Abstract
Objective:
Cutaneous neonatal lupus (cNL) occurs in possibly 5-16% of anti-Ro±anti-La antibody exposed infants. Data suggest in utero exposure to hydroxychloroquine (HCQ) may prevent cardiac-NL. The aims were to assess whether in utero exposure to HCQ decreases the risk of cNL and/or delays onset.
Methods:
A multicenter case-control study was performed with 122 cNL cases and 434 controls born to women with a rheumatologic disease who had documentation of maternal anti-Ro±anti-La antibodies at pregnancy and confirmation of medication use and the child's outcome. A secondary analysis was performed on 262 cNL cases, irrespective of maternal diagnosis, to determine if HCQ delayed time to cNL onset.
Results:
Twenty (16%) cNL cases were exposed to HCQ compared to 146 (34%) controls (OR 0.4 [95%CI 0.2-0.6]; p<0.01). Exposure to HCQ was associated with a reduced risk of cNL; exposure to anti-La antibody and female gender were associated with an increased risk of cNL. Exposure to HCQ remained significantly associated with a reduced cNL risk in the analyses limited to mothers with Systemic Lupus Erythematosus and those who developed rash ≤1 month. When analyzing all 262 cNL cases, HCQ-exposed infants were older (6.0 [95%CI 5.7-6.3] weeks) at cNL onset vs. HCQ-non-exposed infants (4.4 [95%CI: 3.9-5.0] weeks) but the difference was not statistically significant (p=0.21).
Conclusion:
Exposure to HCQ was associated with a reduced risk of cNL. Among cNL cases, those exposed to HCQ tend to have later onset of rash. Both findings suggest a protective effect of HCQ on cNL.
Keywords: Neonatal lupus, cutaneous neonatal lupus, hydroxychlororquine, pregnancy
INTRODUCTION
Neonatal lupus (NL) is an autoimmune disease associated with the transplacental passage of maternal anti-Ro±anti-La antibodies. Cutaneous involvement is one of the most common non-cardiac manifestations of NL, affecting possibly 5-16% of anti-Ro±anti-La antibody exposed infants [1,2] and the recurrence rate of cNL approaches 23% [3]. Biopsy specimens of affected areas usually show interface dermatitis [4]. Cutaneous neonatal lupus (cNL) is a transient condition that generally heals spontaneously as maternal autoantibodies are cleared from the child's circulation [5], although permanent scarring can develop in cases where the lesions are extensive [6]. In addition, if misdiagnosed, it may lead to unnecessary investigations and treatments.
Hydroxychloroquine (HCQ) is a drug frequently used in women with systemic autoimmune rheumatic diseases (SARD) [7]. HCQ has been show to prevent disease flares in pregnant women with systemic lupus erythematosus (SLE) [8]. Transplacental passage of HCQ has been demonstrated in pregnant women with detectable levels in cord blood and is considered safe during pregnancy [9,10]. HCQ is thought to act via inhibition of toll like receptors (TLRs) which have been implicated in the pathogenesis of cardiac-NL and cutaneous lupus [11-13]. Although HCQ has not been specifically studied for prevention of cNL, there are encouraging data on its association with a reduced risk of cardiac-NL [14-17]. Accordingly, the primary objective of this study was to assess if prenatal exposure to HCQ lowered the risk of cNL with the secondary objective evaluating for a delay in cNL rash onset in HCQ exposed infants who develop cutaneous involvement.
METHODS
Study population
All cases of cNL and controls found in three data sources were collected for this multicenter retrospective study: the SickKids Neonatal Lupus Erythematosus Database (SickKids NLE Database), the Research Registry for Neonatal Lupus (RRNL), and the French Registry of Neonatal Lupus (French RNL). The SickKids NLE Database was created in 1984 and contains prospectively collected data on anti-Ro±anti-La antibody exposed infants and their mothers living in the Greater Toronto Area, irrespective of the infants NL status [17]. The RRNL, established in 1994, and the French RNL, established in 2000, both enroll women that are anti-Ro±anti-La antibody positive, living in the United States and France, respectively, and have had at least one child with any manifestation of NL. In addition, these two registries collected data on whether siblings of the affected NL children had NL. These registries comprise data collected both prospectively and retrospectively [18,19].
Inclusion and exclusion criteria
Inclusion criteria for the case-control analyses were: (1) infant born to a woman positive for anti-Ro±anti-La antibodies, documented prior to or at the time of pregnancy, (2) known age of onset of cNL and (3) documentation of medication taken during pregnancy. For the primary analysis, a maternal diagnosis of a SARD in which HCQ could be used was required: SLE, Sjogren's syndrome (SS), dermatomyositis, rheumatoid arthritis (RA) or juvenile idiopathic arthritis (JIA). Diagnosis had to be made prior to conception to allow time for maternal HCQ blood level to increase. For the secondary analysis related to onset of rash, all cases of cNL irrespective of the maternal diagnosis were included which meant that mothers could be clinically asymptomatic. The diagnosis of cNL in both analyses had to be made by a rheumatologist, dermatologist±pediatrician. Infants with cardiac-NL were excluded from all analyses because data from the three registries regarding the effects of HCQ on cardiac-NL have already been reported [14,15,17].
Study Design, Outcome Measure and Data collection
The primary analysis was a case–control study to determine whether exposure to HCQ in mothers with a known SARD reduced the risk of cNL. The primary outcome was the development of cNL. A secondary analysis used a cohort of all children that developed cNL, irrespective of maternal diagnosis, to assess if in utero exposure to HCQ delayed the onset of cNL.
