We identified a novel Arabidopsis ammonium tolerance 1 (amot1) mutant and reveal that blocking of ethylene signaling reduces tissue H2O2 accumulation and enhances seedling tolerance to ammonium stress.
Keywords: Ammonium stress, amot1 mutant, Arabidopsis, AMOT1/EIN3, H2O2, peroxidases
Abstract
Ammonium (NH4+) toxicity inhibits shoot growth in Arabidopsis, but the underlying mechanisms remain poorly characterized. Here, we show that a novel Arabidopsis mutant, ammonium tolerance 1 (amot1), exhibits enhanced shoot growth tolerance to NH4+. Molecular cloning revealed that amot1 is a new allele of EIN3, a key regulator of ethylene responses. The amot1 mutant and the allelic ein3-1 mutants show greater NH4+ tolerance than the wild type. Moreover, transgenic plants overexpressing EIN3 (EIN3ox) are more sensitive to NH4+ toxicity The ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) increases shoot sensitivity to NH4+, whereas the ethylene perception inhibitor Ag+ decreases sensitivity. NH4+ induces ACC and ethylene accumulation. Furthermore, ethylene-insensitive mutants such as etr1-3 and ein3eil1 display enhanced NH4+ tolerance. In contrast, the ethylene overproduction mutant eto1-1 exhibits decreased ammonium tolerance. AMOT1/EIN3 positively regulates shoot ROS accumulation, leading to oxidative stress under NH4+ stress, a trait that may be related to increased expression of peroxidase-encoding genes. These findings demonstrate the role of AMOT1/EIN3 in NH4+ tolerance and confirm the strong link between NH4+ toxicity symptoms and the accumulation of hydrogen peroxide.
Introduction
Ammonium (NH4+), an important source of nitrogen for many species (Kronzucker et al., 1997; Balkos et al., 2010), is frequently present in soils and in the atmosphere in significant quantities (Britto and Kronzucker, 2002; Dupre et al., 2009). However, NH4+ is toxic at moderate levels, frequently achieved in soils, to most plants, in particular those used in temperate agriculture, with stunted root and leaf growth as major symptoms of toxicity (Britto and Kronzucker, 2002; Coskun et al., 2013). Several important physiological processes have been linked to excessive NH4+ exposure, such as ionic imbalance, relationships with carbon biochemistry, energy consumption, and modifications of hormonal balance (Britto and Kronzucker, 2002; Coskun et al., 2013). Ethylene production has been shown to increase linearly with tissue NH4+ accumulation (Barker, 1999a), concurrent with the development of toxicity symptoms (You and Barker, 2002, 2005). In addition, the application of ethylene synthesis and ethylene action inhibitors can ameliorate NH4+ toxicity symptoms (Barker and Corey, 1991; Feng and Barker, 1992a, b; You and Barker, 2005; G. Li et al., 2013). Ethylene is synthesized from S-adenosyl-l-methionine (SAM) via 1-aminocyclopropane-1-carboxylic acid (ACC). The rate-limiting step in ethylene biosynthesis lies in ACC production via ACC synthase (ACS), followed by ACC conversion to ethylene by ACC oxidase (ACO) (Adams and Yang, 1979). ACS and ACO are encoded by multigene families and are regulated by both developmental and environmental factors. Evolution of ethylene in response to biotic and abiotic stress resulting from ACS and ACO up-regulation is a common phenomenon (Yamagami et al., 2003; Schellingen et al., 2014). In Arabidopsis, the downstream component of the ethylene signaling pathway includes ethylene receptor ETRs (e.g. ETR1), CTR1 (constitutive triple response 1), EIN2 (ethylene-insensitive 2), EIN3/EIL (ethylene-insensitive 3/EIN3-like1), and ERF1 (ethylene-response factor 1) transcription factors (Lei et al., 2011). Ethylene signaling is transduced into the nucleus to cause the accumulation of two master transcriptional activators EIN3 and EIL1, which initiate transcriptional re-programming in various ethylene responses (Yoo et al., 2009; Tao et al., 2015). However, the detailed mechanisms of ethylene biosynthesis and signaling in NH4+ stress responses remain unclear.
Reactive oxygen species (ROS) are induced under a wide range of environmental stresses; they can cause oxidative damage leading to injury and death, depending on cellular concentrations. NH4+ was found to induce higher levels of H2O2 and oxidative stress response reactions in leaves of the aquatic plant Vallisneria natans (Wang et al., 2008), Arabidopsis (Podgórska et al., 2013), and other species (Podgórska and Szal, 2015). However, data regarding the pathways involved in NH4+ regulation of H2O2 production are still rare. ROS can be generated in the apoplast via the activity of NADPH oxidases under stress (Mittler et al., 2004). A group of NADPH oxidases and respiratory burst oxidase homologs (RBOHs) have been identified in Arabidopsis (Sagi and Fluhr, 2006). ROS are tightly regulated via a production/scavenging equilibrium. The expression of genes involved in the ROS regulatory network, including APX1 (ascorbate peroxidase 1) and CAT1 (catalase 1) in Arabidopsis (Davletova et al., 2005; Xing et al., 2008), also affects ROS levels. Peroxidases (PODs) have been proposed as alternative producers of ROS (Apel and Hirt, 2004; Bindschedler et al., 2006). PODs catalyze the oxidoreduction of various substrates using H2O2. PODs, rather than NADPH oxidase, have been proposed as the major ROS producer in French bean (Phaseolus vulgaris) treated with a cell wall elicitor of Colletotrichum lindemuthianum, the fungus that causes anthracnose (Bolwell et al., 1998). Kim et al. (2010) found that a POD contributes to ROS production during the Arabidopsis root response to potassium deficiency, showing the POD to be a component of the low potassium signal transduction pathway. Recently, Balzergue et al. (2017) showed that –Pi induces root tip ROS accumulation, indicating that PODs play a role. Further, the POD inhibitor salicylhydroxamic acid (SHAM) restored root growth and reduced ROS accumulation under –Pi conditions (Balzergue et al., 2017). Although ethylene and ROS have been reported to be involved in NH4+ sensitivity, there has been no study to evaluate the role of ethylene in high-NH4+-induced ROS production in leaves, and further research is necessary to clarify the circumstances under which NH4+ causes oxidative stress in plants.
One approach to elucidating mechanisms of NH4+ toxicity in plants is to use mutant lines. Qin et al. (2008) isolated the first NH4+-sensitive root elongation mutant, vtc1 (vitamin C defective 1), disrupted in GDP-mannose pyrophosphorylase (GMPase). Recently, several genetic regulators controlling root sensitivity to NH4+ have been identified in Arabidopsis, such as aux1 (auxin resistant 1) (Cao et al., 1993; Li et al., 2011), trh1 (tiny root hair 1) (Zou et al., 2012), dpms1 (dolichol phosphate mannose synthase1) (Jadid et al., 2011), and gsa1 (gravitropism sensitive to ammonium1) (Zou et al., 2013). Elucidation of the function of these genetic regulators in determining the root sensitivity to NH4+ offered insight into the molecular basis of historically described physiological responses to NH4+ toxicity. In contrast, the underlying mechanisms of impaired leaf growth under NH4+ toxicity are still largely unknown. Reduced shoot biomass and leaf chlorosis are important symptoms (Britto and Kronzucker, 2002). Based on their chlorotic phenotypes, ammonium-overly-sensitive 1 (amos1) (B. Li et al., 2012) and amos2 (G. Li et al., 2012) mutants were recently identified: the AMOS1 locus is identical to EGY1 (ethylene-dependent gravitropism-deficient and yellow-green-like protein1), which encodes a membrane-bound, ATP-independent metalloprotease localized to plastids, required for chloroplast biogenesis (Chen et al., 2005). However, the genetic locus responsible for the amos2 mutation has not been identified. These studies, in combination, provide a significantly improved understanding of the process of NH4+ toxicity in plants.
Here, we report a novel Arabidopsis thaliana mutant, amot1 (ammonium tolerance 1), which displays enhanced shoot growth in response to NH4+ stress. Gene cloning shows amot1 to be allelic to EIN3. Our results demonstrate that the disruption of AMOT1/EIN3 reduces high-NH4+-induced ROS accumulation in leaves, leading to reduced oxidative stress in the shoot. Moreover, AMOT1/EIN3 up-regulates shoot expression of the genes coding for PODs, previously shown to correlate positively with NH4+-induced changes in ROS content and cell growth inhibition.
Materials and methods
Plant materials and growth conditions
Plant materials used in this work included wild-type (WT) A. thaliana L. (Col-0 ecotype) and genetic mutants derived from the Col-0 background. The mutants ein3-1 (Chao et al., 1997), eil1-1 (Alonso et al., 2003), ein3-1eil1-1 (Alonso et al., 2003), EIN3ox (35S:EIN3) (Chao et al., 1997), and 5×EBS:GUS/Col-0 transgenic plants (He et al., 2011) were described previously. The eto1-1 (Alonso and Stepanova, 2004), etr1-3 (Guzmán and Ecker, 1990), and rbohd mutants were obtained from the Arabidopsis Biological Resource Center (ABRC). Seeds were surface-sterilized and cold-treated at 4 °C for 48 h prior to being sown onto standard growth medium. The standard growth medium was described previously (G. Li et al., 2013) and was composed of 2 mM KH2PO4, 5 mM NaNO3, 2 mM MgSO4, 1 mM CaCl2, 0.1 mM Fe-EDTA, 50 μM H3BO3, 12 μM MnSO4, 1 μM ZnCl2, 1 μM CuSO4, 0.2 μM Na2MoO4, 0.5 g l–1 MES, 1% sucrose, and 0.8% agarose (pH 5.7, adjusted with 1 M NaOH). The day of sowing was considered day 0. Seedlings were grown, oriented vertically on the surface of the medium in a growth chamber, under a 16 h light/8 h dark photoperiod, an irradiance of 100 μmol m−2 s−1, and a constant temperature of 23±1 °C. Other chemical treatments were provided as additions to the growth medium, as indicated.
Screening conditions
Transfer DNA (T-DNA) lines were constructed in the laboratories of D. Weigel and C. Somerville using the pSKI15 vector. Approximately 7500 independent lines (stock no. N21995) were provided by the ABRC. After surface sterilization, seeds were sown and grown on vertically oriented growth medium plates. After 5 d, seedlings were transferred to growth medium plates supplemented with 20 mM (NH4)2SO4. Potential NH4+ tolerance mutants were selected after 6 d and rescued, transferred to soil, and allowed to self-fertilize. The homozygous M4amot1 mutant was backcrossed to the WT Col-0, and the resulting F1 generation was crossed with WT Col-0 twice to remove unlinked mutations caused by the mutagenesis.
