Abstract
Background/aims
The small bowel is affected in more than half of patients with Crohn’s disease (CD) at the time of diagnosis, and small bowel involvement has a negative impact on the long-term outcome. Many patients reportedly have active lesions in the small intestine even in patients in clinical remission. This study was performed to compare findings of magnetic resonance enterography (MRE) and ileocolonoscopy.
Methods
A single-center retrospective study was conducted in 50 patients (60 imaging series) with CD, for whom MRE was additionally performed during the bowel preparation for subsequent ileocolonoscopy. Endoscopic remission was defined as a Simple Endoscopic Score for CD (SES-CD) of <5. MRE remission was defined as a Magnetic Resonance Index of Activity (MaRIA) score of <50. The time to treatment escalation was assessed by the log-rank test.
Results
Importantly, 7 of 29 patients (24.1%) with endoscopic remission had a MaRIA score of ≥50. Both SES-CD and MaRIA correlated with the need for treatment escalation (P = 0.025, P = 0.009, respectively). MRE predicted the need for treatment escalation even in patients with endoscopic remission. Although no correlation was present between SES-CD and MaRIA score in patients with structuring/penetrating disease, or insufficient ileal insertion (<10cm), a high MaRIA score still correlated with the need for treatment escalation in stricturing or penetrating disease (P = 0.0306).
Conclusions
The MaRIA score predicts the need for treatment escalation even in patients with endoscopic remission, indicating that addition of MRE to conventional ileocolonoscopy alone can be a useful, noninvasive tool for monitoring CD especially in stricturing or penetrating disease.
Introduction
Crohn’s disease (CD) is a chronic inflammatory disease of the gastrointestinal tract that may flare and remit over time, and the number of affected patients is increasing. Persistent inflammation results in disease progression due to structural bowel damage, which often requires surgical bowel resection. Therefore, it is important to closely monitor the disease activity and achieve sustained remission and mucosal healing to prevent the progression of bowel damage.
The small bowel is reportedly affected in 53% of patients with CD at the time of diagnosis (ileal and ileocolonic involvement in 27% and 26% of patients, respectively) according to the Montreal classification[1]. Small bowel involvement increases to 61% after 5 years[1]. The assessment of small intestinal lesions is important; one study showed that 43% to 60% of patients with established CD had suspected small bowel involvement[2]. Small bowel involvement negatively affects the long-term outcome of CD but is less associated with C-reactive protein and fecal calprotectin[3–6]. Ileal involvement in patients with CD is a significant risk factor[7, 8].
Conventional ileocolonoscopy is a valuable tool in the assessment of CD; it is widely accessible, however, it may not be sufficient to evaluate the entire small bowel involvement[9]. Balloon-assisted enteroscopy enables observation beyond the reach of conventional ileocolonoscopy, but it requires specific devices and highly trained endoscopists[2]. Capsule endoscopy is another endoscopic tool but not an easy first-line examination because of the risk of capsule retention in patients with stricturing disease[10]. These endoscopic methods enable to accurately detect the luminal inflammation with high sensitivity[11], whereas their common weakness can be related to the transmural nature of inflammation caused by CD[12]. MRE is a noninvasive cross-sectional examination technique that may overcome this weakness of endoscopy, but it is less sensitive than endoscopy in detecting stenosis and superficial inflammation[6, 13–15].
Taken together, we hypothesized that adding MRE to conventional ileocolonoscopy during the bowel preparation might be beneficial to compensate their weakness. In the present study, we retrospectively compared findings of ileocolonoscopy and MRE to evaluate the possible usefulness of this procedure.
Methods
Patients
We performed a retrospective chart review of 365 series of ileocolonoscopy and 140 series of MRE conducted in patients with CD from January 2013 to November 2017 at Kitasato University Kitasato Institute Hospital. Fifty patients (60 imaging series) in whom both examinations were performed on the same day were included in this study. The patients underwent evaluation of clinical severity (CD activity index [CDAI] and Harvey–Bradshaw Index [HBI])[16, 17], measurement of laboratory parameters including C-reactive protein (CRP), and performance of ileocolonoscopy combined with MRE. Treatment escalation was defined by the addition of corticosteroids, anti-tumor necrosis factor agents, immunomodulators, and surgery. The need for treatment escalation was determined based on the attending physician’s evaluation of aggravated symptoms in the patients. Patients with an ileoanal pouch or ileorectal anastomosis were excluded. Ileocolonoscopy and MRE were performed to assess the severity, location, and extent of disease or to carry out clinical follow-up of CD.
Ethical consideration
The Research Ethics Committee of Kitasato University Kitasato Institute Hospital approved the study protocol and all documents (approval number: 17042).
