Abstract
This article comments on the following paper:
Liu Y, Yu Y, Sun J, Cao Q, Tang Z, Liu M, Xu T, Ma D, Li Z, Sun J. 2019. Root-zone-specific sensitivity of K+- and Ca2+-permeable channels to H2O2 determines ion homeostasis in salinized diploid and hexaploid Ipomoea trifida. Journal of Experimental Botany 70, 1389–1405.
Keywords: Ca2+ transport, H2O2, K+ retention, K+/Na+ homeostasis, Na+ exclusion, polyploid, salinity stress
Polyploidy is considered to be a driving force in plant evolution that enabled adaptation to adverse environmental conditions such as soil salinity. This phenomenon is examined by Liu et al. (2019) in relation to root-zone-specific ion transport, and can be explained by more efficient operation of an NADPH-dependent ‘ROS–Ca2+ hub’ and desensitization of ROS-inducible cation channels in polyploid lines. Two hypotheses include that non-selective cation channels in polyploid lines are formed of chimeric tetramers, with some subunits having modified thiol groups (hence, reduced sensitivity to H2O2), or alternatively that inactivation of Ca2+ channels and higher Ca2+-ATPase pump activity may reduce the level of cytosolic free Ca2+ and provide a negative control over NADPH oxidase operation.
Whole-genome duplication, or polyploidy, is considered to be a driving force in plant evolution that enabled better adaptation to some adverse environmental conditions (Adams and Wendel, 2005; Parisod et al., 2010). Polyploid plants demonstrate enhanced tolerance to a range of biotic and abiotic stresses, including soil salinity (Chao et al., 2013). A good example is hexaploid bread wheat (Triticum aestivum; genome BBAADD) that is more salt tolerant than its tetraploid wheat progenitor (T. turgidum) or durum wheat (T. durum) (Munns and James, 2003). Genome duplication improved rice resistance to salt stress (Tu et al., 2014), and citrus tetraploid genotypes are more tolerant of moderate saline stress than diploids (Saleh et al., 2008; Mouhaya et al., 2010). The link between ploidy level and salinity tolerance seems to be reciprocal, with the recent report by Barkla et al. (2018) showing that salt treatment led to a significant increase in ploidy levels in the epidermal bladder cells of the halophyte Mesembryanthemum crystallinum.
The physiological mechanisms explaining improved salt tolerance with increasing level of ploidy remain obscure. Yang et al. (2014) showed superior salinity stress tolerance in a synthetic allohexaploid wheat (neo-6×) compared with its tetraploid (T. turgidum; BBAA) and diploid (Aegilops tauschii; DD) parents, and attributed this to regulatory transition of the HKT1;5 gene from constitutive high basal expression to induced high expression upon salt stress. However, no HKT1;5 activity was measured, and the only evidence provided was a difference in the xylem Na concentration and minor variations in HKT1;5 expression in leaves at one specific timepoint (with a plethora of other reported differences in gene expression between genotypes).
Recent years have witnessed a paradigm shift towards recognition of plant tissue tolerance (e.g. a capacity of tissues to function while containing a high internal Na+ and Cl– concentration; Munns et al., 2016) as a key determinant of overall salinity stress tolerance. Cytosolic K+ retention, i.e. an ability of root and mesophyll cells to prevent NaCl-induced K+ efflux, has been shown to be an essential component of the tissue tolerance mechanism (Shabala and Pottosin, 2014; Shabala et al., 2016; Wu et al., 2018). Recently Chao et al. (2013) analyzed the elemental composition of leaves from 349 Arabidopsis accessions and 89 RILs and reported a strong correlation between the ploidy level and leaf K+ content. Can this be an explanation for superior salinity tolerance in polyploids? And if so, how is this trait regulated?
Root-zone ion transport
Liu et al. (2019) conducted a comprehensive study of the relationship between the ploidy level of Ipomoea trifida plants and root-zone-specific ion transport under saline conditions. They convincingly showed that superior tolerance of autohexaploid (6×) I. trifida as compared with diploid (2×) plants was conferred by reduced sensitivity of plasma membrane K+-permeable channels in the meristem root zone and increased sensitivity of Ca2+-permeable channels in the elongation and mature root zones to H2O2. This differential ROS sensitivity confers superior K+ retention and Na+ exclusion under salt stress, explaining the salt-tolerant phenotype in hexaploid plants. As the reported H2O2 levels were the same in double- and hexaploid lines, the above difference cannot be attributed to higher activity of antioxidant enzymes and suggests changes in sensitization of ROS-activated ion channels in the root epidermis.