Thermal asymmetric interlaced PCR
DNA for PCR amplification was extracted according to Weigel and Glazebrook (2002). Plant T-DNA-flanking sequences were amplified by PCR according to the protocols of Rodrigues et al. (2009).
The following primers were used: SKI1, 5'-AATTGGTAATTACTCTTTCTTTTCCTCCATATTGA-3'; SKI2, 5'-ATATTGACCATCATACTCATTGCTGATCCAT-3'; SKI3, 5'-TGATCCATGTAGATTTCCCGGACATGAA-3'; AD1, 5'-TG(AT)G(ACGT)AG(GC)A(ACGT)CA(GC)AGA-3'; AD2, 5'-(ACGT)TCGA(GC)T(AT)T(GC)G(AT)GTT-3'; AD3, 5'-(ACGT)GTCGA(GC)(AT)GA(ACGT)A(AT)GAA-3'; AD4, 5'-AG(AT)-G(ACGT)AG(AT)A(ACGT)CA(AT)AGG-3'; AD5, 5'-(AT)GTG(ACGT)AG-(AT)A(ACGT)CA(ACGT)AGA-3'; and AD6, 5'-(GC)TTG(ACGT)TA(GC)T-(ACGT)CT(ACGT)TGC-3'.
Growth assays
For high-NH4+ stress experiments, 5-day-old seedlings were transferred onto growth medium containing various concentrations of (NH4)2SO4. Following 6 d of treatment, photographs were taken, and relative rosette size and shoot biomass were measured. To study the effect of precursors or inhibitors, the medium was supplemented with NH4+ plus the indicated concentrations of ACC (Sigma), AgNO3 (Shanghai yuanye biotechnology Co. Ltd, Shanghai, China), H2O2 (Shanghai yuanye biotechnology Co. Ltd), or SHAM (Shanghai yuanye biotechnology Co. Ltd). The ratio of average rosette size on NH4+-stressed plates to the average rosette size on control plates was calculated as relative rosette size, according to Lei et al. (2011). The fresh weight of each individual shoot was measured immediately after harvest using a high-precision balance (0.000001) (XP105, Mettler Toledo).
NH4+, H2O2, MDA, and ACC content, and peroxidase and glutamine synthetase activity assay
Shoots (30–50 mg FW) of each sample were washed with 10 mM CaSO4, frozen in liquid nitrogen, and then extracted with 1 ml of 10 mM formic acid for the NH4+ content assay by HPLC, following derivatization with o-phthaldialdehyde (Sigma) as described previously (G. Li et al., 2012). H2O2 content was determined by the POD-coupled assay according to Veljovic-Jovanovic et al. (2002). Arabidopsis shoots were ground in liquid nitrogen, and the powder was extracted in 2 ml of 1 M HClO4 in the presence of insoluble polyvinylpyrrolidone (5%). The homogenate was centrifuged at 12 000 g for 10 min, and the supernatant was neutralized with 5 M K2CO3 to pH 5.6 in the presence of 100 ml of 0.3 M phosphate buffer (pH 5.6). The solution was centrifuged at 12 000 g for 1 min, and the sample was incubated for 10 min with 1 U of ascorbate oxidase (Shanghai yuanye biotechnology Co. Ltd) to oxidize ascorbate prior to use in the assay. The reaction mixture consisted of 0.1 M phosphate buffer (pH 6.5), 3.3 mM DMAB (Shanghai yuanye biotechnology Co. Ltd), 0.07 mM MBTH (Shanghai yuanye biotechnology Co. Ltd), and 0.3 U of POD (Shanghai yuanye biotechnology Co. Ltd). The reaction was initiated by the addition of the sample. The absorbance change at 590 nm was monitored at 25 °C. The malondialdehyde (MDA) level was measured using a thiobarbituric acid-reactive substance (TBARS) assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) (Ren et al., 2015). Shoot ACC content was detected by negative ion chemical ionization (NICI) GC-MS (Schellingen et al., 2014). Data are expressed in nmol g–1 FW. POD activity was detected by a micro-POD assay kit (BC0095, Solarbio, Beijing, China) according to the manufacturer’s recommendations. POD activity is expressed as U mg–1 based on protein content. Glutamine synthetase (GS) activity was detected by a GS kit (BC0910, Solarbio) (Zhao et al., 2017). The specific enzyme activity (U g–1 FW) was defined as the amount of enzyme units catalyzing the transformation of 1 μM substrate per minute by the amount of fresh weight in grams.
Real-time quantitative PCR analysis
Total RNA was extracted from Arabidopsis shoots. Gene sequences were provided by the National Center of Biotechnology Information (NCBI), and gene-specific primers for real-time quantitative PCR (qRT-PCR) were designed using Primer-5 software (see Supplementary Table S1 at JXB online). CBP20 (nuclear-encoded cap-binding protein) and ACTIN2 were used as the internal reference genes, and relative RNA abundance was normalized to the CBP20 or ACTIN2 internal control ([mRNA]gene/[mRNA]CBP20 or [mRNA]gene/[mRNA]ACTIN2).
Histochemical staining and image analysis
Histochemical staining of H2O2 was performed as previously described (Dong et al., 2009) with minor modifications. Shoots were vacuum-infiltrated with 0.1 mg ml–1 3,3′-diaminobenzidine (DAB) in 50 mM Tris-acetate buffer, at pH 5.0. Samples were incubated for 24 h at room temperature in the dark prior to transfer to 80% ethanol. Histochemical analysis of β-glucuronidase (GUS) reporter enzyme activity was performed as described by Weigel and Glazebrook (2002). GUS or H2O2 staining in the shoot was carried out using an Olympus SZX10 stereo microscope. Intensities of the GUS- and DAB-stained zone were quantified using Image-J software. All staining and image analysis procedures were repeated at least twice.
Ethylene measurements
After seedling exposure to 40 mM NH4+ for varying durations, as indicated, shoots from the control and treatments were weighed and put into 5 ml gas-tight vials containing 1 ml of agar medium (0.7% agar). Headspace samples (1 ml) were withdrawn and analyzed using a GC-6850 gas chromatograph (Agilent Technologies Japan, Ltd), which was equipped with an FID detector.
Yeast one-hybrid (Y1H) analysis
Promoter fragments from At1g49570 (2217 bp) and At5g19890 (1692 bp) were cloned into the pAbAi vector to produce the bait constructs pAbAi-At1g49570 and pAbAi-At5g19890, respectively. The coding seuqence (CDS) of AMOT1/EIN3 was fused to the pGADT7 vector to generate a prey construct, AD-EIN3. The bait construct and the empty vector (AD) served as the negative control; p53-AbAi/pGAD-p53 were used as a positive control and transformed separately into yeast cells. Transformed yeast cells were diluted with a 10× dilution series and dotted onto SD plates lacking Ura and Leu (with or without antibiotic).
Statistical and graphical analyses
For all experiments, data were statistically analyzed using the SPSS 13.0 program (SPSS Chicago, IL, USA). Details are shown in the figure legends. Graphs were produced using Origin 8.0. All graphs and images were arranged using Adobe Photoshop 7.0.
Results
Enhanced tolerance of the amot1 mutant to ammonium toxicity
Under our growth conditions, NH4+, at concentrations of 20–30 mM, caused slight reductions in shoot size and biomass (Supplementary Fig. S1). A concentration of 40 mM significantly inhibited shoot size and biomass (Supplementary Fig. S1). To explore the mechanisms of NH4+-induced shoot growth inhibition, we performed a forward genetic screen for seedlings that show a shoot phenotype that was more resistant than WT plants when grown on medium containing 40 mM NH4+. Seedlings that appeared similar to the WT without NH4+ but displayed significantly higher resistance of shoot growth to NH4+ were amot (ammonium tolerance) mutants. We present the characterization of the amot1 mutant.
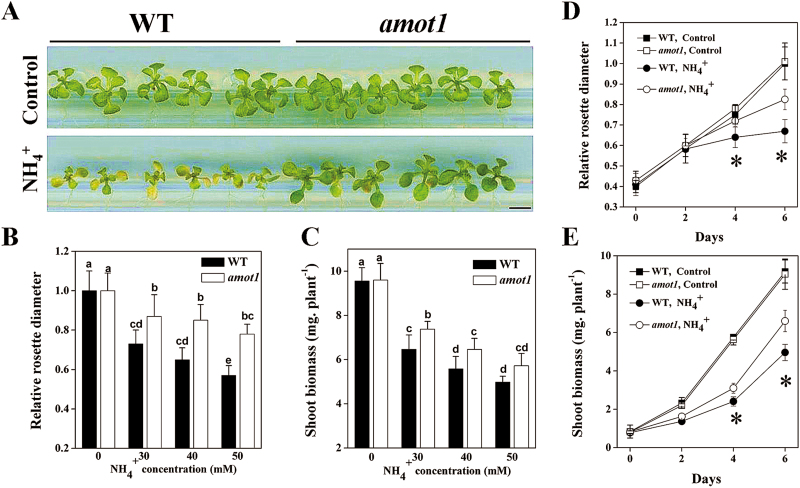
In agar plates without NH4+, the amot1 shoot growth phenotype was indistinguishable from that of the WT (Fig. 1A). However, when grown in the presence of high NH4+, amot1 shoot growth displayed greater resistance to NH4+ than the WT (Fig. 1A).
Fig. 1.
Isolation and characterization of the ammonium-tolerant amot1 mutant. (A) Appearance of Arabidopsis thaliana wild-type (WT) and amot1 mutant plants following treatment with NH4+. Five-day-old plants were transferred to control and 40 mM NH4+ concentration for 6 d, and then pictures were taken. Scale bars=0.5 cm. (B and C) Relative rosette diameter and fresh shoot weight of A. thaliana WT and amot1 mutant plants following treatment with various NH4+ concentrations for 6 d. The rosette diameter on control nutrient solution was considered as 1. Values are the means ±SD, n=8–11. Different letters indicate statistical differences at P<0.05 (one-way ANOVA with Duncan post-hoc test). (D and E) Relative rosette diameter and fresh shoot weight of A. thaliana WT and amot1 mutant plants following treatment with 40 mM NH4+ for 0, 2, 4, and 6 d. Values are the means ±SD, n=6–10. Asterisks indicate statistical differences between the WT and amot1 under NH4+ treatment at the indicated times (independent samples t-test, *P<0.05). (This figure is available in color at JXB online.)