Endoscopic procedure
All patients ingested 1000 mL of polyethylene glycol (PEG) before MRE. After MRE, the patients were required to orally ingest 0 to 1000 mL of additional PEG for a total of 1000 to 2000 ml as a standard bowel preparation regimen for ileocolonoscopy. A long slim colonoscope (PCF-PQ260L; Olympus Medical Systems, Tokyo, Japan) was routinely utilized to enable deeper insertion through possible strictures in daily clinical practice for patients with CD. All segments were retrospectively and separately scored by the Simple Endoscopic Score for CD (SES-CD), and the scores of each segment were calculated to include the sum of the scores in five segments[18]. A segment was scored as 0 if it could not be reached by ileocolonoscopy. Endoscopic remission was defined as an SES-CD of <5[19]. SES-CD was retrospectively scored blinded to the results of MRE.
MRE procedure and evaluation of CD
All patients were instructed to orally ingest 1000 mL of PEG within 45 to 60 minutes before MRE. MRE was performed using a 1.5-T magnetic resonance imaging unit (Signa HDx; GE Healthcare, Tokyo, Japan). Patients were placed in the supine position on the magnetic resonance imaging table using a previously described protocol[20] (S1 Table). The presence of stricture, fistulae, and abscesses was also assessed on MRE. Relative contrast enhancement (RCE) was calculated as previously reported[13].
The images were retrospectively evaluated by a radiologist (N.K) with more than 10 years of experience blinded to the clinical information and the results of the endoscopic examination. The severity and extent of inflammatory lesions were evaluated using the Magnetic Resonance Index of Activity (MaRIA) score[18, 21]. The overall MaRIA score was calculated as the sum of MaRIA in six segments (distal ileum, ascending, transverse, descending, sigmoid colon, and rectum). MRE remission was defined as a MaRIA score of <50[22]. Restricted maximum likelihood estimation is performed using all of the data in the MaRIA score, even if missing values are present. Multiple imputation was used to impute overall MaRIA for those missing data[23, 24].
Statistical methods
The mean and standard deviation or the median and range were calculated for parametric and nonparametric data, respectively. Between-group differences were evaluated for time to treatment escalation. Continuous data are summarized as median and interquartile range (IQR), while categorical data are summarized as count and percentage. Nonparametric Spearman rank correlation (rs) was used to assess continuous and ordinal bivariate relationships between MRE findings (MaRIA score) and endoscopic findings (SES-CD). A Kaplan–Meier evaluation was carried out to compare treatment escalation between the two groups of patients, with identified differences evaluated using the log-rank statistics. A Cox proportional hazards model was then applied to assess the time to treatment escalation. A P-value of <0.05 was considered significant, and correction by Bonferroni’s method was applied when needed. All statistical analyses were carried out using the JMP software program, version 14.1 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the study population
The patients’ characteristics are presented in Table 1 (34 men, 16 women). Their median age was 34.5 years (IQR, 26.0–42.0 years). The median follow-up period was 449 days (IQR, 183–853.75 days). No serious adverse events related to either MRE or ileocolonoscopy were observed.
Table 1. Patients’ characteristics (n = 50).
| Sex | |
| Male | 34 (68.0) |
| Female | 16 (32.0) |
| Age, years | 34.5 (26.0–42.0) |
| Montreal classification | |
| Age at diagnosis | |
| <17 years (A1) | 11 (22.0) |
| ≥17 to ≤40 years (A2) | 33 (66.0) |
| >40 years (A3) | 6 (12.0) |
| Behavior | |
| Nonstricturing, nonpenetrating (B1) | 21 (42.0) |
| Stricturing (B2) | 15 (30.0) |
| Penetrating (B3) | 14 (28.0) |
| Disease location | |
| Ileum only (L1) | 16 (32.0) |
| Colon only (L2) | 3 (6.0) |
| Ileum and colon (L3) | 31 (62.0) |
| Proximal GI tract (L4) | 6 (12.0) |
| Perianal GI tract (p) | 12 (24.0) |
| Body mass index, kg/m2 | 20.8 (18.7–22.8) |
| Surgical history | |
| Appendectomy | 10 (20.0) |
| Crohn’s disease-related surgery | 20 (40.0) |
| Duration of disease, mo | 108 (10.5–226) |
| CDAI | 113.7 ± 73.2 |
| Harvey–Bradshaw Index | 2.0 ± 2.3 |
| Overall MaRIA | 37.9 (29.1–51.5) |
| SES-CD | 5 (0–11) |
| Medication profile | |
| 5-Aminosalicylic acid | 37 (74.0) |
| Glucocorticoid | 6 (12.0) |
| Azathioprine | 10 (20.0) |
| 6-Mercaptoprine | 14 (28.0) |
| Infliximab | 12 (24.0) |
| Adalimumab | 3 (6.0) |
| Elemental diet | 16 (32.0) |
| Antibiotics | 3 (6.0) |
| None | 3 (6.0) |
| Laboratory tests | 13.1 ± 1.9 |
| Hemoglobin, g/dL | 0.15 (0.04–1.01) |
| CRP, mg/dL | |
| Smoking habits | |
| Active smoking | 5 (10.0) |
| Past smoking | 11 (22.0) |
| Never smoking | 34 (68.0) |
| Family history of Crohn’s disease | 4 (8.0) |
Data are presented as n (%), median (interquartile range), or mean ± standard deviation.