Information was extracted on infants (gender, year of birth, age of onset of cNL and siblings with cNL±cardiac NL) and their mothers (age at delivery, diagnosis, anti-Ro±anti-La antibody positivity, medication intake including HCQ, fluorinated and non-fluorinated steroids, azathioprine, intravenous immunoglobulins [IVIG] and plasmapheresis). Children were considered exposed to HCQ if their mother had documented intake of ≥200 mg per day throughout the entire pregnancy. Maternal intake of fluorinated and non-fluorinated steroids, azathioprine and IVIG was defined as intake of any dose at least once during pregnancy. Anti-Ro and anti-La antibodies were measured locally using the following assays: RRNL, commercial CLIA approved labs but in majority of cases confirmed in Buyon Clancy lab using recombinant Ro 52 and La 48 and native Ro60 in Elisa; SickKids NLE Database, commercially available Elisa assays using affinity-purified Ro and La antigens; and French RNL, counterimmunoelectrophoresis and/or by using INNO-LIAT ANA Update (Innogenetics, provided by InGen, Rungis, France in most of the cases). Research Ethics Board approval was obtained at each participating institutions.
Statistical analysis
For the primary analyses, characteristics of cases and controls were compared using Chi-square, Fisher's exact and Mann-Whitney tests as appropriate. To account for within family data correlation, generalized estimating equations (GEE) were used for data analyses [20,21]. The following ten covariates were tested in univariable GEE as potential factors associated with cNL: maternal age at delivery, maternal diagnosis of primary or secondary SS (higher levels of anti-Ro±anti-La antibodies±epitope specificity±genetic background in women with SS may put them at a higher risk of having an affected child) [22-24], maternal anti-La antibody positive, maternal use of HCQ, maternal use of fluorinated steroids±IVIG±plasmapheresis (use to treat fetuses), maternal intake of non-fluorinated steroids±azathioprine (use to treat mothers), infants gender, infant born ≥year 2000 (a time when the safety of HCQ use during pregnancy had been recognized), sibling with cNL±cardiac-NL (identify families that may be at increased risk of NL) and registry source (RRNL and French RNL grouped as have same enrolment criteria). Variables with a p value ≤0.05 were selected for inclusion in the multivariable GEE model. A p value <0.05 in the final multivariable model was considered statistically significant. Two a priori subgroup analyses were performed. The first included only infants born to SLE mothers, to account for possible confounding by indication, as women with SLE may be more likely to be prescribed HCQ than women with other types of SARD. The second, limiting cases to those who developed cNL within ≤4 weeks of life. The rationale was that neonates are not continued on HCQ after delivery, therefore HCQ levels are expected to decline.
The secondary analysis was limited to cNL cases, irrespective of maternal health. Kaplan-Meier survival analysis was performed to assess if HCQ delayed the onset of cNL. A p value <0.05 by the Gehan-Breslow-Wilcoxon test was considered statistically significant. Data analysis was performed using IBM SPSS Statistics Version 21.0. (Armonk, NY: IBM Corp.) and R Version 3.1.1 (Vienna, Austria: R Foundation for Statistical Computing).
RESULTS
Included and excluded patients
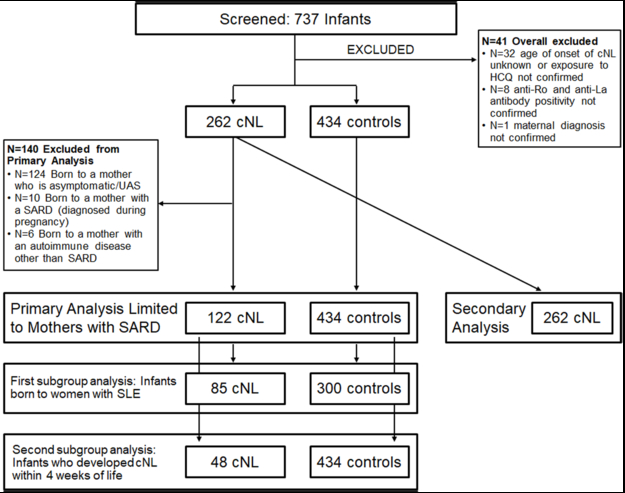
A total of 737 infants were screened (Figure 1). Forty-one were excluded due to missing key variables. The overall study population consisted of 696 infants: 262 cNL cases and 434 controls. Among the 262 cNL cases, 122 infants were born to women with a SARD diagnosed before pregnancy and were therefore included into the primary analysis. The first subgroup analysis included only infants born to women with SLE (cNL cases=85; controls=300). The second subgroup analysis was restricted to infants who developed cNL within 4 weeks of life (cNL cases=48; controls=434). All 262 cNL cases were included into the secondary analysis.
Figure 1. Diagram of included and excluded patients.
A total of 737 infants were screened. Forty-one infants were excluded for missing key variables. A total of 696 infants were eligible: 262 cNL cases and 434 controls. Among the 262 cNL cases, 122 were born to women with a SARD diagnosed before pregnancy and were therefore included into the primary analysis, with 434 controls. The first subgroup analysis included only infants born to women with SLE (cNL cases=85; controls=300). The second subgroup analysis was restricted to cNL cases with onset of rash in the first 4 weeks after birth (cNL cases=48; controls=434). The secondary analysis included 262 cNL cases, regardless of maternal diagnosis: 122 infants born to women with a SARD diagnosed before pregnancy, 10 infants born to women with a SARD diagnosed during pregnancy, 6 infants born to women with autoimmune diseases other than SARD and 124 infants born to women who were asymptomatic or had an undifferentiated autoimmune syndrome (UAS).