NH4+-treated WT and amot1 plants showed a dose-dependent inhibitory effect of NH4+ on the growth of aerial parts in response to a range of NH4+ concentrations, but WT shoot growth was inhibited more than in amot1 at the concentrations used (Fig. 1B, C). Furthermore, we analyzed the shoot phenotypes of amot1 and WT seedlings in response to 40 mM NH4+ over time. The shoot growth between WT and amot1 seedlings remained similar 2 d after NH4+ addition, but the difference was clearly accentuated under prolonged NH4+ treatment, with the mutant maintaining significantly higher growth rates (Fig. 1D, E). Considering these results together, amot1 emerges as the first NH4+-resistant mutant, displaying superior shoot growth.
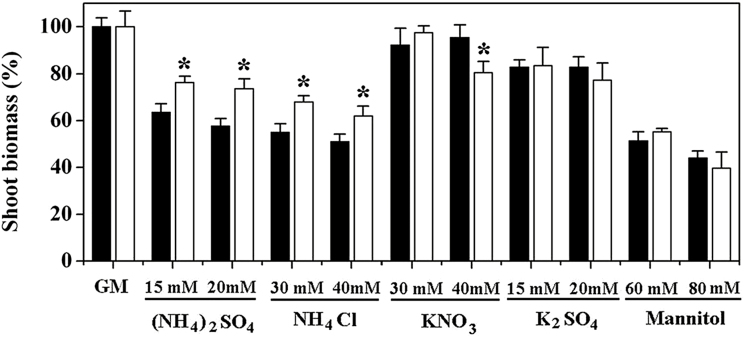
The WT and amot1 seedlings were also treated on medium enriched with a variety of ions and molecules, and the results indicate that amot1 seedlings are highly resistant to both (NH4)2SO4 and NH4Cl, but responded to 15 mM and 20 mM K2SO4 or 60 mM and 80 mM mannitol in a similar pattern to the WT (Fig. 2).
Fig. 2.
Specificity of the amot1 mutant to NH4+. WT and amot1 seedlings were grown for 5 d on growth medium (GM) and then transferred to medium supplemented with salts and osmotica as indicated. Shoot fresh weight was measured 6 d after transfer. Growth on control nutrient (GM) was considered as 100%. Values are the means ±SD, n=10–14. Asterisks indicate statistical differences between the mutant and WT (independent samples t-test, *P<0.05).
The amot1 mutant is a novel ein3 allele, and loss of EIN3 function enhances ammonium tolerance
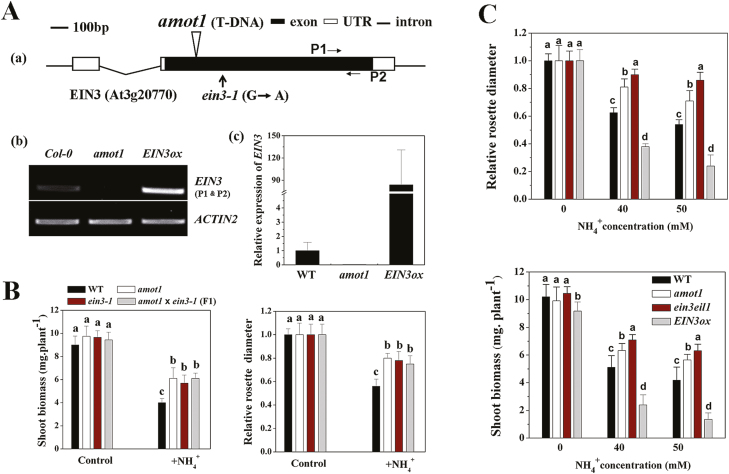
The WT as female parent was crossed with the homozygous mutant as the pollen donor. F1 plants were selfed to obtain F2 seeds. Both F1 and F2 seeds were assayed for growth on NH4+ medium. All examined F1 progeny (45 seedlings) displayed the same phenotypes as the WT. In the F2 population, the amot1 phenotype segregated at an approximate 1:3 ratio (amot1:WT=54:142; x2=0.55, P>0.05), indicating that amot1 is a recessive mutation at a single nuclear locus. T-DNA-flanking sequences were isolated from the mutant by thermal asymmetric interlaced PCR, and sequence analysis revealed that the pSKI15 T-DNA was inserted into the exon of the EIN3 gene (At3g20770) in amot1, 192 bp downstream of the start codon, ATG [Fig. 3A(a)]. EIN3 gene transcripts were greatly reduced in amot1 compared with the WT [Fig. 3A(b) and (c)]. To ascertain further whether the NH4+-resistant phenotype in amot1 is due to the mutation in the EIN3 gene, we analyzed the previously reported allele ein3-1 (Chao et al., 1997) and crossed the amot1 mutant with ein3-1 plants. With exposure to 40 mM NH4+, shoot size and biomass in ein3-1 seedlings were indeed similar to those of amot1 seedlings (Fig. 3B). Furthermore, the amot1 mutant was crossed to ein3-1, and the F1 progeny showed a phenotype similar to that of the parents in the presence of NH4+ (Fig. 3B). Collectively, these results show that the amot1 mutant is a new loss-of-function allele of the EIN3 gene.
Fig. 3.
Molecular characterization of the Arabidopsis thaliana amot1 mutant. (A) (a) Diagram illustrating the genomic coding sequence of the Arabidopsis AMOT1/EIN3 gene and the locations of the mutant alleles amot1 and ein3-1. UTR, untranslated region. (b) RT-PCR analysis of EIN3 transcripts in WT, EIN3ox, and the amot1 mutant plants. The ACTIN2 gene was used as an internal control. (c) Expression of EIN3 in WT, EIN3ox, and the amot1 mutant plants analyzed by qRT-PCR analysis. Values are means ±SD of three replicates. ACTIN2 was used as the internal reference gene, and EIN3 expression of the WT was considered as 1. (B) amot1 is allelic to the ein3-1 mutant. WT, amot1, ein3-1, and F1 progeny from crosses between amot1 and ein3-1 were grown on 40 mM NH4+ for 6 d. The relative rosette diameter on control nutrient solution was considered as 1. Values are the means ±SD, n=7–12. (C) Effect of various NH4+ concentrations on relative rosette diameter, and fresh shoot weight of WT, ein3eil1, amot1, and EIN3ox seedlings. Five-day-old plants were transferred to control and 40 mM NH4+ concentration for 6 d. Values are the means ±SD, n=5–8. Different letters indicate statistical differences at P<0.05 (one-way ANOVA analysis with Duncan post-hoc test). (This figure is available in color at JXB online.)
AMOT1/EIN3 is a member of a protein family that includes EIN3-like (EIL) proteins (Chao et al., 1997) and initiates transcriptional re-programming in various ethylene responses (Guo and Ecker, 2004; Peng et al., 2014). We sought to determine the role of AMOT1/EIN3 in NH4+ resistance. Because AMOT1/EIN3 and its close homolog EIL1 functionally overlap (Chao et al., 1997; An et al., 2010), we examined the ein3-1eil1-1 double mutant seedling response under various NH4+ concentrations. Under high NH4+, ein3-1eil1-1 had superior tolerance to NH4+ compared with the WT (26% reduction in ein3-1eil1-1 versus 52% in the WT at 40 mM NH4+) (Fig. 3C). Furthermore, in contrast to the ein3 single mutant’s tolerant phenotype, the ein3-1eil1-1 double mutant exhibited a more tolerant phenotype than the amot1 mutant (Fig. 3C). We also examined the phenotype of the eil1 single mutant upon treatment with high NH4+; however, the eil1-1 mutant was similar to the WT under high NH4+ (Supplementary Fig. S2). Next, we examined the NH4+-responsive phenotype of a transgenic line overexpressing EIN3 under the control of the 35S promoter (35S:EIN3/Col-0 or EIN3ox), which displays an enhanced ethylene response (Chao et al., 1997; An et al., 2010; Z. Li et al., 2013). The transcripts of AMOT1/EIN3 were significantly increased in the EIN3ox seedlings [Z. Li et al., 2013; Fig. 3A(b) and (c)]. Under high NH4+, EIN3ox plants displayed increased sensitivity, based on shoot size and biomass, when compared with their WT counterparts (73% shoot biomass reduction in EIN3ox plants versus 52% in the WT at 40 mM NH4+) (Fig. 3C). Together, these results suggest that constitutive overexpression of AMOT1/EIN3 leads to elevated shoot NH4+ sensitivity in Arabidopsis. Consistent with a previous report (G. Li et al., 2013), the ein3eil1 lateral root number was also more resistant than that of the WT to high-NH4+ stress (Supplementary Fig. S3).
Enhanced shoot ethylene evolution is involved in ammonium-mediated inhibition of shoot growth
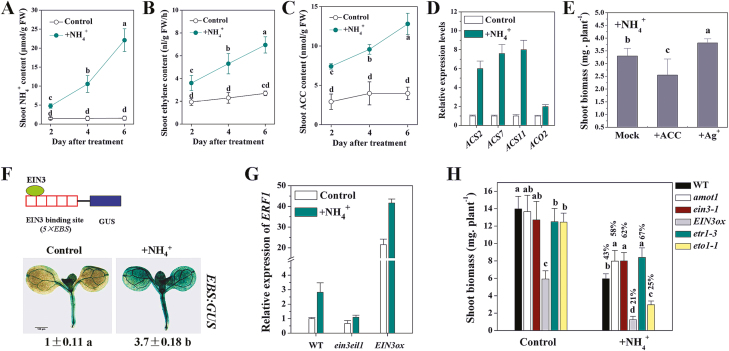
Aerial tissue NH4+ content was determined, and the NH4+ content increased gradually with treatment time compared with that in untreated shoots (Fig. 4A). Shoot ethylene production under NH4+ exposure was also significantly greater than without NH4+ and increased linearly with NH4+ treatment time (Fig. 4B), consistent with Barker (1999b). Ethylene is synthesized from SAM via ACC, which is catalyzed by the enzyme ACS (Adams and Yang, 1979). ACC amounts in untreated and treated seedlings are presented in Fig. 4C. Consistent with previous reports, ACC amounts increased linearly with NH4+ treatment time. As ACS and ACO are key enzymes of the ethylene biosynthetic pathway in plants, AtACS2, AtACS7, AtACS11, and AtACO2 expression was examined. Expression of the four genes was rapidly up-regulated in response to high NH4+ (Fig. 4D).