GI, gastrointestinal; CDAI, Crohn’s Disease Activity Index; the Magnetic Resonance Index of Activity, MaRIA; the Simple Endoscopic Score for CD, SES-CD; CRP, C-reactive protein.
The CDAI consists eight factors, with each factor totaled after adjustment using a weighing factor ranging from 1 to 30. The CDAI ranges from approximately 0 to 600, with higher scores indicating more severe disease activity.
The glucocorticoids included budesonide.
Relationship between SES-CD and MaRIA score
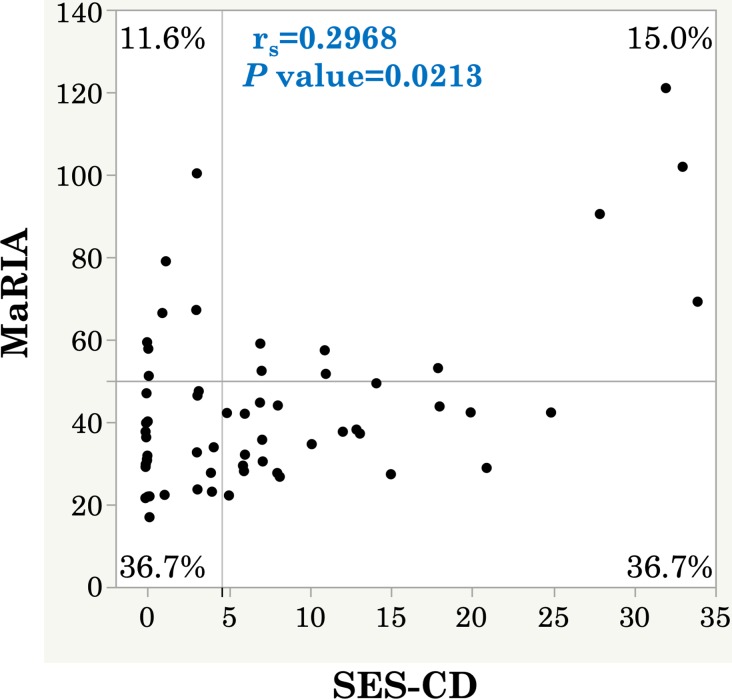
Next, we evaluated the relationship between the SES-CD and MaRIA score. The Spearman rank correlation coefficient between the SES-CD and MaRIA score showed a low significant correlation (rs = 0.2968, P = 0.0213) (Fig 1). The correlations between the segmental SES-CD and segmental MaRIA score in six segments were shown in S1 Fig. Importantly, 7 of 29 patients (24.1%) with a negative SES-CD had an overall MaRIA score of ≥50.
Fig 1. Correlation between SES-CD and MaRIA score (per patient).
SES-CD and MaRIA score are associated with a time to treatment escalation
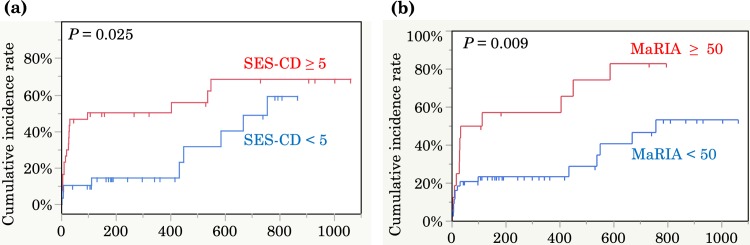
Kaplan–Meier curves revealed a longer time to treatment escalation in patients with endoscopic remission (SES-CD of <5) than in patients without endoscopic remission (SES-CD of ≥5) (hazard ratio, 2.43; 95% confidence interval, 1.09–5.42; P = 0.0302) (Fig 2A). Furthermore, active disease in MRE (MaRIA score of ≥50) was associated with a higher incidence of treatment escalation than was disease remission (MaRIA score of <50) (hazard ratio, 2.65; 95% confidence interval, 1.23–5.70; P = 0.0121) (Fig 2B).
Fig 2. Kaplan–Meier estimates of the cumulative incidence of treatment escalation according to endoscopic findings and MRE findings.
(a) Patients with endoscopic remission (blue line, n = 29) showed a significantly longer time to treatment escalation than patients with endoscopically active disease (red line, n = 31) (P = 0.025). (b) Patients with a low MaRIA score (blue line, n = 44) showed a significantly longer time to treatment escalation than patients with a high MaRIA score (red line, n = 16) (P = 0.009).