Primary analysis: Does exposure to HCQ in mothers with a known SARD reduce the risk of cNL
Patient characteristics
The study population consisted of 556 infants: 122 cNL cases and 434 controls. There were 10 twin pairs: cNL status was concordant in 8/10 pairs (both twins unaffected). Thirty-eight percent (N=209) of the study population were siblings: 89 sibling pairs, 7 groups of 3 siblings and 2 groups of 5 siblings. Thirty-seven percent (N=208) of the study population had at least one sibling with cutaneous (N=85), cardiac (N=107) or both NL features (N=16).
Demographics of the primary analysis study population are shown in Table 1. A diagnosis of SLE (48.4%) and SS (48.4%) was found with equal frequency in mothers of cNL cases as compared to mothers of controls for which SLE was the leading diagnosis (62.0%). Nearly all women were anti-Ro antibody positive (99%) but the proportion of women with positive anti-La antibody was significantly higher in mothers of cNL cases (73%) than mothers of controls (48%); p<0.01. In utero exposure to HCQ and to non-fluorinated steroids±azathioprine was higher in controls (34% HCQ exposed and 44% non-fluorinated steroids±azathioprine exposed) than cNL cases (16% HCQ exposed and 25% non-fluorinated steroids±azathioprine exposed); p<0.01. Characteristics of the 556 infants were similar. The RRNL and French RNL had more cNL cases (65%) vs. the SickKids NLE Database (35%). The median age of onset of cNL was 6 (range 0-21) weeks old.
Table 1.
Characteristics of the study population, primary aim
| Patients | Cases | Controls | P value | |
|---|---|---|---|---|
| Maternal characteristics | N=546 | N=122 | N=424 | |
| Age at delivery (years [IQR]) | 533 | 31 (29-35) | 32 (29-35) | 0.33 |
| Diagnosis | 0.03 | |||
| SLE | 322 | 59 (48.4) | 263 (62.0) | |
| Primary or secondary SS | 204 | 59 (48.4) | 145 (34.2) | |
| RA or JIA | 19 | 4 (3.2) | 15 (3.6) | |
| Dermatomyositis | 1 | 0 | 1 (0.2) | |
| Anti-Ro antibody positive | 545 | 121 (100) | 419 (99) | 0.59 |
| Anti-La antibody positive | 518 | 88 (73) | 192 (48) | <0.01 |
| Medication use | ||||
| Hydroxychloroquinea | 546 | 20 (16) | 146 (34) | < 0.01 |
| Fluorinated steroids±IVIG±plasmapheresis | 546 | 7 (6) | 17 (4) | 0.41 |
| Non-fluorinated steroids±azathioprine | 543 | 31 (25) | 186 (44) | < 0.01 |
| Children characteristics | N=556 | N=122 | N=434 | |
| Female:Male; (%) | 554 | 58:42 | 48:52 | 0.05 |
| Born ≥year 2000 | 556 | 75 (62) | 277 (64) | 0.63 |
| Registry, French/RRNL: SickKids (%) | 556 | 65:35 | 47:53 | <0.01b |
| French RNL | 6 | 106 | ||
| RRNL | 73 | 97 | ||
| SickKids NLE Database | 43 | 231 |
Data presented as number (percentage) unless otherwise specified. N, number; IVIG, intravenous immunoglobulin; JIA, juvenile idiopathic arthritis; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome.
The proportion of women taking HCQ throughout pregnancy among the three datasources were as follows: RRNL N=30/168 (18%), SickKids NLE Database N=76/267 (28%) and French RNL N=60/111 (54%);
pvalue, French RNL/RRNL compared to SickKids NLE Database.
Main analysis: Factors associated with development of cNL
Association between the ten predefined covariates and cNL was first explored with univariable GEE analyses (Table 2A). Six variables were found to have a p value ≤0.05 and were selected for the multivariable GEE model. In the final model, the odds of in utero exposure to HCQ were significantly lower in cNL cases than in controls (OR 0.4 [95%CI 0.2-0.7]; p<0.01) (Table 3A). A similar association was found for exposure to non-fluorinated steroids±azathioprine (OR 0.5 [0.3-0.8]; p=0.01). Cases with cNL were more likely to be female (OR 1.7 [95%CI 1.1-2.6]; p=0.02), exposed to maternal anti-La antibody (2.7 [95%CI 1.7-4.3]; p<0.01) and come from the French or RRNL Registries (OR 2.0 [95%CI 1.1-3.6]; p=0.02).
Table 2A.
Patients characteristics associated with cNL in univariable GEE analysis.
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Age at delivery | 1.0 (0.9-1.1) | 0.39 |
| Primary or secondary SS | 1.9 (1.2-2.9) | < 0.01 |
| Anti-La antibody positive | 3.0 (1.8-4.7) | < 0.01 |
| Hydroxychloroquine | 0.4 (0.2-0.6) | < 0.01 |
| Fluorinated steroids±IVIG±plasmapheresis | 1.4 (0.6-3.5) | 0.47 |
| Non-fluorinated steroids±azathioprine | 0.5 (0.3-0.7) | < 0.01 |
| Children characteristics | ||
| Female gender | 1.5 (1.0-2.3) | 0.04 |
| Born ≥year 2000 | 1.1 (0.7-1.8) | 0.54 |
| Sibling with cNL and/or cardiac NL | 1.5 (0.9-2.4) | 0.10 |
| Registrya | 2.1 (1.4-3.3) | < 0.01 |
cNL, cutaneous neonatal lupus; GEE, generalized estimating equations; IVIG, intravenous immunoglobulin; NL, neonatal lupus; OR (95% CI), odds ratio (95% confidence interval); RRNL, Research Registry for Neonatal Lupus; SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
Table 3A.