The mechanisms of ion channel activation by ROS are poorly understood. It is generally assumed that the major targets of ROS-induced modification of proteins are reactive cysteine residues (Alansary et al., 2016). A reactive cysteine contains a thiolate group (S-) which reacts with H2O2 while the thiol groups (SH) do not react physiologically with H2O2 unless the reaction is catalyzed (Forman et al., 2004). The direct proof for this comes from experiments by Garcia-Mata et al. (2010), who used a heterologous expression system to show that the K+ outward-rectifying SKOR channel was activated by by H2O2 via targeted oxidation of Cys168 at the S3 α-helix within the channel’s voltage sensor. Thus, the difference in ROS-induced K+ and Ca2+ fluxes between 2× and 6× plants in Liu et al. (2019) may potentially be explained by desensitization of the appropriate transport system to H2O2 resulting from modification of thiol groups in the sensory domain.
A ‘ROS–Ca2+ hub’
Another important observation by Liu et al. (2019) was that the magnitude of NaCl-induced K+ efflux in the diploid line was reduced by twofold in plants treated with DPI, a known inhibitor of NADPH oxidase. NADPH oxidase is a plasma-membrane-bound enzyme complex from the NOX family, which faces the extracellular space (Marino et al., 2012). Discovered first as part of the plant hypersensitive (HR) response to pathogens, this enzyme has recently emerged as a critical component of stress signaling mechanisms in response to a broad range of abiotic stresses, including salinity (Miller et al., 2010; Ma et al., 2012; Shabala et al., 2015). NADPH oxidase can stabilize SOS1 transcripts (Chung et al., 2008), thus assisting plants in reducing the salt load, and is involved in generating the stress-induced Ca2+ ‘signatures’ that mediate rapid systemic signalling (Miller et al., 2010).
The concept of a ROS–Ca2+ hub was recently put forward (Demidchik and Shabala, 2018; Demidchik et al., 2018) and implies that Ca2+-activated NADPH oxidases work in concert with ROS-activated Ca2+-permeable cation channels to generate and amplify stress-induced Ca2+ and ROS signals (Box 1). Interestingly, an effect of DPI on K+ fluxes was not observed in the 6× line (Liu et al., 2019), suggesting that NADPH oxidase was already inactivated in the polyploid. This inactivation may be a result of either decreased NADPH oxidase phosphorylation by BIK1 (Kadota et al., 2014; Box 1) or low activity of Rac/Rop GTPases (Baxter-Burrell et al., 2002). More active Ca2+-ATPase activity in a hexaploid line or inactivation of Ca2+ channels resulting from its interaction with CaM (DeFalco et al., 2016) or decreased CDPK-catalyzed phosphorylation (Zhou et al., 2014) may also be the reason for ROS–Ca2+ hub activity ceasing (Box 1).
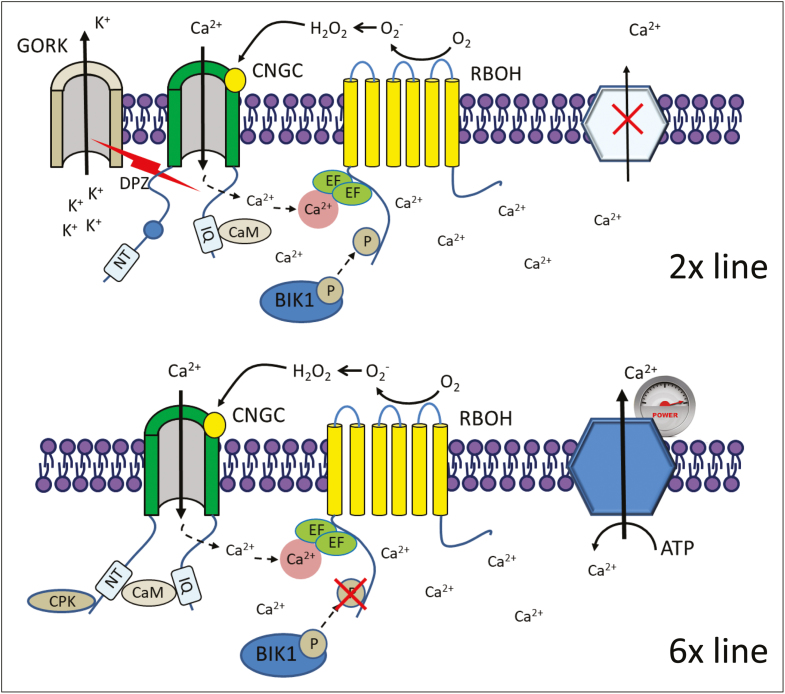