Fig. 4.
Effects of ethylene on shoot growth tolerance to NH4+. (A) NH4+ content in Arabidopsis shoots for the duration of the NH4+ treatment. (B) Ethylene evolution in Arabidopsis shoots for the duration of the NH4+ treatment. (C) 1-Aminocyclopropane-1-carboxylic acid (ACC) content in Arabidopsis shoots for the duration of the NH4+ treatment. Seedlings at 5 d after germination were exposed to NH4+ for varying treatment times, and NH4+ content (A), ethylene evolution (B), and ACC content were determined. Values are means ±SD of three replicates. Different letters indicate statistical differences at P<0.05 (one-way ANOVA with Duncan post-hoc test). (D) Effect of NH4+ treatment on shoot ACS and ACO genes expression by qRT-PCR for 6 h. Values are means ±SD of three replicates. CBP20 was used as the internal reference gene, and the control was considered as 1. (E) Effect of supplied ethylene inhibitors 30 μM AgNO3 and 25 μM ACC on shoot biomass of WT seedlings grown in 40 mM NH4+ treatment medium. Values are the means ±SD, n=10–12. (F) Schematic diagram of the EIN3 activity reporter system showing the EIN3 protein, five tandem repeats of the EBS (5×EBS), and the GUS gene. Expression of 5×EBS:GUS in leaves of the WT under control conditions and 24 h NH4+ treatment. One representative sample from each treatment (10 plants) is shown. GUS staining intensity was quantified using Image J software, and the control was considered as 1. Values are means ±SD of three replicates. (G) Effect of NH4+ treatment on shoot ERF1 gene expression of WT, ein3eil1, and EIN3ox lines by qRT-PCR for 6 h. Values are means ±SD of three replicates. ACTIN2 was used as the internal reference gene, and the WT control was considered as 1. (H) Effect of NH4+ treatment for 6 d on shoot fresh weight of WT, amot1, ein3-1, EIN3ox, etr1-3, and eto1-1 seedlings. Values are the means ±SD, n=12. Different letters indicate statistical differences at P<0.05 of control and NH4+ treatment, respectively (one-way ANOVA with Duncan post-hoc test).
We further investigated the activity of AMOT1/EIN3 in response to NH4+ in shoot. A transgenic reporter line harboring the GUS gene driven by five tandem repeats of the EIN3-binding site (EBS) followed by a minimal 35S promoter (5×EBS:GUS/Col-0) has been used to monitor the transcriptional activity of EIN3 (Stepanova et al., 2007; He et al., 2011). Following NH4+ treatment, GUS staining became intensified in the cotyledons of 5×EBS:GUS/Col-0 plants (Fig. 4F), indicative of elevated levels of AMOT1/EIN3 activity. We also observed that the expression of the ethylene-responsive gene ERF1 was up-regulated by NH4+ in the WT (Fig. 4G). In keeping with the results on AMOT1/EIN3 activity, expression of ERF1, a direct target gene of EIN3 (Solano et al., 1998), was also lower in the NH4+-treated ein3eil1 mutant, but higher in EIN3ox lines, compared with the WT (Fig. 4G).
The WT plants treated with the ethylene biosynthetic precursor ACC displayed decreased tolerance to NH4+ (Fig. 4E). Consistent with this, the ethylene overproduction mutant eto1-1 (ethylene overproducer 1) also showed reduced NH4+ tolerance compared with the WT (Fig. 4H). In the presence of the ethylene receptor antagonist Ag+, shoot growth of the WT was significantly increased when the plants were exposed to NH4+ stress (Fig. 4E). As ethylene is known to activate downstream signaling pathways by binding to ethylene receptors (e.g. ETR1), we examined whether ethylene regulates shoot growth sensitivity to NH4+ in such a way. Shoot growth in the ethylene-insensitive (ethylene receptor) mutant etr1-3 and positive regulator mutants in ethylene signaling, amot1 and ein3-1, was more tolerant to NH4+ than that of the WT; consistent with this, EIN3ox lines displayed increased shoot growth sensitivity (Fig. 4H). These results indicate that ethylene has a negative effect on NH4+ tolerance in Arabidopsis shoot growth.
AMOT1/EIN3 regulates ammonium-induced ROS accumulation in shoots
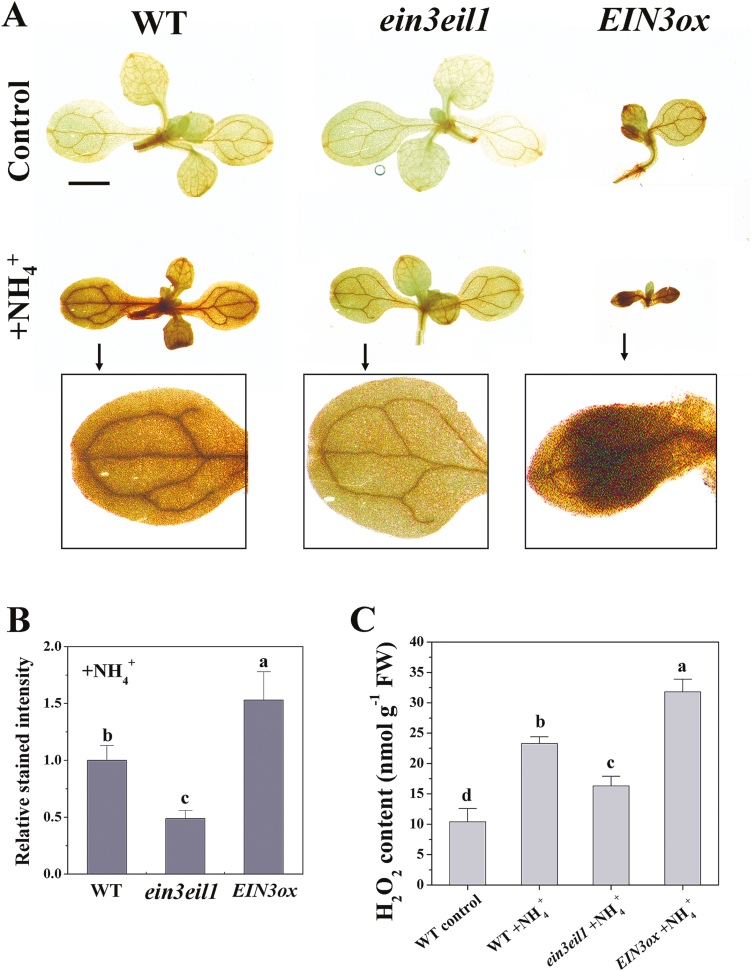
High NH4+ induces an increase in ROS in plants; however, the biological mechanism of NH4+-induced ROS accumulation remains largely unknown. Here, we examined the levels of endogenous H2O2 in the WT, ein3eil1, and EIN3ox in response to high-NH4+ treatment. NH4+ stress increased H2O2 accumulation in the cotyledons of the WT, indicated by DAB staining (Fig. 5A, B). We further found higher levels of DAB staining in EIN3ox but lower levels in ein3eil1 than in the WT following NH4+ stress (Fig. 5A, B), in accordance with the NH4+ tolerance phenotypes of these genotypes (Fig. 3C). We also measured the H2O2 contents in shoots. As inferred from DAB staining assays, high-NH4+ treatment induced accumulation of H2O2 in the WT. However, the level was lower in ein3eil1 and higher in EIN3ox shoots compared with Col-0 under NH4+ stress (Fig. 5C). The H2O2 content under control conditions was not significantly different among the three ecotypes (Supplementary Fig. S4). A split-shoot experiment was devised to examine further the relationship between NH4+-induced AMOT1/EIN3 transcriptional activity and ROS accumulation (Supplementary Fig. S5A). The cotyledon supplied with NH4+ displayed significantly increased EBS:GUS expression compared with the untreated cotyledon (Supplementary Fig. S5B). Consistent with EBS:GUS inductive sites, a higher intensity of DAB staining was also detected in the NH4+-treated cotyledon (Supplementary Fig. S5C).
Fig. 5.
Effects of EIN3 on NH4+-induced H2O2 accumulation in shoots. (A) In situ detection of WT, ein3eil1, and EIN3ox leaf H2O2. Seedlings at 5 d were exposed to 40 mM NH4+ for 3 d, and then DAB staining of shoots was performed. Scale bars=1 mm. The inserts show images of partial enlargement, as indicated by arrows. (B) The mean relative DAB staining intensity in the WT, ein3eil1, and EIN3ox of the NH4+-treated shoots in (A), and the WT was considered as 1. Values are the means ±SD, n=10–15. (C) H2O2 concentration in the WT, EIN3ox, and ein3eil1 shoot tissue. Seedlings at 5 d were exposed to 40 mM NH4+ for 3 d, and the contents of H2O2 were determined as described in the Materials and methods. Values are means ±SD of three replicates. Different letters indicate statistical differences at P<0.05 (one-way ANOVA with Duncan post-hoc test).