Patients characteristics associated with cNL in multivariable GEE analyses
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Anti-La antibody positive | 2.7 (1.7-4.3) | < 0.01 |
| Hydroxychloroquine | 0.4 (0.2-0.7) | < 0.01 |
| Non-fluorinated steroids±azathioprine | 0.5 (0.3-0.8) | 0.01 |
| Children characteristics | ||
| Female gender | 1.7 (1.1-2.6) | 0.02 |
| Registrya | 2.0 (1.1-3.6) | 0.02 |
GEE, generalized estimating equations; OR (95% CI), odds ratio (95% confidence interval); SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
First subgroup analysis: Infants born to women with SLE
This analysis included the 385 infants (cNL cases=85, controls=300) born to women with SLE. Results of the univariable and multivariable GEEs were similar to those observed for the main analysis (Tables 2B and 3B) with significantly lower odds of HCQ exposure in cNL cases than in controls (OR 0.4 [95%CI 0.2-0.7]; p<0.01). In the subgroup of SLE mothers, secondary SS was positively associated with cNL (OR 2.4 [95%CI 1.1-5.3]; p=0.03) but female gender was no longer significant in the multivariable analysis.
Table 2B.
Univariable GEE analysis for factors associated with cNL in infants born to women with SLE.
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Age at delivery | 1.0 (0.9-1.1) | 0.49 |
| Primary or secondary SS | 4.4 (2.4-7.9) | < 0.01 |
| Anti-La antibody positive | 3.8 (2.1-6.9) | < 0.01 |
| Hydroxychloroquine | 0.3 (0.2-0.6) | < 0.01 |
| Fluorinated steroids±IVIG±plasmapheresis | 1.7 (0.6-4.5) | 0.32 |
| Non-fluorinated steroids±azathioprine | 0.5 (0.3-0.8) | < 0.01 |
| Children characteristics | ||
| Female gender | 1.5 (0.9-2.4) | 0.13 |
| Born ≥year 2000 | 1.3 (0.8-2.1) | 0.31 |
| Sibling with cNL and/or cardiac NL | 2.5 (1.4-4.5) | < 0.01 |
| Registrya | 2.8 (1.6-4.7) | < 0.01 |
cNL, cutaneous neonatal lupus; GEE, generalized estimating equations; IVIG, intravenous immunoglobulin; NL, neonatal lupus; OR (95% CI), odds ratio (95% confidence interval); RRNL, Research Registry for Neonatal Lupus; SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
Table 3B.
Multivariable GEE analysis for factors associated with cNL in infants born to women with SLE
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Secondary SS | 2.4 (1.1-5.3) | 0.03 |
| Anti-La antibody positive | 3.4 (1.8-6.3) | < 0.01 |
| Hydroxychloroquine | 0.4 (0.2-0.7) | < 0.01 |
| Non-fluorinated steroids±azathioprine | 0.4 (0.2-0.8) | < 0.01 |
| Children characteristics | ||
| Registrya | 3.5 (1.6-7.6) | < 0.01 |
GEE, generalized estimating equations; OR (95% CI), odds ratio (95% confidence interval); SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
Second subgroup analysis: Infants who developed cNL within 4 weeks of life
A total of 482 infants were included (cNL cases=48, controls=434). In univariable analysis, cNL cases were more likely to be exposed to maternal anti-La antibody (OR 3.7 [95%CI 1.7-7.8]; p<0.01) and come from the French or RRNL Registries (OR 2.1 [95%CI 1.1-4.0]; p=0.02) but less likely to be exposed to HCQ (OR 0.2 [95%CI 0.1-0.6]; p<0.01) (Table 2C). Maternal anti-La antibody positivity (OR 3.2 [95%CI 1.5-6.8]; p<0.01) and exposure to HCQ (OR 0.2 [95%CI 0.1-0.5]; p<0.01) remained significant in the multivariable GEE model (Table 3C).
Table 2C.
Univariable GEE analysis for factors associated with early onset cNL.
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Age at delivery | 1.0 (0.9-1.1) | 0.89 |
| Primary or secondary SS | 1.8 (1.0-3.3) | 0.06 |
| Anti-La antibody positive | 3.7 (1.7-7.8) | < 0.01 |
| Hydroxychloroquine | 0.2 (0.1-0.6) | < 0.01 |
| Fluorinated steroids±IVIG±plasmapheresis | 2.1 (0.7-6.4) | 0.17 |
| Non-fluorinated steroids±azathioprine | 0.7 (0.4-1.3) | 0.27 |
| Children characteristics | ||
| Female gender | 1.5 (0.8-2.8) | 0.18 |
| Born ≥year 2000 | 1.1 (0.6-2.0) | 0.83 |
| Sibling with cNL and/or cardiac NL | 1.6 (0.8-3.0) | 0.16 |
| Registrya | 2.1 (1.1-4.0) | 0.02 |
cNL, cutaneous neonatal lupus; GEE, generalized estimating equations; IVIG, intravenous immunoglobulin; NL, neonatal lupus; OR (95% CI), odds ratio (95% confidence interval); RRNL, Research Registry for Neonatal Lupus; SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
Table 3C.
