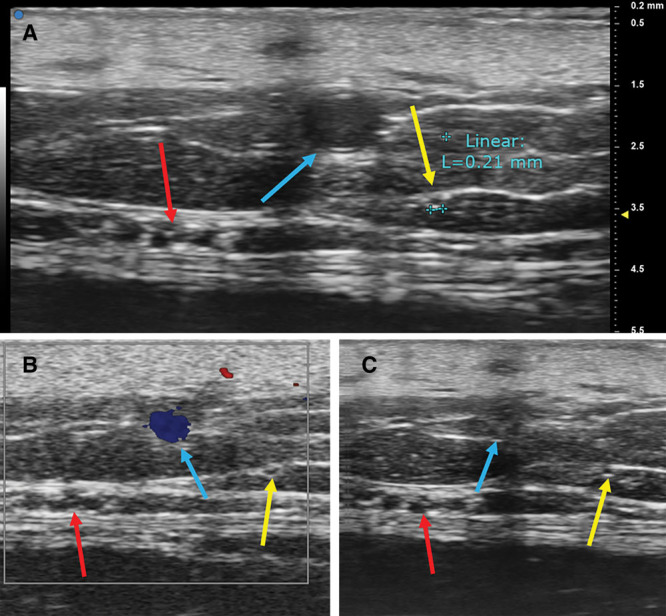
A, UHFUS showed clear image of lymphatic vessels (yellow arrow), great saphenous vein (blue arrow), and saphenous nerve (red arrow) in the medial aspect of lower leg. The diameter of lymphatic vessels was around 0.2 mm. B, Lymphatic vessel was not colored in color Doppler mode. C, Vein was collapsed when the transducer was pushed against skin of examined site, while lymphatic vessel was less likely to collapse.
Fig. 5.
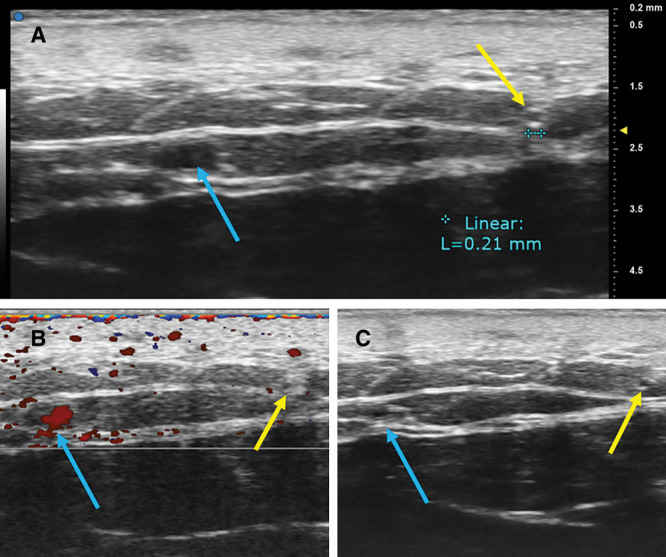
A, UHFUS showed clear image of lymphatic vessels (yellow arrow) and cephalic vein (blue arrow) in the volar aspect of forearm. The diameter of lymphatic vessels was around 0.2 mm. B, Lymphatic vessel was not colored in color Doppler mode. C, Vein was collapsed when the transducer was pushed against skin of examined site, while lymphatic vessel was less likely to collapse.
Video Graphic 1.
See video, Supplemental Digital Content 1, which displays UHFUS in the medial aspect of lower leg showed that vein was collapsed when the transducer was pushed against skin of examined site, while lymphatic vessel was less likely to collapse, http://links.lww.com/PRSGO/A951.
Video Graphic 2.
See video, Supplemental Digital Content 2, which displays UHFUS in the volar aspect of forearm showed that vein was collapsed when the transducer was pushed against skin of examined site, while lymphatic vessel was less likely to collapse, http://links.lww.com/PRSGO/A952.
Video Graphic 3.
See video, Supplemental Digital Content 3, which displays UHFUS in the medial aspect of thigh showed that lymphatic fluid moving with valve functioning, http://links.lww.com/PRSGO/A953.