Differential roles of ileocolonoscopy and MRE
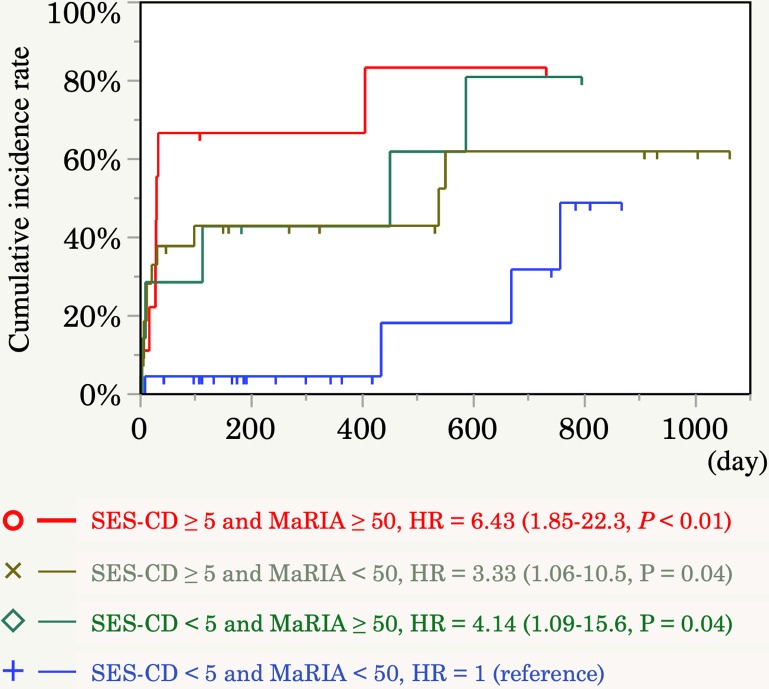
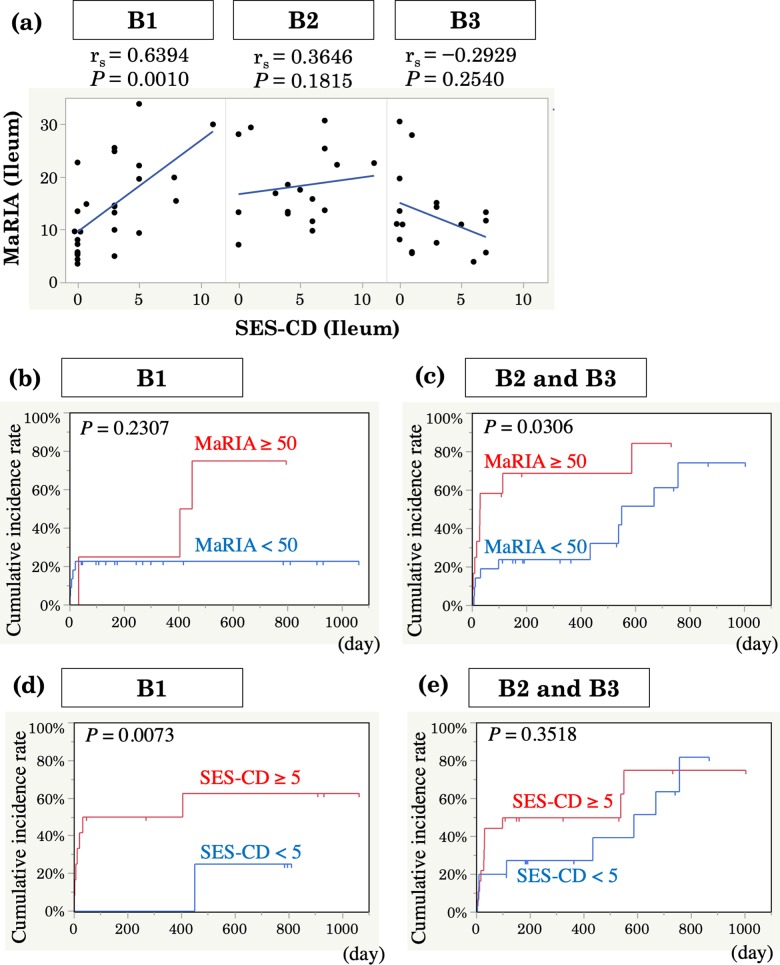
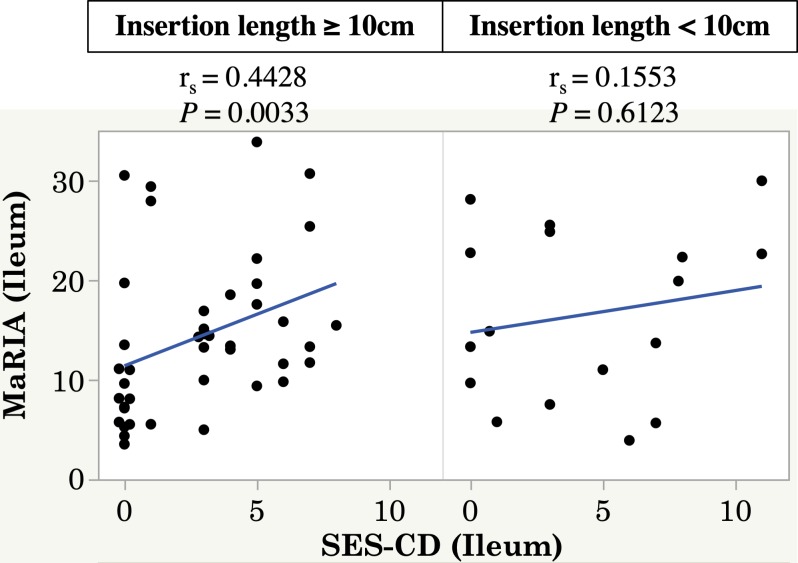
Since there were some patients still needed treatment escalation despite SES-CD<5 or MaRIA<50 (Fig 2A and 2B). we next assessed combined efficacy in predicting the need for treatment escalation. Patients with positive findings in both endoscopy and MRE showed the highest need for treatment escalation, indicating that both MRE and endoscopic findings are important (hazard ratio, 6.43; 95% confidence interval, 1.85–22.3; P <0.01) (Fig 3). The influence of active lesions in endoscopy in patients with MRE remission is shown in Fig 3 (hazard ratio, 3.33; 95% confidence interval, 1.06–10.5; P = 0.04). Active disease on MRE (defined as a MaRIA score of ≥50) was associated with a shorter time to treatment escalation, even in patients with endoscopic remission (hazard ratio, 4.14; 95% confidence interval, 1.09–15.6; P = 0.04) (Fig 3). Next, we investigated the correlation between the SES-CD and MaRIA score in each disease category according to the Montreal classification to explain the discrepancy between the SES-CD and MaRIA score. The Spearman rank correlation coefficient between the SES-CD and MaRIA score was strong (rs = 0.6394) (P = 0.001) in patients with inflammatory disease (B1), whereas there was no correlation in patients with stricturing or penetrating disease (B2, B3) (Fig 4A). A high MaRIA score predicted the early need for treatment escalation in patients with lesions (B2, B3) (Fig 4B and 4C), while a high SES-CD in patients with B1 disease was associated with the early need for treatment escalation but not in patients with B2/B3 disease (Fig 4D and 4E). In addition, we found that SES-CD significantly correlated with MaRIA when ileal insertion was sufficient (≥ 10cm) (Fig 5), suggesting the importance of lesions beyond the reach of colonoscopy.
Fig 3. Kaplan–Meier estimates of the cumulative incidence of treatment escalation according to MRE findings and endoscopic findings.
Probability of treatment escalation according to active disease in both ileocolonoscopy and MRE (red line, n = 9), active disease only in MRE (green line, n = 7), active disease only in ileocolonoscopy (gray line, n = 22), or remission in both ileocolonoscopy and MRE (blue line, n = 22). A low MaRIA score was associated with a longer incidence-free duration even in patients with endoscopic remission. Hazard ratio (HR) with 95% confidence interval (CI) and P value are shown. Blue line (both remission finding in SES-CD and MaRIA) represents control group).