Multivariable GEE analysis for factors associated with early onset cNL
| OR (95% CI) | P value | |
|---|---|---|
| Maternal characteristics | ||
| Anti-La antibody positive | 3.2 (1.5-6.8) | <0.01 |
| Hydroxychloroquine | 0.2 (0.1-0.5) | <0.01 |
GEE, generalized estimating equations; OR (95% CI), odds ratio (95% confidence interval); SS, Sjogren's syndrome.
Comparator is the SickKids NLE Database Registry.
Secondary analysis: Does in utero exposure to HCQ delay the onset of cNL
Patient characteristics
The study population consisted of 262 cNL patients. Twenty-three (8.8%) infants were exposed to HCQ. Demographics of the study cohort are shown in Table 4. Characteristics of infants exposed to HCQ differed from those non-exposed with respect to the following variables: maternal diagnoses of SLE or cutaneous lupus (52.2% vs. 24.4%; p<0.01) or Sjogren's syndrome (43.5% vs. 21.8%; p=0.04) were more frequent but the proportion of asymptomatic/undifferentiated autoimmune syndrome was lower (4.3% vs. 51.7%; p<0.01), prenatal exposure to non-fluorinated steroids±azathioprine (43.5% vs. 9.2%; p<0.01) and proportion of infants born ≥year 2000 (91.3% vs. 53.1%; p<0.01) were higher.
Table 4.
Characteristics of the cNL population, secondary aim
| Patients | Exposed to HCQ |
Non-exposed to HCQ |
P value | |
|---|---|---|---|---|
| Maternal characteristics | N=262 | N=23 | N=239 | |
| Diagnosis | 261 | <0.01 | ||
| Asymptomatic/UAS | 1 (4.3) | 123 (51.7) | ||
| SLE or cutaneous lupus | 12 (52.2) | 58 (24.4) | ||
| Primary or secondary SS | 10 (43.5) | 52 (21.8) | ||
| Overlap connective tissue disease | 0 | 1 (0.4) | ||
| RA or JIA | 0 | 4 (1.7) | ||
| Antibody status | ||||
| Anti-Ro antibody positive | 262 | 23 (100) | 239 (100) | - |
| Anti-La antibody positive | 255 | 19 (86) | 173 (74) | 0.21 |
| Medication intake | ||||
| Fluorinated steroids±IVIG | 262 | 2 (9) | 9 (4) | 0.25 |
| Non-fluorinated steroids±azatdioprine | 262 | 10 (44) | 22 (9) | <0.01 |
| Children characteristics | N=262 | N=23 | N=239 | |
| Female:male; (%) | 262 | 52:48 | 59:41 | 0.55 |
| Bom ≥year 2000 | 262 | 21 (91) | 127 (53) | <0.01 |
| Registry, French/RRNL:SickKids (%) | 262 | 65:35 | 63:37 | a0.82 |
| French RNL | 5 | 9 | ||
| RRNL | 10 | 141 | ||
| SickKids NLE Database | 8 | 89 |
Data presented as number (percentage) unless otherwise specified. N, number; IVIG, intravenous immunoglobulin; JIA, juvenile idiopathic arthritis; RA, rheumatoid arthritis; RRNL, Research Registry for Neonatal Lupus; SLE, systemic lupus erythematosus; SS, Sjogren's syndrome; UAS, undifferentiated autoimmune syndrome.
pvalue, French RNL/RRNL compared to SickKids NLE Database.
Effect of in utero exposure to HCQ on the age of onset of cNL
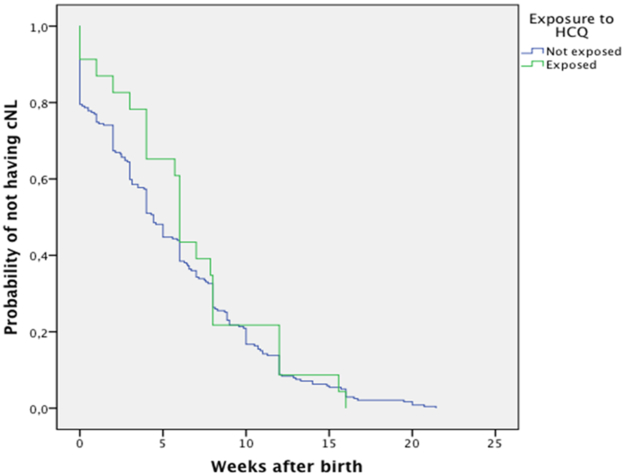
Kaplan-Meier analysis showed no statistically significant difference in the median number of weeks to cNL onset: HCQ exposed 6.0 (95%CI: 5.7-6.3) vs. non-exposed 4.4 (95%CI: 3.9-5.0) weeks; p=0.21 (Figure 2). Although no statistically significant difference was seen, the survival curves showed a delayed onset of rash during the first 6 weeks in infants exposed to HCQ.
Figure 2. Kaplan-Meier analysis of time of onset of cNL in HCQ exposed vs. non-exposed infants.