Box 1 A tentative model for the operation of an NADPH-dependent ‘ROS–Ca2+ hub’ in diploid and hexaploid lines
In the 2× line, apoplastic H2O2 produced by NADPH oxidase stimulates Ca2+ uptake through non-selective cation channels (CNGC in the model) and forms a positive feedback loop, resulting in an avalanche-like increase in cytosolic free Ca2+. Because of the massive Ca2+ influx into the cell, the plasma membrane is depolarized, triggering K+ efflux through the GORK channel. NADPH oxidase operation requires the phosphorylation of one of its terminal domains, mediated by BIK1 (Kadota et al., 2014). Operation of CNGC is also dependent on binding of calmodulin (CaM) to the IQ motif in the C terminus (DeFalco et al., 2016). In a hexaploid line, inactivation of Ca2+ channels resulting from its interaction with CaM (DeFalco et al., 2016) or decreased CDPK-catalyzed phosphorylation (Zhou et al., 2014) reduce NADPH oxidase activity. Higher Ca2+-ATPase pump activity also reduces the level of cytosolic free Ca2+ and provides a negative control over NADPH oxidase operation. BIK1, the plasma-membrane-associated kinase; CPK, calcium-dependent protein kinase; NT, a putative CaM-binding motif; DPZ, depolarization; CaM, calmodulin; IQ, a conserved isoleucine–glutamine motif in the C terminus.
Contrary to animal systems, plant genomes do not encode any Ca2+-selective ion channels (Demidchik et al., 2018), with Ca2+ transport across the plasma membrane mediated by non-selective cation channels (NSCCs). While the genetic origin of NSCCs remains unknown, two major classes – CNGCs (cyclic nucleotide-gated channels) and GLRs (glutamate receptors) – are known in Arabidopsis (with 20 members in each class; Maser et al., 2001). NSCCs can be activated by ROS (Demidchik et al., 2018). GLRs are believed to be tetramers consisting of different subunits (Price et al., 2013). CNGCs can also form chimeric channels (Zhong et al., 2003), and plants harbouring the ATCNGC11/12 gene showed a phenotype with constitutively activated (ROS-burst-related) defence responses to pathogens (Yoshioka et al., 2006). Keeping this in mind, one may hypothesize that polyploid lines may encode chimeric NSCCs with altered ligand-gated properties and reduced sensitivity to H2O2 (Box 2). It was shown that replacement of the positively charged lysine (Lys1110) with the neutrally charged asparagine (K1110N) or the negatively charged amino acid glutamic acid (K1110E) in the mammalian TRPM2 channel generated mutants that failed to induce an increase in free cytosolic calcium concentration in response to H2O2 (Kim et al., 2013). It remains to be shown if the similar substitution of one or several amino acids in chimeric NSCCs may desensitize them, thus altering ROS–Ca2+ hub operation kinetics and affecting plant salt stress signaling and ionic homeostasis, explaining salt-tolerant phenotype in polyploid lines.