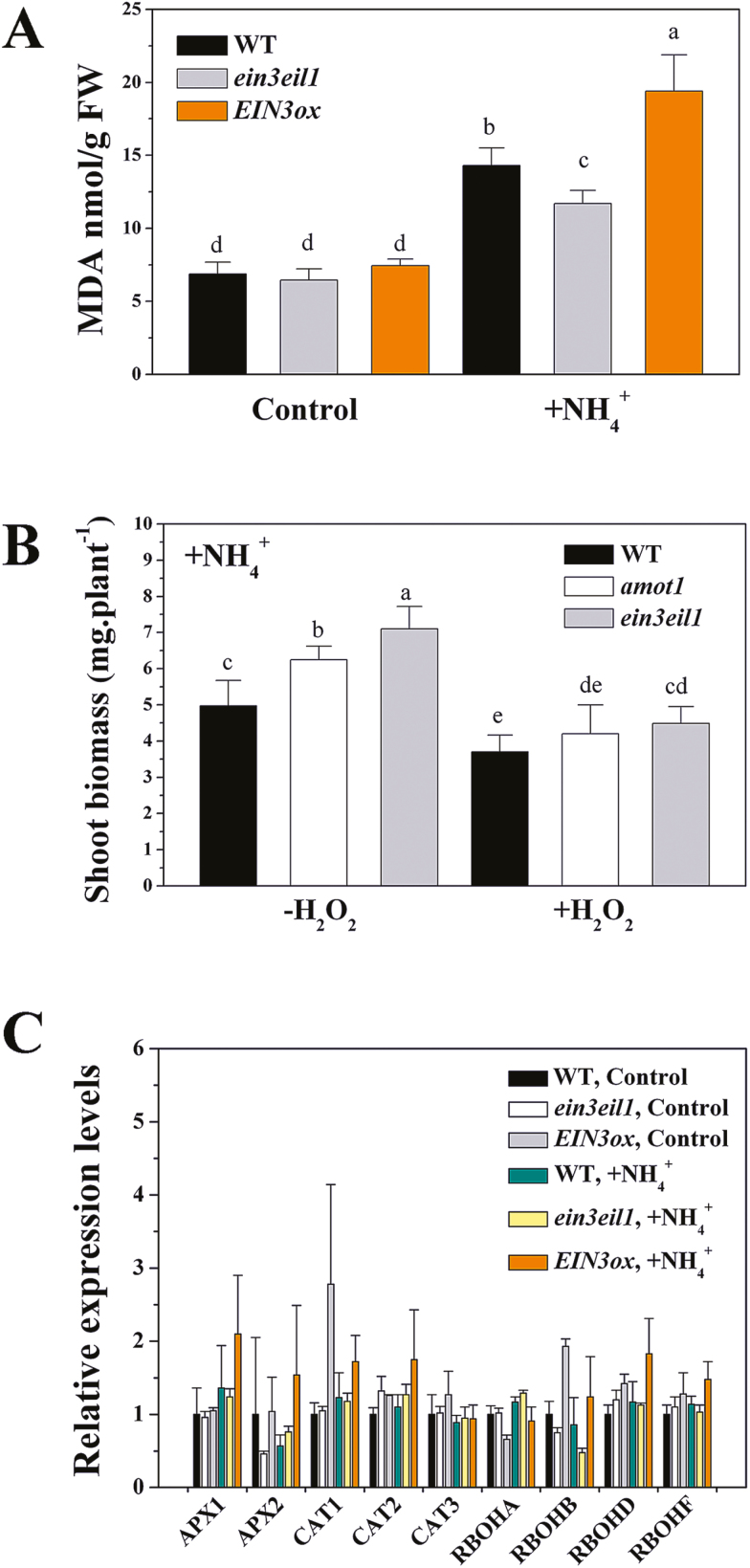
In this study, the concentration of MDA equivalents was increased in NH4+-treated leaves (Fig. 6A). However, we observed a higher MDA level in EIN3ox, and a lower level in ein3eil1 than in the WT following NH4+ stress (Fig. 6A). The effects of NH4+ on shoot growth were also examined in combination with external H2O2. The combined treatment with NH4+ and H2O2 in the medium markedly inhibited shoot growth compared with NH4+ alone, and the combined treatment inhibited shoot growth more significantly in amot1 and ein3eil1 (Fig. 6B).
Fig. 6.
Effects of EIN3 on NH4+-induced lipid peroxidation in shoots and related gene expression. (A) Lipid peroxidation (MDA content) in the WT, ein3eil1, and EIN3ox shoot tissue. Seedlings at 5 d were exposed to 40 mM NH4+ for 6 d. Values are means ±SD of three replicates. (B) Effect of external H2O2 on shoot biomass of WT, ein3eil1, and amot1 plants under NH4+ treatment. Five-day-old seedlings were transferred to medium supplemented with NH4+ alone or in combination with 2 mM H2O2 for 6 d. n=7–10. (C) Effect of NH4+ on gene expression of WT, EIN3ox, and ein3eil1 shoot tissue by qRT-PCR for 6 h. Values are means ±SD of three replicates. ACTIN2 was used as the internal reference gene, and the WT control was considered as 1. Different letters indicate statistical differences at P<0.05 (one-way ANOVA with Duncan post-hoc test).
We further examined the expression of genes encoding antioxidant metabolic enzymes, such as APX1, APX2, CAT1, CAT2, and CAT3. NH4+ stress did not induce APX and CAT gene expression in WT shoots, and these genes were also not much affected in ein3eil1 and EIN3ox seedlings under NH4+ stress (Fig. 6C), suggesting that AMOT1/EIN3 regulation of NH4+-induced ROS accumulation might not be related to APX1-, APX2-, CAT1-, CAT2-, and CAT3-mediated antioxidant activity. Previous studies showed that drought stress increases RBOH transcript levels (Lee et al., 2012). However, the expression patterns of RBOHA, RBOHB, RBOHD, and RBOHF under control and high-NH4+ stress were similar, and they were also not much affected in the ein3eil1 mutant and in EIN3ox lines compared with the WT (Fig. 6C). Furthermore, DAB staining and shoot growth in response to high-NH4+ stress were also similar between the WT and the rbohd mutant (Supplementary Fig. S6).
AMOT1/EIN3 induces the transcription of peroxidases and increases their activity
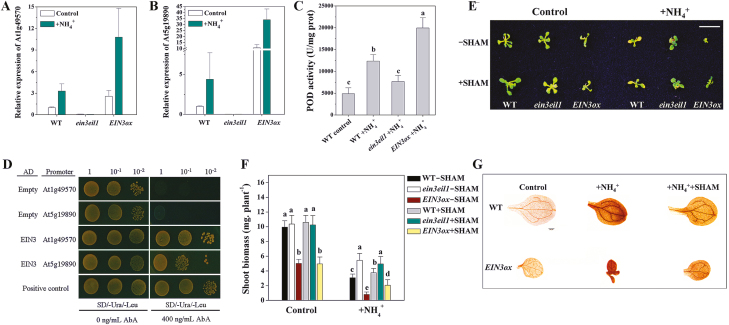
Podgórska et al. (2015) proposed that higher POD levels are positively correlated with NH4+-induced ROS generation and cell growth inhibition. We found that the expression of two of the genes encoding PODs, At5g19890 and At1g48570, was induced by NH4+ treatment in the WT, and expression was more elevated in EIN3ox while it was reduced in ein3eil1 with or without NH4+ (Fig. 7A, B). The transcript levels of other POD-encoding genes, such as At5g42180, At2g18980, At4g11290, and At3g49960, were not increased by NH4+ treatment in the WT, and were also not significantly altered in EIN3ox and ein3eil1 seedlings, under either normal or NH4+ stress conditions (Supplementary Fig. S7). The POD activity assay also showed that POD activity was significantly elevated in EIN3ox seedlings compared with the WT and ein3eil1 under NH4+ stress (Fig. 7C). POD activity in EIN3ox seedlings under control conditions was also slightly elevated compared with the WT and with ein3eil1 (Supplementary Fig. S8). We next analyzed the promoter regions of two POD genes (At5g19890 and At1g48570) and found EBSs (ATGTA) in each promoter (data not shown). To test the interaction between the AMOT1/EIN3 protein and the At5g19890 and At1g48570 promoters, a Y1H assay was performed. As shown in Fig. 7D, bait yeast cells co-transformed with the empty vector (AD) or the fusion vector (AD-EIN3) grew well on synthetic dropout medium (SD) without Ura and Leu. However, only the yeast cells co-transformed with the fusion vector AD-EIN3 survive on the selective medium supplemented with 400 ng ml–1 aureobasidin A (AbA; Fig. 7D). The data suggest that the AMOT1/EIN3 protein interacts with the At5g19890 and At1g48570 promoters in the yeast system. SHAM is a widely used POD inhibitor (Balzergue et al., 2017). The shoot biomass of the WT and EIN3ox lines was increased under NH4+ stress after SHAM treatment, but no effects on ein3eil1 lines were observed (Fig. 7E, F). Furthermore, the POD inhibitor SHAM could decrease NH4+-induced DAB staining, indicating H2O2 accumulation in both WT and EIN3ox leaves (Fig. 7G; Supplementary Fig. S9).
Fig. 7.
EIN3 increases activity of PODs through transcriptional regulation of POD genes. (A and B) qRT-PCR analysis of the expression of two POD genes (At5g19890 and At1g48570) in WT, EIN3ox, and ein3eil1 shoot tissue after NH4+ treatment for 6 h. Values are means ±SD of three replicates. ACTIN2 was used as the internal reference gene, and the WT control was considered as 1. (C) Measurement of POD activity of WT, EIN3ox, and ein3eil1 shoot tissue. Seedlings were exposed to 40 mM NH4+ for 5 d. Values are means ±SD of three replicates. (D) Y1H assay showing the EIN3 binding to the promoter of the two POD genes. The yeast expression plasmid pGADT7-EIN3 was reintroduced into the yeast strain Y1H Gold carrying the pAbAi-At1g49570 or pAbAi-At5g19890 vectors. The transformants (with or without dilutions) were screened for their growth on yeast synthetic defined medium (SD/-Ura -Leu) in the presence of 400 ng ml–1 AbA (antibiotic) for stringent selection. The empty vectors pGADT7 and p53-AbAi/pGAD-p53 were used as a negative and positive control, respectively. (E and F) Effect of salicylhydroxamic acid (SHAM) on the shoot biomass of the WT, EIN3ox, and ein3eil1. Five-day-old seedlings were transferred to medium alone or in combination with 10 μM SHAM for 6 d. A photograph of representative seedlings is shown in (E). Scale bars=1 cm. The shoot biomass is shown in (F). Values are the means ±SD, n=12. Different letters indicate statistical differences at P<0.05 of control and NH4+ treatment, respectively (one-way ANOVA with Duncan post-hoc test). (G) Effect of SHAM on the NH4+-induced H2O2 accumulation in shoots of WT and EIN3ox. Seedlings at 5 d were exposed to 40 mM NH4+ with or without 10 μM SHAM for 3 d, and then DAB staining of shoots was performed. Scale bars=200 μm.