Kaplan-Meier analysis of the 262 cNL infants for time to onset of cutaneous involvement in HCQ exposed (represented by the green line) vs. HCQ non-exposed (represented by the blue line) infants. Although HCQ exposed infants were older (6.0 [95%CI 5.7-6.3] weeks) at cNL onset than HCQ non-exposed infants (4.4 [95%CI 3.9-5.0] weeks), the difference was not statistically significant (p=0.21).
DISCUSSION
Cutaneous involvement is one of the most common manifestations of NL. In prospective studies, the reported incidence has ranged from 5-16% [1,2]. Although cutaneous NL is transient and is considered a benign condition, if misdiagnosed, it may lead to unnecessary investigations and treatments. In addition, telangiectasia, epidermal atrophy and/or cutaneous pigmentation changes may appear in 10-38 % of affected children [5,25,26]. There is currently no therapy known to prevent cNL. In this multicenter study of 556 infants born to women with SARD and exposed to anti-Ro±anti-La antibodies, the odds of exposure to HCQ was lower in cNL cases than in controls, suggesting HCQ may protect against cNL.
Hydroxychloroquine may exert a protective effect through inhibition of TLRs activation and signaling [27]. In lupus prone NZBxNZW F1 mice, TLR-7 and −9 were shown to be involved in the initiation and maintenance of interface dermatitis, which is similar to histopathologic changes found in cNL [4,13]. Moreover, inhibiting TLR-7 and −9 signaling improved dermatitis in these mice. In addition, HCQ is effective in treating subacute cutaneous lupus, which closely resembles cNL [7]. Another encouraging link derives from data on the protective effect of HCQ on cardiac-NL. Four reports have shown that prenatal HCQ exposure is associated with a lower risk of cardiac-NL [14-17].
Anti-La antibody positivity has been associated with higher incidence of cNL and maternal high titers of anti-La antibody were found to be more common in infants with non-cardiac NL, who predominantly had cutaneous involvement [1,28]. Binding of human anti-La antibodies to apoptotic cells at the dermal-epidermal junction and around the basal keratinocytes of murine BALB/c fetuses has been demonstrated [29]. In our study, maternal anti-La antibody positivity was significantly associated with cNL in all of our multivariable models, with ORs ranging from 2.7-3.4. Infant's female gender was found to be associated with cNL in our main analysis. This finding may be secondary to the observed increased expression of Ro and La antigen on the membrane surface of keratinocytes exposed to estrogen [30,31].
The impact of maternal health status on the risk of developing cNL remains to be characterized. To our knowledge, there are no studies that have demonstrated that women with Sjogren's syndrome have a higher risk of having a child with cNL as compared to women with SLE. Our subgroup analysis performed in 385 infants born to women with SLE showed that secondary SS was associated with cNL. Maternal and/or fetal genetic factors could explain the mechanism by which maternal disease modulates the risk of skin involvement in their offspring [22-24]. Both the main case-control analysis and the subgroup analysis restricted to SLE mothers identified a lower exposure of non-fluorinated steroids±azathioprine in infants with cNL. A direct effect of these drugs in the infants is not anticipated since the placenta acts as a relative barrier to azathioprine's main metabolites and inactivates non-fluorinated steroids [32,33]. Indirect effects through modulation of maternal disease, lowering of autoantibody titers and/or residual confounding due to the association with maternal disease subtypes are other possible explanations for these findings. In addition, our study was not designed to assess the effect of non-fluorinated steroids±azathioprine on cNL therefore no conclusion on the effect of these two drugs on cNL should be made. Infants from the RRNL and French RNL were more likely to have cNL than those enrolled into the SickKids NLE Database. This is most likely a result of the different enrolment criteria between registries. Inclusion criteria for both the RRNL and French RNL requires at least one infant with NL. In contrast, the SickKids NLE Database includes all children exposed in utero to maternal anti-Ro±anti-La antibodies, irrespective of the infants NL status. Consequently, the SickKids NLE Database had significantly more controls.
The secondary analysis of this study explored the effect of HCQ on the time of onset of cNL. The retrospective multicenter analysis of the 262 cNL infants irrespective of maternal diagnosis suggests that in utero exposure to HCQ did not significantly delay the onset of rash. Despite having a relatively large sample size, there were only 23 cNL infants exposed to HCQ. The lack of a statistically significant effect of HCQ may be explained by the latter limitation. Interestingly, the Kaplan-Meier curves showed a delayed onset of rash in HCQ exposed infants during the first six weeks of life. This suggests that HCQ could have a protective effect early after birth during the time neonatal blood levels are still within a detectable range. Transplacental passage of HCQ has been demonstrated in neonates with measurable drug levels in cord blood [9]. The pharmacokinetics and pharmacodynamics of HCQ in fetuses and neonates has not been studied. Therefore the potential therapeutic dose range required to prevent cNL and time at which infants blood levels fall below this threshold remain unknown. Hydroxychloroquine has been shown to be transfered into breast-milk at very low concentrations resulting in estimated HCQ ingested doses by breastfed infants of 0.1-0.2 mg/kg/day[9,34]. Although we did not collect data on breastfeeding, we believe it is unlikely that the amount of HCQ found into breast-milk had an impact on timing of cNL.