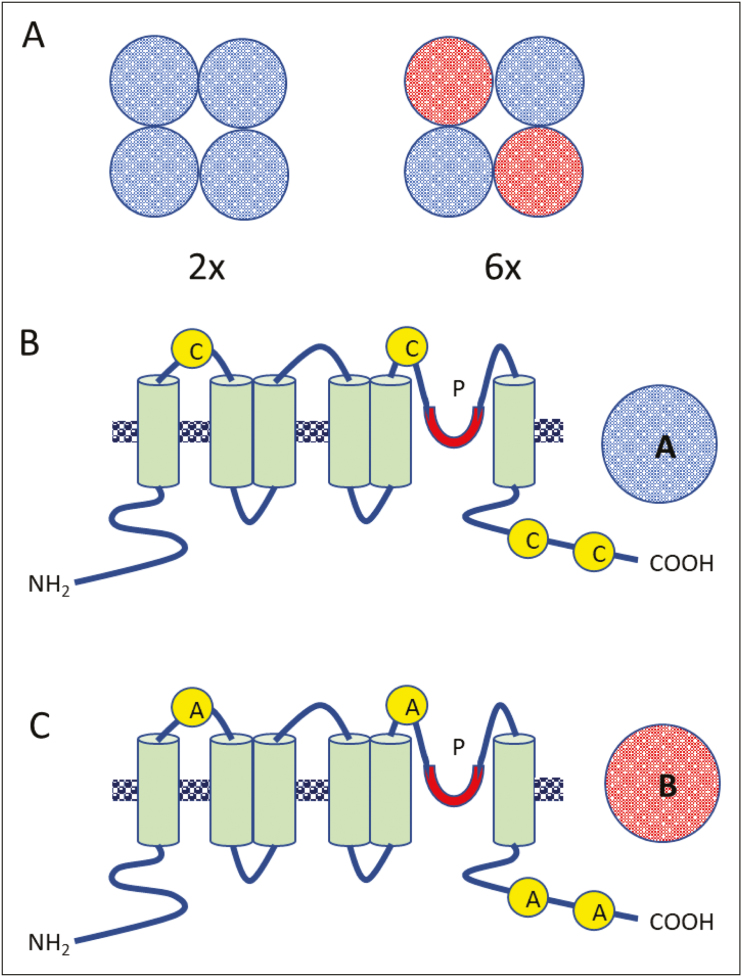
Box 2 Suggested model explaining the desensitization of cation channels in polyploid lines by chimeric protein assembly
The model assumes that Ca2+ and K+ fluxes across the plasma membrane are mediated by cyclic nucleotide-gated channels (CNGCs). Such CNGCs are made up of four subunits, each having one pore region and six transmembrane domains (Demidchik and Shabala, 2018). In a diploid (2×) line, all subunits are identical (panel A; blue) and harbour cysteine (C in the model) residues in both external and enteral loops (panel B) and, thus, can be activated by H2O2 from either the apoplastic or the cytosolic side. In a hexaploid line (6×), two out of four units have cysteine replaced by the neutrally charged asparagine (A in the model; panel C). The chimeric channel is formed of two type A (blue) and two type B (red) subunits with cysteine substituted by asparagine (or with some other non-ROS-binding amino acid). Such a chimeric channel has fewer ligand (H2O2)-binding sites and thus reduced sensitivity to ROS. P, pore.
Acknowledgements
This work was supported by China National Science Foundation (project 31870249) and Australian Research Council (DP170100430) grants to S.S.
References
- Adams KL, Wendel JF.. 2005. Polyploidy and genome evolution in plants. Current Opinion in Plant Biology 8, 135–141. [DOI] [PubMed] [Google Scholar]
- Alansary D, Schmidt B, Dörr K, Bogeski I, Rieger H, Kless A, Niemeyer BA.. 2016. Thiol dependent intramolecular locking of Orai1 channels. Scientific Reports 6, 33347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkla BJ, Rhodes T, Tran KT, Wijesinghege C, Larkin JC, Dassanayake M.. 2018. Making epidermal bladder cells bigger: developmental- and salinity-induced endopolyploidy in a model halophyte. Plant Physiology 177, 615–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J.. 2002. RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296, 2026–2028. [DOI] [PubMed] [Google Scholar]
- Chao DY, Dilkes B, Luo H, Douglas A, Yakubova E, Lahner B, Salt DE.. 2013. Polyploids exhibit higher potassium uptake and salinity tolerance in Arabidopsis. Science 341, 658–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi H.. 2008. Reactive oxygen species mediate Na+-induced SOS1 mRNA stability in Arabidopsis. The Plant Journal 53, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Marshall CB, Munro K, Kang HG, Moeder W, Ikura M, Snedden WA, Yoshioka K.. 2016. Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. The Plant Cell 28, 1738–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I.. 2018. Calcium transport across plant membranes: mechanisms and functions. New Phytologist 220, 49–69. [DOI] [PubMed] [Google Scholar]
- Demidchik V, Shabala S.. 2018. Mechanisms of cytosolic calcium elevation in plants: the role of ion channels, calcium extrusion systems and NADPH oxidase-mediated ‘ROS–Ca2+ Hub’. Functional Plant Biology 45, 9–27. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Fukuto JM, Torres M.. 2004. Redox signaling: thiol chemistry defines which reactive oxygen and nitrogen species can act as second messengers. American Journal of Physiology. Cell Physiology 287, C246–C256. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata C, Wang J, Gajdanowicz P, Gonzalez W, Hills A, Donald N, Riedelsberger J, Amtmann A, Dreyer I, Blatt MR.. 2010. A minimal cysteine motif required to activate the SKOR K+ channel of Arabidopsis by the reactive oxygen species H2O2. The Journal of Biological Chemistry 285, 29286–29294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, et al. 2014. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Molecular Cell 54, 43–55. [DOI] [PubMed] [Google Scholar]