Fig 4. Usefulness of MRE in predicting early need for treatment escalation in patients with stricturing or penetrating disease.
(a) Correlation between the SES-CD and MaRIA score in the distal ileum according to the Montreal classification. (b) Kaplan–Meier estimates of the cumulative incidence of treatment escalation according to MRE findings in patients with B1 (without strictures or penetrating disease, n = 26) or (c) B2/3 (with strictures or penetrating disease, n = 34) disease. (d) Kaplan–Meier estimates of the cumulative incidence of treatment escalation according to endoscopic findings in patients with B1 or (e) B2/3 disease.
Fig 5. SES-CD correlated with MaRIA score when ileocolonoscopy was inserted >10cm.
Correlation between the SES-CD and MaRIA score in the distal ileum according to insertion length. (with observing 10 cm or more of distal ileum, n = 17) or (with observing less than 10 cm of distal ileum, n = 43).
Discussion
In this study, we retrospectively compared ileocolonoscopy and MRE findings and assessed the usefulness of adding MRE during bowel preparation for ileocolonoscopy.
Clinical and serological remission cannot exclude the presence of active lesions in MRE, ileocolonoscopy balloon-assisted enteroscopy, or video capsule endoscopy [6, 25]. Thus, clinical symptoms and the CRP level are not sensitive enough to detect active lesions in patients with CD[5, 6, 26, 27].
MRE is one of the most widely used imaging techniques for monitoring small intestinal CD lesions, predicting the outcome of CD, and helping physicians to make clinical decisions[6, 28, 29]. According to a previous study, it has poorer sensitivity than endoscopy for detecting active lesions[13]. However, a recent study revealed that not only ulcers but also milder lesions such as erosions and redness can be detected in MRE by adding relative contrast enhancement and diffusion-weighted imaging[30]. Evaluation of MaRIA add the presence of disease activity from endoscopic activity[12]. These previous findings support our data showing that MRE is considered a useful additional diagnostic technique, particularly in patients with transmural inflammation.