The amot1 mutant accumulates less NH4+ in shoot tissue under NH4+ toxicity
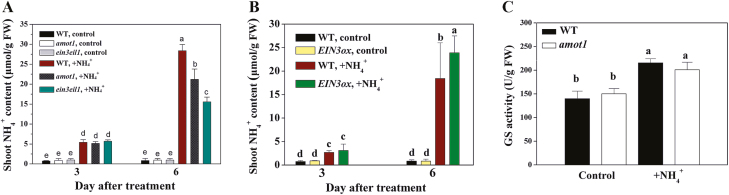
There was no difference in shoot NH4+ content of the WT, amot1, and ein3eil1 with 3 d of NH4+ treatment (Fig. 8A), although ROS accumulation was greater in WT shoots than in those of ein3eil1 at this treatment time (Fig. 5). However, higher levels of NH4+ in the WT, but lower levels in amot1 and ein3eil1, than in the WT were recorded following high-NH4+ stress during a 6 d treatment (Fig. 8A). Consistently, NH4+ accumulation was slightly higher in EIN3ox shoots than in the WT following the treatment (Fig. 8B). Activities of the enzyme GS, centrally involved in the NH4+ assimilation process (Kronzucker et al., 1995; Hirano et al., 2008), was determined in shoots of the WT and amot1. GS activity was induced by external NH4+ in NH4+-fed WT and amot1 plants, but this increase was not significantly different in the two genotypes (Fig.8C). This indicates that NH4+ metabolism was not affected by the AMOT1 mutation.
Fig. 8.
Effects of NH4+ treatment on shoot NH4+ content and GS activity. (A) NH4+ contents in the shoot tissues of WT, ein3eil1, and amot1 seedlings. Five-day-old WT, ein3eil1, and amot1 seedlings were grown on growth medium and transferred to fresh medium with control or NH4+ for 3 d and 6 d of growth, and then NH4+ tissue content was determined. Values are means ±SD of three replicates. (B) NH4+ contents in the shoot tissues of WT and EIN3ox seedlings. Five-day-old WT and EIN3ox seedlings were grown on growth medium and transferred to fresh medium with control or NH4+ for 3 d and 6 d of growth, and then NH4+ tissue content was determined. Values are means ±SD of three replicates. (C) GS activities in the shoots of WT and amot1 seedlings. Five-day-old WT and amot1 seedlings were grown on growth medium and transferred to fresh medium with control or NH4+ for 6 d, and then GS activity was determined. Values are means ±SD of three replicates. Different letters indicate statistical differences at P<0.05 (one-way ANOVA with Duncan post-hoc test).
Discussion
Stunted root system and decreased leaf bomass are among the most visible phenotypic manifestations of NH4+ toxicity in higher plants (Britto and Kronzucker, 2002). Several genetic regulators controlling root sensitivity to NH4+ have been identified in Arabidopsis (Li et al., 2014); however, little is known about the specific targets and pathways that lead to impaired leaf growth in the context of NH4+ toxicity. To gain insight into the mechanisms of the effects of NH4+ on shoot growth, we employed a molecular genetics approach, based on a mutant screen for altered response to NH4+. In the current work, enhanced NH4+ tolerance of shoot growth was found in amot1. We further revealed that the nuclear AMOT1 locus is identical to EIN3, which encodes a transcriptional activator required for initiating transcriptional re-programming in various ethylene responses (Guo and Ecker, 2004; Yoo et al., 2009). It was found that amot1 and ein3-1, a reported allele, showed enhanced shoot growth tolerance compared with the WT, but the transgenic line overexpressing EIN3 (EIN3ox) was more sensitive. The activity of AMOT1/EIN3, indicated by using EBS:GUS in shoots, was markedly enhanced on NH4+ (Fig. 4F). These results suggest that AMOT1/EIN3 plays an important role in the NH4+-induced impairment of shoot growth. It was demonstrated furthermore that this inhibitory effect is related to enhanced shoot ACC and ethylene accumulation. More importantly, it was found that AMOT1/EIN3 positively regulates shoot ROS accumulation, which leads to oxidative stress in Arabidopsis shoots under NH4+ stress, and up-regulates the shoot expression of the genes coding for PODs previously shown to correlate positively with NH4+-induced increases in ROS content and cell growth inhibition (Podgórska et al., 2015).
A role for ethylene evolution has long been suggested under NH4+ excess (Barker and Corey, 1991; Barker, 1999a, b), but its involvement remains incompletely understood. The present data indicate that ethylene evolution increases linearly with NH4+ treatment time (Fig. 4B), consistent with previous reports (Barker 1999b). Foliar ethylene evolution increased sharply in tomato when foliar NH4+ accumulation passed a critical value (Barker, 1999a). Our present and previous data also link the stimulation of EBS:GUS activity to NH4+ exposure (G. Li et al., 2013; Supplementary Fig. S5). The present data show that shoot NH4+ content increases linearly with increased treatment time (Fig. 4A). Furthermore, a previous observation showed high ethylene evolution to correlate with high tissue NH4+ but to be independent of nitrogen form and pH regime (Feng and Barker, 1992c). Hence, together with previous reports, our study suggests that greatly increased shoot NH4+ content may be the intrinsic trigger leading to enhanced ethylene evolution under NH4+ stress. The rate-limiting step in ethylene biosynthesis lies in the production of ACC by ACS (Schellingen et al., 2014). Shoot ACC amounts also increased linearly with NH4+ treatment time (Fig. 4C). Furthermore, we show here that AtACS2, AtACS7, AtACS11, and AtACO2, which encode ACS and ACO, the two key enzymes responsible for ethylene synthesis, are transcriptionally up-regulated by NH4+ treatment (Fig. 4D). Therefore, it is conceivable that the increased ACC biosynthesis resulting from up-regulation of ACS and ACO gene expression is involved in NH4+-induced ethylene evolution. Further study on the detailed mechanisms of NH4+-regulated ethylene evolution is warranted.
In our study, we provide several lines of evidence supporting the notion that ethylene biosynthesis and signaling play a negative role in the adaptation of Arabidopsis shoot growth to NH4+ stress. ACC content, ethylene production, and AMOT1/EIN3 activity, and the expression of genes encoding key enzymes responsible for ethylene synthesis in the Arabidopsis shoot showed dramatic increases after NH4+ treatment. The dual evidence that shoot biomass was inhibited by NH4+ to a greater extent in the ethylene overproduction mutant eto1-1 and the EIN3ox line, compared with the WT, and that mutations in ethylene receptors (e.g. etr1-3) and key positive regulators in ethylene signaling (e.g. amot1 and ein3-1) showed increased shoot growth compared with the WT under NH4+ stress (Figs 3, 4H), supports this notion. The observations that the externally supplied ethylene inhibitor Ag+ alleviated the phenotypic manifestation of toxicity, but that ACC, a precursor of ethylene, aggravated NH4+-suppressed shoot growth in the WT (Fig. 4E), further demonstrate the important role of shoot ethylene signaling. Our findings are in excellent agreement with a previous study showing that Ag+ improved plant growth on NH4+ (Barker and Corey, 1991). Therefore, the data suggest that ethylene biosynthesis and signaling negatively regulate NH4+ tolerance of shoot growth in Arabidopsis.
The underlying mechanisms determining ROS accumulation by NH4+ in leaves are only partially understood (Bittsánszky et al., 2015). Our results show that AMOT1/EIN3 is involved in H2O2 metabolism in leaves under NH4+ stress. First, a higher level of H2O2 cytochemical staining in EIN3ox was found, while a lower level of H2O2 staining was seen in ein3eil1 than in the WT following NH4+ stress (Fig. 5A, B), in accordance with the NH4+ tolerance phenotypes and lipid peroxidation profiles of these genotypes (Figs 3, 6A). In agreement with the above results, NH4+ stress increased leaf H2O2 concentrations in the WT, while these were lower in ein3eil1 and higher in EIN3ox under identical treatment conditions (Fig. 5C). Moreover, our split-shoot experiment showed that a higher DAB staining intensity was detected in the components of EBS:GUS cotyledons exposed to NH4+ (Supplementary Fig. S5). These results suggest that AMOT1/EIN3 positively regulates NH4+-induced leaf H2O2 accumulation. Investigations on whether oxidative stress is involved in NH4+ phytotoxicity have led to conflicting conclusions. The results of Domínguez-Valdivia et al. (2008) in spinach and pea suggest that stress originating from applying NH4+ as the only nitrogen source is not an oxidative stress. However, evidence is accumulating that NH4+ can induce oxidative stress in leaves, including in Arabidopsis (Podgórska and Szal, 2015), the aquatic plant Hydrilla verticillata (Wang et al., 2010), and duckweed (Huang et al., 2013). Our present results show that externally supplied H2O2 increases shoot growth sensitivity of amot1 and ein3eil1 to NH4+ stress (Fig. 6B). The extent of lipid peroxidation, estimated by monitoring the decomposition product MDA, has also been reported as elevated in NH4+-grown seedlings (Hachiya et al., 2010; Podgórska et al., 2013; Fig. 6A). Together, these findings indicate that increased H2O2 accumulation and oxidative stress in leaves under NH4+ stress at least partially result from elevated shoot AMOT1/EIN3 activity, providing a molecular basis for NH4+-induced accumulation of H2O2 in leaves. However, the present study shows that, although lower, there was still increased H2O2 accumulation and oxidative stress in ein3eil1 mutant leaves under NH4+ stress (Figs 5, 6A), suggesting that there are pathways through which AMOT1/EIN3 functions independently to regulate shoot H2O2 accumulation and oxidative stress in response to NH4+ stress.