Our study is the first to address the effect of HCQ exposure on cNL. Cutaneous involvement in NL may result in unnecessary medical consultations, investigations and treatments and lead to permanent scarring [5,25,26]. Moreover the risk of cardiac NL in a pregnancy following the birth of a child with cNL has been estimated to be 13% [3]. Using an inexpensive and safe medication to prevent cNL and therefore minimize these issues is definitely appealing. In addition, we were able to assemble a relatively large sample size for a NL study as our data came from three of the largest NL database/registries. Despite inconclusive findings regarding HCQ's effect on timing of cNL, results of this work are valuable as the survival curves showed a delayed onset of rash during the first six weeks of life in infants exposed to HCQ in utero. All analyses performed suggest that prenatal exposure to HCQ may have an impact on the development of cNL and support the need for further studies addressing the underlying pathophysiology of cNL and potential preventive use of maternal HCQ for cNL.
Potential limitations need to be acknowledged. Medication adherence in mothers was not measured specifically during pregnancy but women were queried about medication intake during routine prenatal rheumatology follow-up and upon enrolment into their respective database. It is therefore possible that some children categorized as exposed to HCQ were not exposed throughout gestation due to maternal non-adherence to therapy [35]. Because women with SLE are more likely to be prescribed HCQ than women with other SARD, confounding by indication could have occurred in our study. To account for this potential issue, a subgroup analysis was performed and results were consistent with that of the primary analysis. For the secondary analysis, a larger sample size may have been required to show a significant difference. The age of onset of cNL was obtained from parents during postnatal clinic follow up. Because early cNL may be very subtle, parents might not have noticed the cutaneous lesions when they first appeared and the age of onset reported could have been incorrect.
In this large multicenter study exposure to HCQ in utero was associated with a reduced risk of cNL and this association remained in subgroup analyses limited to mothers with SLE and in infants who developed cNL within 4 weeks of life. In addition, although not statically significant, cNL cases exposed to HCQ tend to have later onset of cNL perhaps due to protection conferred by HCQ when neonatal HCQ blood levels still remain within a detectable range.
ACKNOWLEDGMENTS
The authors thank the following individuals for assitance with data collection and extraction: SickKids NLE Database, Michelle Wang and Shazia Ali; RRNL, Tishaun Middleton and French RNL, Ada Clarke. We wish to thank all the rheumatologists working in the Greater Toronto Area for referring patients to Dr. Laskin and all of the obstetricians at Mount Sinai Hospital High Risk Unit. Similarly, we want to acknowledge physicians who refered patients to the RRNL and French Registry of Neonatal Lupus. Lastly, the authors thank families who agreed to be part of all three registries.
FUNDING
Funding for RRNL was provided by National Institute of Arthritis and Musculoskeletal and Skin Disease (NIAMS) contract N01-AR-4-2220-11-0-1 and grant 5R37AR042455, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) grants R01 HD079951-01A1 and R03 HD069986, and a Lupus Foundation of America LFA Lifeline Program grant to JPB.
Footnotes
COMPETING INTERESTS
All authors have no competing interests to declare.
ETHICS APPROVAL
The study was approved by the Hospital for Sick Children Ethics Boards (REB1000037488), RRNL IRB (Protocol Number: 7820), French RNL IRB Pitié-Salpetriére.
REFERENCES
- 1.Cimaz R, Spence DL, Hornberger L et al. Incidence and spectrum of neonatal lupus erythematosus: a prospective study of infants born to mothers with anti-Ro autoantibodies. J Pediatr 2003;142(6):678–83. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DM, Kim MY, Copel JA, et al. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation 2008;117(4):485–93. [DOI] [PubMed] [Google Scholar]
- 3.Izmirly PM, Llanos C, Lee LA et al. Cutaneous manifestations of neonatal lupus and risk of subsequent congenital heart block. Arthritis Rheum 2010;62(4):1153–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penate Y, Guillermo N, Rodriguez J, et al. Histopathologic characteristics of neonatal cutaneous lupus erythematosus: description of five cases and literature review. J Cutan Pathol 2009;36(6):660–7. [DOI] [PubMed] [Google Scholar]
- 5.Neiman AR, Lee LA, Weston WL et al. Cutaneous manifestations of neonatal lupus without heart block: characteristics of mothers and children enrolled in a national registry. J Pediatr 2000;137(5):674–80. [DOI] [PubMed] [Google Scholar]
- 6.High WA, Costner MI. Persistent scarring, atrophy, and dyspigmentation in a preteen girl with neonatal lupus erythematosus. J Am Acad Dermatol 2003;48(4):626–8. [DOI] [PubMed] [Google Scholar]
- 7.Frances C, Cosnes A, Duhaut P, et al. Low blood concentration of hydroxychloroquine in patients with refractory cutaneous lupus erythematosus: a French multicenter prospective study. Arch Dermatol 2012;148(4):479–84. [DOI] [PubMed] [Google Scholar]
- 8.Clowse ME, Magder L, Witter F et al. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum 2006;54(11):3640–7. [DOI] [PubMed] [Google Scholar]