The accuracy of MRE for assessment of CD location, activity and complications has been confirmed using CDEIS, SES-CD, clinical index, histopathological findings, and panel diagnosis as reference standards[6, 22, 31, 32]. However, in our study, the correlation between SES-CD and MaRIA in the entire cohort was not strong. There are a few possible reasons that could explain this discrepancy. First, we have more patients with stricturing/penetrating disease compared with previous publications[21, 22, 33]. As shown in Fig 4, MaRIA still correlated with SES-CD in B1 disease but not in B2/3. Furthermore, SES-CD predicted need for treatment escalation in MRE-negative B1 disease, while MaRIA was superior to ileocolonoscopy in predicting need for treatment escalation in B2/3 disease. This is clinically relevant considering the cross-sectional feature of MRE and its capability of assessing transmural inflammation. Second possible explanation is sufficient observation of distal ileum by MRE when the endoscope cannot be inserted deep enough. Our analysis showed the discrepancy between segmental SES-CD and MaRIA score in the distal ileum when the insertion was less than 10cm from the ileocecal valve. Third, we did not give any transanal preparation as previously proposed using warm water enema. This might have worsened the accuracy of MRE in the distal colorectum and caused this discrepancy[34]. Poorly distended segments may mimic bowel wall thickening or mucosal hyperenhancement, thereby falsely assessing the presence of inflammatory changes[34]. In some cases, elimination of solid stools was not enough to get precise details of the mucosa. However, this should be overcome by ileocolonoscopy combined with MRE in our procedure.
A discrepancy between ileocolonoscopy and MRE findings may in fact justify combination of ileocolonoscopy and MRE. Our study shows that positive findings in MRE predict treatment escalation in patients with negative ileocolonoscopy findings. Therefore, in clinical practice, the combination of ileocolonoscopy and MRE might be useful especially in patients at high risk of intestinal complications. This finding is inconsistent with a previous study showing that MRE is less sensitive in detecting strictures[33]. These differences in patients with stricturing or penetrating disease can be explained by the scoring system for the SES-CD, in which the presence of a stricture increases the score. Using the SES-CD active score with exclusion of the scoring item “strictures” from the original SES-CD might be useful to eliminate this discrepancy[14, 18]. Because the proportion of patients with intestinal complications (B2, B3) increases over time[35], the combination of ileocolonoscopy and MRE might be highly significant for patients with CD exhibiting a long disease duration.
Our bowel preparation regimen for ileocolonoscopy requires 2 L of PEG; thus, MRE can be performed during preparation without additional PEG intake. Performing the two methods in combination has various advantages. First, MRE may be able to detect ileal lesions that cannot be observed by ileocolonoscopy as well as transmural lesions. Second, double-checking of the distal ileum by both ileocolonoscopy and MRE may help to screen the distal ileum, which CD most frequently affects, with a higher sensitivity. Third, ileocolonoscopy allows for detailed mucosal observation and histopathological evaluation. Fourth, the ability to perform the procedure in 1 day may improve the patient’s acceptability by reducing the burden of bowel preparation. Undergoing bowel preparation twice (before both ileocolonoscopy and MRE) was considered burdensome and performing both endoscopy and MRE in a short period of time contributed to lower acceptance of repeated examinations in previous studies[22, 36]. Our 1-day procedure might relieve the burden of undergoing ileocolonoscopy and MRE on different days.
The current study has some limitations. The study is susceptible to limitations inherent to the retrospective design. Whether abnormal findings lead to additional therapy or additional therapy was added in the future in patients with abnormal findings remains uncertain. In addition, patient selection might include the selection bias; we cannot fully exclude the possibility that enrolled patients received this combination of ileocolonoscopy and MRE when they were considered treatment escalation for other reasons. The other limitation is the lack of fecal biomarkers in our study. Increasing evidence suggests that fecal calprotectin is useful in monitoring CD activity and predicting relapse[37]. We used ileocolonoscopy and MRE but used neither balloon-assisted enteroscopy nor capsule endoscopy. Both examination techniques have risk of false-negative results in the jejunum, and ileocolonoscopy has a risk of false-negative results in the ileum or more proximal small bowel because they are out of reach.
Conclusions
The present study suggests the possible benefit of combining MRE with ileocolonoscopy in patients with complicated CD. A prospective study with a larger sample size and more clinically relevant endpoints, such as the rate of hospitalization and operation rate, is warranted.
Supporting information
(TIFF)
(DOCX)
Acknowledgments
We are grateful to Tadae Mori, Toyomi Ishibashi, and Yuki Watanabe for assisting with this study. We thank Satoshi Suzuki for his technical advice. We also thank Angela Morben, DVM, ELS, from Edanz Group (www.edanzediting.com/ac), for editing a draft of this manuscript.
Data Availability
All data files are available from the GitHub database (https://github.com/Shintaro-Sagami/PLOS-ONE-2018).
Funding Statement
The authors received no specific funding for this work.