Drought can promote ROS biosynthesis by inducing expression of several Atrboh genes, such as AtrbohA, AtrbohD, and AtrbohE (Lee et al., 2012). However, Podgórska et al. (2015) found that expression of the RBOHD gene is not induced in leaves under NH4+ stress. In this study, we show that there is no increase in RBOHA, RBOHB, RBOHD, or RBOHF expression in NH4+-treated WT, EIN3ox, and ein3eil1 leaves. These results suggest that AMOT1/EIN3 is not involved through modulating expression of Atrboh genes under high NH4+, such as RBOHA, RBOHB, RBOHD, and RBOHF. However, we cannot exclude NADPH oxidase as a potential ROS source in NH4+-treated plants, as other RBOH genes might be up-regulated. NH4+-mediated changes in apoplastic pH (Husted and Schjoerring, 1995) may induce ROS generation, possibly through the modulation of the activities of cell wall PODs (Lager et al., 2010). Furthermore, under low-Pi conditions, increased POD activities also inhibited Arabidopsis root growth by regulating ROS levels and cell wall stiffening, and the POD inhibitor SHAM could restore root growth and reduce ROS accumulation under –Pi (Balzergue et al., 2017). Podgórska et al. (2015) showed that higher POD levels positively correlate with NH4+-induced ROS content and cell growth inhibition. Consistent with this, by examining POD gene expression and POD activity under NH4+ conditions, we show that NH4+ stress increases expression of some POD genes (At5g19890 and At1g48570) and POD activity in WT shoots (Fig. 7A–C). Moreover, the peroxidase inhibitor SHAM could indeed alleviate NH4+-induced shoot ROS accumulation (Fig. 7G; Supplementary Fig. S9) and growth inhibition (Fig. 7E, F). This result, together with previous reports, confirms that NH4+ induces accumulation of ROS and suggests that POD expression and activity may play an important role. Our qRT-PCR analyses show that two POD genes (At5g19890 and At1g48570) were constitutively up-regulated in EIN3ox but down-regulated in the ein3eil1 double mutant, regardless of NH4+ (Fig. 7A). Moreover, NH4+-induced POD activity was positively correlated with the expression of EIN3 genes, as shown for ein3eil1 and EIN3ox seedlings (Fig. 7B). Further studies revealed that the key transcription factor AMOT1/EIN3 may directly target the two POD genes (At5g19890 and At1g48570), as the AMOT1/EIN3 protein could specifically bind to the promoters of the At5g19890 and At1g48570 genes, as revealed by the Y1H assay. Further supporting our finding of increased POD activity and DAB staining, indicating H2O2 accumulation in EIN3ox, the POD inhibitor SHAM was shown to enhance EIN3ox shoot growth and reduce DAB staining used to indicate H2O2 accumulation under NH4+ stress (Fig. 7; Supplementary Fig. S9). Collectively, these data indicate that the ethylene signaling-mediated NH4+ response of Arabidopsis shoot growth is brought about, at least partially, through POD genes (e.g. At5g19890 and At1g48570), via AMOT1/EIN3.
The accumulation of free shoot NH4+ is widely considered to be critical to the development of NH4+ toxicity (Gerendas et al., 1997; Szczerba et al., 2008). Because H2O2 accumulation was greater in WT shoots than in ein3eil1, but higher in EIN3ox than in the WT, after a 3 d treatment with high NH4+, we hypothesized that loss of function or overexpression of AMOT1/EIN3 may entail reduced or enhanced NH4+ content in the shoot at the 3 d treatment time, respectively. However, there was no difference in shoot NH4+ content between the WT, amot1, ein3eil1, and EIN3ox (Fig. 8A). These results further highlight that AMOT1/EIN3 plays an important role in regulating NH4+-induced shoot ROS accumulation and rules out that reduced shoot ROS accumulation during the early phase of exposure (within 3 d in our study) by loss of function of AMOT1/EIN3 resulted from reduced NH4+ content. However, a higher NH4+ content in EIN3ox and the WT, and a lower content in amot1 and ein3eil1 were observed following high-NH4+ stress for prolonged treatment times (6 d) (Fig. 8A, B). Our results indicate that GS activity was not affected by the mutation in AMOT1/EIN3 (Fig. 8C), showing that GS is not responsible for the lower NH4+ accumulation in the amot1 mutant. Alternatively, oxidative stress itself, induced by H2O2, can increase cellular NH4+ concentrations by inducing proteolytic activity (Sweetlove et al., 2002). It is not clear how AMOT1/EIN3 mediates shoot NH4+ accumulation over longer periods of treatment, and more research is warranted to examine this.
In summary, we have identified and characterized a novel gene, AMOT1/EIN3, that controls shoot NH4+ sensitivity and propose a model for ethylene–AMOT1/EIN3 functions in shoot NH4+ sensitivity (Fig. 9). Our study shows the importance of EIN3 in shoot inhibition under high-NH4+ stress, providing strong genetic evidence in support of the role of the ethylene biosynthesis and signaling pathway in regulating shoot NH4+ sensitivity. Under NH4+ stress, ethylene is perceived and transduced, affecting the transcription factors EIN3/EIL and initiating the ethylene response. Additionally, our work highlights the roles of AMOT1/EIN3 in regulating ROS accumulation in Arabidopsis shoots under NH4+ stress. In the ein3eil1 mutant, high-NH4+-induced ROS accumulation is reduced, which leads to reduced oxidative stress in the shoot. However, EIN3ox shoots accumulated more ROS and displayed higher sensitivity to NH4+ stress. Elucidation of this interaction between AMOT1/EIN3 and H2O2 signaling provides novel insight into our understanding on how EIN3 regulates shoot growth under high-NH4+ stress. Moreover, it was found that AMOT1/EIN3 can up-regulate shoot expression of the genes coding for PODs (e.g. At5g19890 and At1g48570) previously shown to correlate positively with NH4+-induced ROS accumulation and cell growth inhibition. Further studies using molecular approaches to investigate the transcriptional network regulated by AMOT1/EIN3 will provide important new insights into the process of acclimation and of adaptation to NH4+ stress in plants.
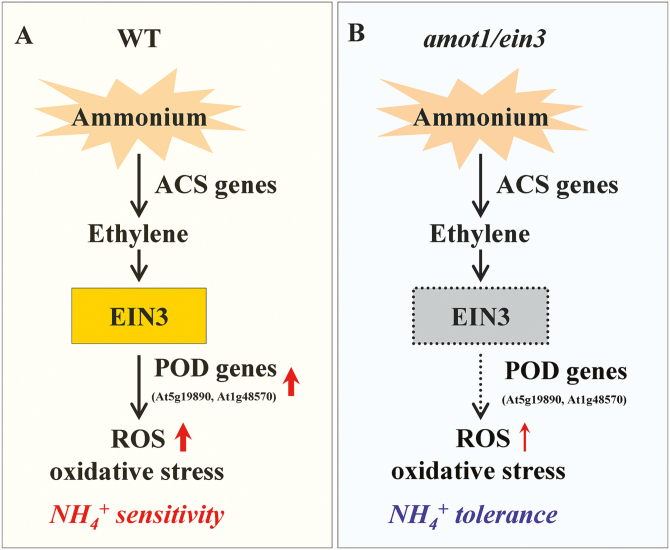
Fig. 9.
A proposed model for ethylene–EIN3 function in shoot NH4+ sensitivity. Based on our study and previous reports (Chao et al., 1997; G. Li et al., 2013; Podgorska et al., 2013, 2015), we established a model for ethylene–EIN3 function in shoot NH4+ sensitivity. (A) In the wild type, NH4+ stress enhanced the expression of ACS and ACO genes, encoding ACS and ACO, the two key enzymes responsible for ethylene synthesis. Under NH4+ stress, ethylene is perceived and transduced, affecting the transcription factor EIN3, and initiating the ethylene response. EIN3 regulates ROS accumulation, which leads to oxidative stress in Arabidopsis shoots under NH4+ stress. The expression of EIN3-mediated POD genes (e.g. At5g19890 and At1g48570) is involved in NH4+ stress-induced shoot ROS accumulation. (B) In the amot1/ein3 mutant, expression of the AMOT1/EIN3-dependent POD genes (e.g. At5g19890 and At1g48570) in the shoot is blocked under NH4+ stress. Ethylene regulation of ROS accumulation and oxidative stress is lowered. Orthogons in orange represent known EIN3 functions, and orthogons in gray with dashed lines represent the inhibition of EIN3 functions due to the amot1/ein3 mutation. Red arrows indicate increased POD gene expression, ROS accumulation, and oxidative stress, and thick and thin red arrows indicate, respectively, a high or low ROS accumulation and oxidative stress.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Rosette diameter and fresh shoot weight of Arabidopsis thaliana wild-type (WT, Col-0) plants following treatment with various NH4+ concentrations.
Fig. S2. Relative rosette diameter (A) and fresh shoot weight (B) of Arabidopsis thaliana WT and eil1 mutant plants following treatment with NH4+ for 6 d.
Fig. S3. Lateral root number of Arabidopsis thaliana WT and ein3eil1 mutant plants following treatment with high NH4+ for 6 d.
Fig. S4. H2O2 content in WT, EIN3ox, and ein3eil1 shoot tissue under control conditions.
Fig. S5. EBS:GUS expression and DAB staining in the split-shoot experiment.
Fig. S6. Effect of NH4+ treatment on shoot DAB staining and biomass of the WT and the Atrbohd mutant.
Fig. S7. qRT-PCR analysis of POD gene expression in WT, EIN3ox, and ein3eil1 shoot tissue under NH4+ treatment for 6 h.
Fig. S8. Measurement of POD activity of WT, EIN3ox, and ein3eil1 shoot tissue under control conditions.
Fig. S9. Mean relative DAB staining intensity in WT (A) and EIN3ox (B) shoots treated with NH4+ and NH4+ plus SHAM.
Table S1. Gene-specific primers used for qRT-PCR.
Acknowledgements
We thank Dr J. Alonso at North Carolina State University for providing seeds of the EBS:GUS reporter lines which were generated by Dr Anna Stepanova in Dr Joe Ecker’s lab. We thank Dr Hongwei Guo for providing the seeds of ein3-1, eil1-1, ein3eil1, and EIN3ox transgenic lines, and the ABRC of Ohio State University for etr1-3, eto1-1, and rbohd mutant seeds. This work was supported by the National Natural Science Foundation of China (no. 31430095), the National Key R&D Program of China (no. 2017YFD0200103), the Chinese Academy of Sciences Innovation Program (no. CAS ISSASIP1604), and the University of Melbourne.