DISCUSSION
This study revealed high sensitivity and specificity of UHFUS for detection of the lymphatic vessels when compared with those of CHFUS. This study also showed UHFUS could detect the lymphatic vessels with smaller diameter and larger number of lymphatic vessels in the upper and lower extremities. In addition, from the results of this study, this advanced ultrasound showed not only unprecedented clear image of the lymphatic vessels, even ones with diameters smaller than 0.3 mm, but also new characteristics and imaging findings that could not be observed using CHFUS. To our knowledge, this is the first attempt to detect lymphatic vessels using UHFUS.
Several techniques using CHFUS have been reported for detection of the lymphatic vessels using ultrasound. Goldberg et al.26 reported contrast-enhanced ultrasonographic imaging of the lymphatic vessel. They reported that lymphosonography can be used to detect lymphatic drainage pathways in a variety of animal models using contrast medium. Sever et al.27 reported ultrasonographic imaging of human breast lymphatic vessels in patients with breast cancer using contrast medium. In our previous report, ultrasound visualization of lymphatic vessels in the lower leg of healthy volunteers was demonstrated without the use of contrast medium: intermittent homogeneous, hypoechoic, and specular misshapen images were the characteristic findings of lymphatic vessels.19 We also revealed that ultrasonography could also detect the lymphatic vessels in the limbs affected with lymphedema where dermal backflow patterns were shown with ICG lymphography.
Although ultrasound visualization of the lymphatic vessels has made great progress using CHFUS, there are several disadvantages of CHFUS system with an upper frequency of 15–18 MHz: (1) it is highly operator dependent; and (2) it is difficult to distinguish the lymphatic vessels from subcutaneous vasculature or nerves.20 Precise imaging of small size anatomical structures is often difficult with conventional ultrasound.
In recent years, research on small animals promoted advances in diagnostic imaging technologies, such as UHFUS, which has recently been approved.28,29 This novel device has recently been approved for use in humans.30 The transducer with 70 MHz permits more precise detection of small size anatomical structures. We explored the use of ultra high-resolution ultrasound for detecting the lymphatic vessels, and the present study revealed that UHFUS provides unprecedented clear images of the lymphatic vessels along with new valuable information of the ultrasonographic images of the lymphatic vessels. In our study, we believe that these advantages of UHFUS allowed detection of larger number of the smaller diameter lymphatic vessels particularly in the superficial layer.
This study showed that lymphatic vessels were less likely to collapse when pressure was applied to the skin with the transducer. Macdonald et al.31 reported that amount of lymphatic flow positively correlated with regional tissue pressure in the previous study. From this viewpoint and our results, we discovered that expansion of the lymphatic vessel could be observed when pressure was applied to the vessel via the transducer. Thus, the lymphatic vessels can be distinguished from other structures by not only shape and echogenic texture but also adopting this pressure application technique.
Lymphatic fluids moving inside the lumen and functioning valves were visualized in some lymphatic vessels with UHFUS in this study. The lymphatic vessels become sclerotic over time after lymph flow obstruction, and lose their function to drain lymph fluid in peripheral lymphedema patient. Anastomosing the severely sclerotic lymphatic vessels leads to minimum therapeutic effect in LVA. Therefore, identification of functional lymphatic vessels is important for LVA. UHFUS may have the big advantage of identification of functional lymphatic vessels, including ones with diameters smaller than 0.3 mm, for lymphedema cases preoperatively.
One of disadvantages of this new device is its limited reaching distance of ultrasound. The deepest layer from which the device can obtain images is 10 mm from the superficial surface. Although it can clearly visualize the lymphatic vessels in the forearm and the lower leg, where the lymphatic vessels lie in a relatively superficial layer, it cannot visualize the lymphatic vessels in the upper arm and the thigh, where the lymphatic vessels run more deeply. This could have contributed to a result; the difference in the numbers of the lymphatic vessels found at the volar aspect of the upper arm and the medial aspect of the thigh was not statistically significant between UHFUS group and CHFUS group. For detection of the lymphatic vessels running deeper than 10 mm from the skin surface, use of a transducer with 48 MHz (max image depth: 23.5 mm) is recommended.