References
- 1.Henriksen M, Jahnsen J, Lygren I, Aadland E, Schulz T, Vatn MH, et al. Clinical course in Crohn's disease: results of a five-year population-based follow-up study (the IBSEN study). Scandinavian journal of gastroenterology. 2007;42(5):602–10. 10.1080/00365520601076124 [DOI] [PubMed] [Google Scholar]
- 2.Gomollon F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. Journal of Crohn's & colitis. 2017;11(1):3–25. [DOI] [PubMed] [Google Scholar]
- 3.Gauss A, Geib T, Hinz U, Schaefert R, Zwickel P, Zawierucha A, et al. Quality of Life Is Related to Fecal Calprotectin Concentrations in Colonic Crohn Disease and Ulcerative Colitis, but not in Ileal Crohn Disease. Medicine. 2016;95(16):e3477 10.1097/MD.0000000000003477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanauer SB, Sandborn W. Management of Crohn's disease in adults. The American journal of gastroenterology. 2001;96(3):635–43. 10.1111/j.1572-0241.2001.3671_c.x [DOI] [PubMed] [Google Scholar]
- 5.Florin TH, Paterson EW, Fowler EV, Radford-Smith GL. Clinically active Crohn's disease in the presence of a low C-reactive protein. Scandinavian journal of gastroenterology. 2006;41(3):306–11. 10.1080/00365520500217118 [DOI] [PubMed] [Google Scholar]
- 6.Takenaka K, Ohtsuka K, Kitazume Y, Matsuoka K, Nagahori M, Fujii T, et al. Utility of Magnetic Resonance Enterography For Small Bowel Endoscopic Healing in Patients With Crohn's Disease. The American journal of gastroenterology. 2017. [DOI] [PubMed] [Google Scholar]
- 7.Manser CN, Frei P, Grandinetti T, Biedermann L, Mwinyi J, Vavricka SR, et al. Risk factors for repetitive ileocolic resection in patients with Crohn's disease: results of an observational cohort study. Inflammatory bowel diseases. 2014;20(9):1548–54. 10.1097/MIB.0000000000000123 [DOI] [PubMed] [Google Scholar]
- 8.Lakatos PL, Szalay F, Tulassay Z, Molnar T, Kovacs A, Gasztonyi B, et al. Clinical presentation of Crohn's disease. association between familial disease, smoking, disease phenotype, extraintestinal manifestations and need for surgery. Hepato-gastroenterology. 2005;52(63):817–22. [PubMed] [Google Scholar]
- 9.Coremans G, Rutgeerts P, Geboes K, Van den Oord J, Ponette E, Vantrappen G. The value of ileoscopy with biopsy in the diagnosis of intestinal Crohn's disease. Gastrointestinal endoscopy. 1984;30(3):167–72. [DOI] [PubMed] [Google Scholar]
- 10.Kopylov U, Ben-Horin S, Seidman EG, Eliakim R. Video Capsule Endoscopy of the Small Bowel for Monitoring of Crohn's Disease. Inflammatory bowel diseases. 2015;21(11):2726–35. 10.1097/MIB.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 11.Khanna R, Nelson SA, Feagan BG, D'Haens G, Sandborn WJ, Zou GY, et al. Endoscopic scoring indices for evaluation of disease activity in Crohn's disease. The Cochrane database of systematic reviews. 2016;(8):Cd010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia-Bosch O, Ordas I, Aceituno M, Rodriguez S, Ramirez AM, Gallego M, et al. Comparison of Diagnostic Accuracy and Impact of Magnetic Resonance Imaging and Colonoscopy for the Management of Crohn's Disease. Journal of Crohn's & colitis. 2016;10(6):663–9. [DOI] [PubMed] [Google Scholar]
- 13.Rimola J, Rodriguez S, Garcia-Bosch O, Ordas I, Ayala E, Aceituno M, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut. 2009;58(8):1113–20. 10.1136/gut.2008.167957 [DOI] [PubMed] [Google Scholar]
- 14.Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn's disease. Gastroenterology. 2014;147(2):334-42.e3. [DOI] [PubMed] [Google Scholar]
- 15.Yoon HM, Suh CH, Kim JR, Lee JS, Jung AY, Kim KM, et al. Diagnostic Performance of Magnetic Resonance Enterography for Detection of Active Inflammation in Children and Adolescents With Inflammatory Bowel Disease: A Systematic Review and Diagnostic Meta-analysis. JAMA pediatrics. 2017;171(12):1208–16. 10.1001/jamapediatrics.2017.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Best WR, Becktel JM, Singleton JW, Kern F Jr. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976;70(3):439–44. [PubMed] [Google Scholar]
- 17.Harvey RF, Bradshaw JM. A simple index of Crohn's-disease activity. Lancet. 1980;1(8167):514 [DOI] [PubMed] [Google Scholar]
- 18.Daperno M, D'Haens G, Van Assche G, Baert F, Bulois P, Maunoury V, et al. Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointestinal endoscopy. 2004;60(4):505–12. [DOI] [PubMed] [Google Scholar]
- 19.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. Journal of Crohn's & colitis. 2013;7(12):982–1018. [DOI] [PubMed] [Google Scholar]
- 20.Naganuma M, Okuda S, Hisamatsu T, Matsuoka K, Mori K, Hosoe N, et al. Findings of ulceration and severe stricture on MRE can predict prognosis of Crohn's disease in patients treated with anti-TNF treatment. Abdominal radiology (New York). 2017;42(1):141–51. [DOI] [PubMed] [Google Scholar]