- Kim TK, Nam JH, Ahn WG, et al. 2013. Lys1110 of TRPM2 is critical for channel activation. The Biochemical Journal 455, 319–327. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yu Y, Sun J, Cao Q, Tang Z, Liu M, Xu T, Ma D, Li Z, Sun J.. 2019. Root-zone-specific sensitivity of K+- and Ca2+-permeable channels to H2O2 determines ion homeostasis in salinized diploid and hexaploid Ipomoea trifida. Journal of Experimental Botany 70, 1389–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang H, Sun L, Jiao Y, Zhang G, Miao C, Hao F.. 2012. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na⁺/K⁺homeostasis in Arabidopsis under salt stress. Journal of Experimental Botany 63, 305–317. [DOI] [PubMed] [Google Scholar]
- Marino D, Dunand C, Puppo A, Pauly N.. 2012. A burst of plant NADPH oxidases. Trends in Plant Science 17, 9–15. [DOI] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, et al. 2001. Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiology 126, 1646–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R.. 2010. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant, Cell & Environment 33, 453–467. [DOI] [PubMed] [Google Scholar]
- Mouhaya W, Allario T, Brumos J, Andres F, Froelicher Y, Luro F, Talon M, Ollitrault P, Morillon R.. 2010. Sensitivity to high salinity in tetraploid citrus seedlings increases with water availability and correlates with expression of candidate genes. Functional Plant Biology 37, 674–685. [Google Scholar]
- Munns R, James RA.. 2003. Screening methods for salinity tolerance: a case study with tetraploid wheat. Plant and Soil 253, 201–218. [Google Scholar]
- Munns R, James RA, Gilliham M, Flowers TJ, Colmer TD.. 2016. Tissue tolerance: an essential but elusive trait for salt-tolerant crops. Functional Plant Biology 43, 1103–1113. [DOI] [PubMed] [Google Scholar]
- Parisod C, Holderegger R, Brochmann C.. 2010. Evolutionary consequences of autopolyploidy. New Phytologist 186, 5–17. [DOI] [PubMed] [Google Scholar]
- Price MB, Kong D, Okumoto S.. 2013. Inter-subunit interactions between glutamate-like receptors in Arabidopsis. Plant Signaling & Behavior 8, e27034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh B, Allario T, Dambier D, Ollitrault P, Morillon R.. 2008. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. Comptes Rendus Biologies 331, 703–710. [DOI] [PubMed] [Google Scholar]
- Shabala S, Pottosin I.. 2014. Regulation of potassium transport in plants under hostile conditions: implications for abiotic and biotic stress tolerance. Physiologia Plantarum 151, 257–279. [DOI] [PubMed] [Google Scholar]
- Shabala S, Wu H, Bose J.. 2015. Salt stress sensing and early signalling events in plant roots: current knowledge and hypothesis. Plant Science 241, 109–119. [DOI] [PubMed] [Google Scholar]
- Shabala S, Bose J, Fuglsang AT, Pottosin I.. 2016. On a quest for stress tolerance genes: membrane transporters in sensing and adapting to hostile soils. Journal of Experimental Botany 67, 1015–1031. [DOI] [PubMed] [Google Scholar]
- Tu Y, Jiang A, Gan L, et al. 2014. Genome duplication improves rice root resistance to salt stress. Rice 7, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu HH, Zhang XC, Giraldo JP, Shabala S.. 2018. It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant and Soil 431, 1–17. [Google Scholar]
- Yang CW, Zhao L, Zhang HK, Yang ZZ, Wang H, Wen SS, Zhang CY, Rustgi S, von Wettstein D, Liu B.. 2014. Evolution of physiological responses to salt stress in hexaploid wheat. Proceedings of the National Academy of Sciences, USA 111, 11882–11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF.. 2006. The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. The Plant Cell 18, 747–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Lai J, Yau K-W.. 2003. Selective heteromeric assembly of cyclic nucleotide-gated channels. Proceedings of the National Academy of Sciences, USA 100, 5509–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lan W, Jiang Y, Fang W, Luan S.. 2014. A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Molecular Plant 7, 369–376. [DOI] [PubMed] [Google Scholar]