Limitations of the present study included that acquisition of accurate ultrasonographic image was highly operator-dependent. Because our ultrasound scanning of the lymphatic vessels was performed by one examiner, interoperator reliability could not be evaluated. Further studies are required to investigate the learning curve of performing ultrasound and compare it in some operator. Second, the subjects of this study were the extremities not affected by lymphedema. Ultrasonographic findings of the lymphatic vessels in lymphedematous extremities may be different from those in unaffected extremities. Because this device can visualize superficial vessels with diameters smaller than 0.3 mm with high resolution, it may have a potential to assess severity of lymphosclerosis and replace ICG lymphography for the detection of lymphatic vessels in lymphedematous limbs. Third, existence of the lymphatic vessels was not confirmed based on direct intraoperative observation of lymphatic vessels. Further studies are required to reveal correlation between UHFUS findings of the lymphatic vessels and the actual lymphatic vessels.
CONCLUSIONS
UHFUS provides images with extremely high resolution, demonstrating new characteristics of the lymphatic vessels. With UFHUS, detection rate of the lymphatic vessels in nonlymphedematous extremities was higher than that with CHFUS. This advanced technology may open new frontiers in understanding and treatment of lymphedema.
Supplementary Material
Footnotes
Published online 22 January 2019.
Drs. Hayashi and Giacalone contributed equally to this work.
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Sistrunk WE. Modification of the operation for elephantiasis. JAMA. 1918;71:800. [Google Scholar]
- 2.Szuba A, Cooke JP, Yousuf S, et al. Decongestive lymphatic therapy for patients with cancer-related or primary lymphedema. Am J Med. 2000;109:296–300. [DOI] [PubMed] [Google Scholar]
- 3.Homans J. Treatment of elephantiasis of the legs: a preliminary report. N Eng J Med. 1936;215:1099–1104. [Google Scholar]
- 4.Goldsmith HS, De los Santos R, Beattie EJ., Jr. Relief of chronic lymphedema by omental transposition. Ann Surg. 1967;166:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson N. The surgical treatment of advanced postmastectomy lymphoedema of the upper limb. With the late results of treatment by the buried dermis flap operation. Scand J Plast Reconstr Surg. 1969;3:54–60. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien BM. Microlymphaticovenous surgery for obstructive lymphoedema. Aust N Z J Surg. 1977;47:284–291. [DOI] [PubMed] [Google Scholar]
- 7.Kinmonth JB, Hurst PA, Edwards JM, et al. Relief of lymph obstruction by use of a bridge of mesentery and ileum. Br J Surg. 1978;65:829–833. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister RG, Siuda S. Treatment of lymphedemas by microsurgical lymphatic grafting: what is proved? Plast Reconstr Surg. 1990;85:64–74; discussion 75. [DOI] [PubMed] [Google Scholar]
- 9.Cheng MH, Huang JJ, Huang JJ, et al. A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol. 2012;126:93–98. [DOI] [PubMed] [Google Scholar]
- 10.Koshima I, Inagawa K, Urushibara K, et al. Supermicrosurgical lymphaticovenular anastomosis for the treatment of lymphedema in the upper extremities. J Reconstr Microsurg. 2000;16:437–442. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y. Studies on lymphatico-venous anastomoses in lymphedema. Nagoya J Med 1969;32:1–21. [Google Scholar]
- 12.Auba C, Marre D, Rodríguez-Losada G, et al. Lymphaticovenular anastomoses for lymphedema treatment: 18 months postoperative outcomes. Microsurgery. 2012;32:261–268. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto T, Yamamoto N, Yoshimatsu H, et al. Factors associated with lymphosclerosis: an analysis on 962 lymphatic vessels. Plast Reconstr Surg. 2017;140:734–741. [DOI] [PubMed] [Google Scholar]