- 21.Rimola J, Ordas I, Rodriguez S, Garcia-Bosch O, Aceituno M, Llach J, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflammatory bowel diseases. 2011;17(8):1759–68. 10.1002/ibd.21551 [DOI] [PubMed] [Google Scholar]
- 22.Ordás I, Rimola J, Rodríguez S, Paredes JM, Martínez-Pérez MJ, Blanc E, et al. Accuracy of Magnetic Resonance Enterography in Assessing Response to Therapy and Mucosal Healing in Patients With Crohn's Disease. Gastroenterology. 2014;146(2):374-82.e1. [DOI] [PubMed] [Google Scholar]
- 23.van der Heijden GJ, Donders AR, Stijnen T, Moons KG. Imputation of missing values is superior to complete case analysis and the missing-indicator method in multivariable diagnostic research: a clinical example. Journal of clinical epidemiology. 2006;59(10):1102–9. 10.1016/j.jclinepi.2006.01.015 [DOI] [PubMed] [Google Scholar]
- 24.Hayati Rezvan P, Lee KJ, Simpson JA. The rise of multiple imputation: a review of the reporting and implementation of the method in medical research. BMC medical research methodology. 2015;15:30 10.1186/s12874-015-0022-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopylov U, Yablecovitch D, Lahat A, Neuman S, Levhar N, Greener T, et al. Detection of Small Bowel Mucosal Healing and Deep Remission in Patients With Known Small Bowel Crohn's Disease Using Biomarkers, Capsule Endoscopy, and Imaging. The American journal of gastroenterology. 2015;110(9):1316–23. 10.1038/ajg.2015.221 [DOI] [PubMed] [Google Scholar]
- 26.Jones J, Loftus EV Jr., Panaccione R, Chen LS, Peterson S, McConnell J, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2008;6(11):1218–24. [DOI] [PubMed] [Google Scholar]
- 27.De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn's disease: a systematic review. Inflammatory bowel diseases. 2013;19(2):429–44. 10.1002/ibd.22977 [DOI] [PubMed] [Google Scholar]
- 28.Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, et al. Correlation of the Endoscopic and Magnetic Resonance Scoring Systems in the Deep Small Intestine in Crohn's Disease. Inflammatory bowel diseases. 2015;21(8):1832–8. 10.1097/MIB.0000000000000449 [DOI] [PubMed] [Google Scholar]
- 29.Mendoza JL, Gonzalez-Lama Y, Taxonera C, Suarez-Ferrer C, Matute F, Vera MI, et al. Using of magnetic resonance enterography in the management of Crohn's disease of the small intestine: first year of experience. Revista espanola de enfermedades digestivas: organo oficial de la Sociedad Espanola de Patologia Digestiva. 2012;104(11):578–83. [DOI] [PubMed] [Google Scholar]
- 30.Sato H, Tamura C, Narimatsu K, Shimizu M, Takajyo T, Yamashita M, et al. Magnetic resonance enterocolonography in detecting erosion and redness in intestinal mucosa of patients with Crohn's disease. Journal of gastroenterology and hepatology. 2015;30(4):667–73. 10.1111/jgh.12851 [DOI] [PubMed] [Google Scholar]
- 31.Steward MJ, Punwani S, Proctor I, Adjei-Gyamfi Y, Chatterjee F, Bloom S, et al. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. European journal of radiology. 2012;81(9):2080–8. 10.1016/j.ejrad.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 32.Taylor SA, Mallett S, Bhatnagar G, Baldwin-Cleland R, Bloom S, Gupta A, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (METRIC): a multicentre trial. The Lancet Gastroenterology & Hepatology. 2018. 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takenaka K, Ohtsuka K, Kitazume Y, Nagahori M, Fujii T, Saito E, et al. Comparison of magnetic resonance and balloon enteroscopic examination of the small intestine in patients with Crohn's disease. Gastroenterology. 2014;147(2):334–42 e3. [DOI] [PubMed] [Google Scholar]
- 34.Ordas I, Rimola J, Rodriguez S, Gallego M, Ricart E, Panes J. Imaging of the Colon in Inflammatory Bowel Disease: Ready for Prime Time? Current drug targets. 2012;13(10):1252–60. 10.2174/138945012802429714 PubMed PMID: WOS:000307883000006. [DOI] [PubMed] [Google Scholar]
- 35.Louis E, Collard A, Oger AF, Degroote E, Aboul Nasr El Yafi FA, Belaiche J. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut. 2001;49(6):777–82. 10.1136/gut.49.6.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florie J, Horsthuis K, Hommes DW, Nio CY, Reitsma JB, van Deventer SJ, et al. Magnetic resonance imaging compared with ileocolonoscopy in evaluating disease severity in Crohn's disease. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2005;3(12):1221–8. [DOI] [PubMed] [Google Scholar]
- 37.Colombel JF, Panaccione R, Bossuyt P, Lukas M, Baert F, Vanasek T, et al. Effect of tight control management on Crohn's disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390(10114):2779–89. 10.1016/S0140-6736(17)32641-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(DOCX)
Data Availability Statement
All data files are available from the GitHub database (https://github.com/Shintaro-Sagami/PLOS-ONE-2018).