- 9.Costedoat-Chalumeau N, Amoura Z, Aymard G, et al. Evidence of transplacental passage of hydroxychloroquine in humans. Arthritis Rheum 2002;46(4):1123–4. [DOI] [PubMed] [Google Scholar]
- 10.Costedoat-Chalumeau N, Amoura Z, Huong DL et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimm Rev 2005;4(2):111–5. [DOI] [PubMed] [Google Scholar]
- 11.Clancy RM, Alvarez D, Komissarova E et al. Ro60-associated single-stranded RNA links inflammation with fetal cardiac fibrosis via ligation of TLRs: a novel pathway to autoimmune-associated heart block. J Immunol 2010;184(4):2148–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez D, Briassouli P, Clancy RM, et al. A novel role of endothelin-1 in linking Toll-like receptor 7-mediated inflammation to fibrosis in congenital heart block. J Biol Chem 2011;286(35):30444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guiducci C, Tripodo C, Gong M, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med 2010;207(13):2931–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Izmirly PM, Kim MY, Llanos C, et al. Evaluation of the risk of anti-SSA/Ro-SSB/La antibody-associated cardiac manifestations of neonatal lupus in fetuses of mothers with systemic lupus erythematosus exposed to hydroxychloroquine. Ann Rheum Dis 2010;69(10):1827–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izmirly PM, Costedoat-Chalumeau N, Pisoni CN, et al. Maternal use of hydroxychloroquine is associated with a reduced risk of recurrent anti-SSA/Ro-antibody-associated cardiac manifestations of neonatal lupus. Circulation 2012;126(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tunks RD, Clowse ME, Miller SG, et al. Maternal autoantibody levels in congenital heart block and potential prophylaxis with antiinflammatory agents. Am J Obstet Gynecol 2013;208(1):64 e1–7. [DOI] [PubMed] [Google Scholar]
- 17.Barsalou J, Jaeggi E, Laskin CA, et al. Prenatal exposure to antimalarials decreases the risk of cardiac but not non-cardiac neonatal lupus: a single-centre cohort study. Rheumatology 2017;56(9):1552–59. [DOI] [PubMed] [Google Scholar]
- 18.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31(7):1658–66. [DOI] [PubMed] [Google Scholar]
- 19.Levesque K, Morel N, Maltret A, et al. Description of 214 cases of autoimmune congenital heart block: Results of the French neonatal lupus syndrome. Autoimm Rev 2015;14(12):1154–60. [DOI] [PubMed] [Google Scholar]
- 20.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157(4):364–75. [DOI] [PubMed] [Google Scholar]
- 21.Homish GG, Edwards EP, Eiden RD, Leonard KE. Analyzing family data: A GEE approach for substance use researchers. Addict Behav 2010;35(6):558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Molina G, Avila-Casado C, Cardenas-Velazquez F, et al. Similarities and differences between primary and secondary Sjogren's syndrome. J Rheumatol 2010;37(4):800–8. [DOI] [PubMed] [Google Scholar]
- 23.Buyon JP, Slade SG, Reveille JD, Hamel JC, Chan EK. Autoantibody responses to the "native" 52-kDa SS-A/Ro protein in neonatal lupus syndromes, systemic lupus erythematosus, and Sjogren's syndrome. J Immunol 1994;152(7):3675–84. [PubMed] [Google Scholar]
- 24.Colombo G, Brucato A, Coluccio E, et al. DNA typing of maternal HLA in congenital complete heart block: comparison with systemic lupus erythematosus and primary Sjogren's syndrome. Arthritis Rheum 1999;42(8):1757–64. [DOI] [PubMed] [Google Scholar]
- 25.Lin SC, Shyur SD, Wu JY, Huang LH, Yi C. Facial telangiectasia-an unusual complication of neonatal lupus erythematosus: report of one case. Acta Paediatr Taiwan 2004;45(4):246–8. [PubMed] [Google Scholar]
- 26.Weston WL, Morelli JG, Lee LA. The clinical spectrum of anti-Ro-positive cutaneous neonatal lupus erythematosus. J Am Acad Dermatol 1999;40(5 Pt 1):675–81. [DOI] [PubMed] [Google Scholar]
- 27.Wallace DJ, Gudsoorkar VS, Weisman MH, Venuturupalli SR. New insights into mechanisms of therapeutic effects of antimalarial agents in SLE. Nat Rev Rheumatol 2012;8(9):522–33. [DOI] [PubMed] [Google Scholar]
- 28.Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol 2010;55(24):2778–84. [DOI] [PubMed] [Google Scholar]
- 29.Tran HB, Macardle PJ, Hiscock J, et al. Anti-La/SSB antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum 2002;46(6):1572–9. [DOI] [PubMed] [Google Scholar]
- 30.Norris DA, Lee LA. Pathogenesis of cutaneous lupus erythematosus. Clin Dermatol 1985;3(3):20–35. [DOI] [PubMed] [Google Scholar]
- 31.Jones SK. The effects of hormonal and other stimuli on cell-surface Ro/SSA antigen expression by human keratinocytes in vitro: their possible role in the induction of cutaneous lupus lesions. Br J Dermatol 1992;126(6):554–60. [DOI] [PubMed] [Google Scholar]
- 32.Hutson JR, Lubetsky A, Walfisch A, Ballios BG, Garcia-Bournissen F, Koren G. The transfer of 6-mercaptopurine in the dually perfused human placenta. Reprod Toxicol 2011;32(3):349–53. [DOI] [PubMed] [Google Scholar]
- 33.Kemp MW, Newnham JP, Challis JG, Jobe AH, Stock SJ. The clinical use of corticosteroids in pregnancy. Hum Reprod Update 2016;22(2):240–59. [DOI] [PubMed] [Google Scholar]
- 34.Nation RL, Hackett LP, Dusci LJ, Ilett KF. Excretion of hydroxychloroquine in human milk. Br J Clin Pharmacol 1984;17(3):368–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costedoat-Chalumeau N, Houssiau F, Izmirly P, et al. A Prospective International Study on Adherence to Treatment in 305 Patients With Flaring SLE: Assessment by Drug Levels and Self-Administered Questionnaires. Clin Pharmacol Ther 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]