- 14.Chang DW. Lymphaticovenular bypass for lymphedema management in breast cancer patients: a prospective study. Plast Reconstr Surg. 2010;126:752–758. [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto T, Narushima M, Kikuchi K, et al. Lambda-shaped anastomosis with intravascular stenting method for safe and effective lymphaticovenular anastomosis. Plast Reconstr Surg. 2011;127:1987–1992. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Narushima M, Yoshimatsu H, et al. Minimally invasive lymphatic supermicrosurgery (MILS): indocyanine green lymphography-guided simultaneous multisite lymphaticovenular anastomoses via millimeter skin incisions. Ann Plast Surg. 2014;72:67–70. [DOI] [PubMed] [Google Scholar]
- 17.Chang DW, Suami H, Skoracki R. A prospective analysis of 100 consecutive lymphovenous bypass cases for treatment of extremity lymphedema. Plast Reconstr Surg. 2013;132:1305–1314. [DOI] [PubMed] [Google Scholar]
- 18.Ito R, Wu CT, Lin MC, et al. Successful treatment of early-stage lower extremity lymphedema with side-to-end lymphovenous anastomosis with indocyanine green lymphography assisted. Microsurgery. 2016;36:310–315. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi A, Yamamoto T, Yoshimatsu H, et al. Ultrasound visualization of the lymphatic vessels in the lower leg. Microsurgery. 2016;36:397–401. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi A, Hayashi N, Yoshimatsu H, et al. Effective and efficient lymphaticovenular anastomosis using preoperative ultrasound detection technique of lymphatic vessels in lower extremity lymphedema. J Surg Oncol. 2018;117:290–298. [DOI] [PubMed] [Google Scholar]
- 21.Visconti G, Yamamoto T, Hayashi N, et al. Ultrasound-assisted lymphaticovenular anastomosis for the treatment of peripheral lymphedema. Plast Reconstr Surg. 2017;139:1380e–1381e. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto T, Narushima M, Doi K, et al. Characteristic indocyanine green lymphography findings in lower extremity lymphedema: the generation of a novel lymphedema severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;127:1979–1986. [DOI] [PubMed] [Google Scholar]
- 23.Yamamoto T, Matsuda N, Doi K, et al. The earliest finding of indocyanine green lymphography in asymptomatic limbs of lower extremity lymphedema patients secondary to cancer treatment: the modified dermal backflow stage and concept of subclinical lymphedema. Plast Reconstr Surg. 2011;128:314e–321e. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Yamamoto N, Doi K, et al. Indocyanine green-enhanced lymphography for upper extremity lymphedema: a novel severity staging system using dermal backflow patterns. Plast Reconstr Surg. 2011;128:941–947. [DOI] [PubMed] [Google Scholar]
- 25.Ogata F, Narushima M, Mihara M, et al. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg. 2007;59:180–184. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg BB, Merton DA, Liu JB, et al. Contrast-enhanced sonographic imaging of lymphatic channels and sentinel lymph nodes. J Ultrasound Med. 2005;24:953–965. [DOI] [PubMed] [Google Scholar]
- 27.Sever A, Broillet A, Schneider M, et al. Dynamic visualization of lymphatic channels and sentinel lymph nodes using intradermal microbubbles and contrast-enhanced ultrasound in a swine model and patients with breast cancer. J Ultrasound Med. 2010;29:1699–1704. [DOI] [PubMed] [Google Scholar]
- 28.Grassi R, Lagalla R, Rotondo A. Genomics, proteomics, MEMS and SAIF: which role for diagnostic imaging? Radiol Med. 2008;113:775–778. [DOI] [PubMed] [Google Scholar]
- 29.Grassi R, Cavaliere C, Cozzolino S, et al. Small animal imaging facility: new perspectives for the radiologist. Radiol Med. 2009;114:152–167. [DOI] [PubMed] [Google Scholar]
- 30.Belfiore MP, Berritto D, Iacobellis F, et al. A longitudinal study on BIO14.6 hamsters with dilated cardiomyopathy: micro-echocardiographic evaluation. Cardiovasc Ultrasound. 2011;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Macdonald AJ, Arkill KP, Tabor GR, et al. Modeling flow in collecting lymphatic vessels: one-dimensional flow through a series of contractile elements. Am J Physiol Heart Circ Physiol. 2008;295:H305–H313. [DOI] [PubMed] [Google Scholar]