References
- Adams DO, Yang SF. 1979. Ethylene biosynthesis: identification of 1-aminocyclopropane 1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proceedings of the National Academy of Sciences, USA 76, 170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN. 2004. The ethylene signaling pathway. Science 306, 1513–1515. [DOI] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, Ausubel FM, Ecker JR. 2003. Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proceedings of the National Academy of Sciences, USA 100, 2992–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An F, Zhao Q, Ji Y, et al. . 2010. Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. The Plant Cell 22, 2384–2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. [DOI] [PubMed] [Google Scholar]
- Balkos KD, Britto DT, Kronzucker HJ. 2010. Optimization of ammonium acquisition and metabolism by potassium in rice (Oryza sativa L. cv. IR-72). Plant, Cell & Environment 33, 23–34. [DOI] [PubMed] [Google Scholar]
- Balzergue C, Dartevelle T, Godon C, et al. . 2017. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nature Communications 8, 15300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker AV. 1999a Ammonium accumulation and ethylene evolution by tomato infected with root-knot nematode and grown under different regimes of plant nutrition. Communications in Soil Science and Plant Analysis 30, 175–182. [Google Scholar]
- Barker AV. 1999b Foliar ammonium accumulation as an index of stress in plants. Communications in Soil Science and Plant Analysis 30, 167–174. [Google Scholar]
- Barker AV, Corey KA. 1991. Interrelations of ammonium toxicity and ethylene action in tomato. Hortscience 26, 177–180. [Google Scholar]
- Bindschedler LV, Dewdney J, Blee KA, et al. . 2006. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. The Plant Journal 47, 851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittsánszky A, Pilinszky K, Gyulai G, Komives T. 2015. Overcoming ammonium toxicity. Plant Science 231, 184–190. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. 1998. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiology 116, 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britto DT, Kronzucker HJ. 2002. NH4+ toxicity in higher plants: a critical review. Journal of Plant Physiology 159, 567–584. [Google Scholar]
- Cao Y, Glass AD, Crawford NM. 1993. Ammonium inhibition of Arabidopsis root growth can be reversed by potassium and by auxin resistance mutations aux1, axr1, and axr2. Plant Physiology 102, 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. 1997. Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89, 1133–1144. [DOI] [PubMed] [Google Scholar]
- Chen G, Bi YR, Li N. 2005. EGY1 encodes a membrane-associated and ATP-independent metalloprotease that is required for chloroplast development. The Plant Journal 41, 364–375. [DOI] [PubMed] [Google Scholar]
- Coskun D, Britto DT, Li M, Becker A, Kronzucker HJ. 2013. Rapid ammonia gas transport accounts for futile transmembrane cycling under NH3/NH4+ toxicity in plant roots. Plant Physiology 163, 1859–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R. 2005. Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. The Plant Cell 17, 268–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Valdivia MD, Aparicio-Tejo PM, Lamsfus C, Cruz C, Martins-Loução MA, Moran JF. 2008. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and -sensitive plants. Physiologia Plantarum 132, 359–369. [DOI] [PubMed] [Google Scholar]
- Dong CH, Zolman BK, Bartel B, Lee BH, Stevenson B, Agarwal M, Zhu JK. 2009. Disruption of Arabidopsis CHY1 reveals an important role of metabolic status in plant cold stress signaling. Molecular Plant 2, 59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupre C, Stevens CJ, Ranke T, Bleeker A, Peppler-Lisbach C, Gowing DJG, Dise NB, Dorland E, Bobbink R, Diekmann M. 2009. Changes in species richness and composition in European acidic grasslands over the past 70 years: the contribution of cumulative atmospheric nitrogen deposition. Global Change Biology 16, 344–357. [Google Scholar]
- Feng J, Barker AV. 1992a Ethylene evolution and ammonium accumulation by nutrient-stressed tomato plants. Journal of Plant Nutrition 15, 137–153. [Google Scholar]
- Feng J, Barker AV. 1992b Ethylene evolution and ammonium accumulation by nutrient-stressed tomatoes grown with inhibitors of ethylene synthesis or action. Journal of Plant Nutrition 15, 155–167. [Google Scholar]
- Feng J, Barker AV. 1992c Ethylene evolution and ammonium accumulation by tomato plants with various nitrogen forms and regimes of acidity. I. Journal of Plant Nutrition 15, 2457–2469. [Google Scholar]
- Gerendas J, Zhu Z, Bendixen R, Ratcliffe RG, Sattelmacher B. 1997. Physiological and biochemical processes related to ammonium toxicity in higher plants. Zeitschrift für Pflanzenernährung und Bodenkunde 160, 239–251. [Google Scholar]
- Guo H, Ecker JR. 2004. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology 7, 40–49. [DOI] [PubMed] [Google Scholar]
- Guzmán P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachiya T, Watanabe CK, Boom C, Tholen D, Takahara K, Kawai-Yamada M, Uchimiya H, Uesono Y, Terashima I, Noguchi K. 2010. Ammonium-dependent respiratory increase is dependent on the cytochrome pathway in Arabidopsis thaliana shoots. Plant, Cell & Environment 33, 1888–1897. [DOI] [PubMed] [Google Scholar]
- He W, Brumos J, Li H, et al. . 2011. A small-molecule screen identifies l-kynurenine as a competitive inhibitor of TAA1/TAR activity in ethylene-directed auxin biosynthesis and root growth in Arabidopsis. The Plant Cell 23, 3944–3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Satoh Y, Ohki A, Takada R, Arai T, Michiyama H. 2008. Inhibition of ammonium assimilation restores elongation of seminal rice roots repressed by high levels of exogenous ammonium. Physiologia Plantarum 134, 183–190. [DOI] [PubMed] [Google Scholar]
- Huang L, Lu YY, Gao X, Du G, Ma XX, Liu M, Guo JS, Chen YP. 2013. Ammonium-induced oxidative stress on plant growth and antioxidative response of duckweed (Lemna minor L.). Ecological Engineering 58, 355–362. [Google Scholar]
- Husted S, Schjoerring JK. 1995. Apoplastic pH and ammonium concentration in leaves of Brassica napus L. Plant Physiology 109, 1453–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadid N, Mialoundama AS, Heintz D, et al. . 2011. DOLICHOL PHOSPHATE MANNOSE SYNTHASE1 mediates the biogenesis of isoprenyl-linked glycans and influences development, stress response, and ammonium hypersensitivity in Arabidopsis. The Plant Cell 23, 1985–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Ciani S, Schachtman DP. 2010. A peroxidase contributes to ROS production during Arabidopsis root response to potassium deficiency. Molecular Plant 3, 420–427. [DOI] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass A. 1995. Analysis of 13NH4+ efflux in spruce roots (a test case for phase identification in compartmental analysis). Plant Physiology 109, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronzucker HJ, Siddiqi MY, Glass ADM. 1997. Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385, 59–61. [Google Scholar]
- Lager I, Andréasson O, Dunbar TL, Andreasson E, Escobar MA, Rasmusson AG. 2010. Changes in external pH rapidly alter plant gene expression and modulate auxin and elicitor responses. Plant, Cell & Environment 33, 1513–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Seo PJ, Lee HJ, Park CM. 2012. A NAC transcription factor NTL4 promotes reactive oxygen species production during drought-induced leaf senescence in Arabidopsis. The Plant Journal 70, 831–844. [DOI] [PubMed] [Google Scholar]
- Lei G, Shen M, Li ZG, et al. . 2011. EIN2 regulates salt stress response and interacts with a MA3 domain-containing protein ECIP1 in Arabidopsis. Plant, Cell & Environment 34, 1678–1692. [DOI] [PubMed] [Google Scholar]
- Li B, Li Q, Su Y, Chen H, Xiong L, Mi G, Kronzucker HJ, Shi W. 2011. Shoot-supplied ammonium targets the root auxin influx carrier AUX1 and inhibits lateral root emergence in Arabidopsis. Plant, Cell & Environment 34, 933–946. [DOI] [PubMed] [Google Scholar]
- Li B, Li Q, Xiong L, Kronzucker HJ, Krämer U, Shi W. 2012. Arabidopsis plastid AMOS1/EGY1 integrates abscisic acid signaling to regulate global gene expression response to ammonium stress. Plant Physiology 160, 2040–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Li GJ, Kronzucher HJ, Baluška F, Shi WM. 2014. Ammonium stress in Arabidopsis: physiological targets, genetic loci, and signaling pathways. Trends in Plant Science 19, 107–114. [DOI] [PubMed] [Google Scholar]
- Li G, Dong G, Li B, Li Q, Kronzucker HJ, Shi W. 2012. Isolation and characterization of a novel ammonium overly sensitive mutant, amos2, in Arabidopsis thaliana. Planta 235, 239–252. [DOI] [PubMed] [Google Scholar]
- Li G, Li B, Dong G, Feng X, Kronzucker HJ, Shi W. 2013. Ammonium-induced shoot ethylene production is associated with the inhibition of lateral root formation in Arabidopsis. Journal of Experimental Botany 64, 1413–1425. [DOI] [PubMed] [Google Scholar]
- Li Z, Peng J, Wen X, Guo H. 2013. Ethylene-insensitive3 is a senescence-associated gene that accelerates age-dependent leaf senescence by directly repressing miR164 transcription in Arabidopsis. The Plant Cell 25, 3311–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. 2004. Reactive oxygen gene network of plants. Trends in Plant Science 9, 490–498. [DOI] [PubMed] [Google Scholar]
- Peng J, Li Z, Wen X, Li W, Shi H, Yang LS, Zhu HQ, Guo HW. 2014. Salt-induced stabilization of EIN3/EIL1 confers salinity tolerance by deterring ROS accumulation in Arabidopsis. PLoS Genetics 10, e1004664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórska A, Gieczewska K, Łukawska-Kuźma K, Rasmusson AG, Gardeström P, Szal B. 2013. Long-term ammonium nutrition of Arabidopsis increases the extrachloroplastic NAD(P)H/NAD(P)+ ratio and mitochondrial reactive oxygen species level in leaves but does not impair photosynthetic capacity. Plant, Cell & Environment 36, 2034–2045. [DOI] [PubMed] [Google Scholar]
- Podgórska A, Ostaszewska M, Gardeström P, Rasmusson AG, Szal B. 2015. In comparison with nitrate nutrition, ammonium nutrition increases growth of the frostbite1 Arabidopsis mutant. Plant, Cell & Environment 38, 224–237. [DOI] [PubMed] [Google Scholar]
- Podgórska A, Szal B. 2015. The role of reactive oxygen species under ammonium nutrition. In: Gupta KJ, Igamberdiev AU, eds. Reactive oxygen and nitrogen species signaling and communication in plants.Switzerland: Springer International Publishing, 133–153. [Google Scholar]
- Qin C, Qian W, Wang W, Wu Y, Yu C, Jiang X, Wang D, Wu P. 2008. GDP-mannose pyrophosphorylase is a genetic determinant of ammonium sensitivity in Arabidopsis thaliana. Proceedings of the National Academy Sciences, USA 105, 18308–18313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren C, Zhang Y, Cui W, Lu G, Wang Y, Gao H, Huang L, Mu Z. 2015. A polysaccharide extract of mulberry leaf ameliorates hepatic glucose metabolism and insulin signaling in rats with type 2 diabetes induced by high fat-diet and streptozotocin. International Journal of Biological Macromolecules 72, 951–959. [DOI] [PubMed] [Google Scholar]
- Rodrigues A, Santiago J, Rubio S, Saez A, Osmont KS, Gadea J, Hardtke CS, Rodriguez PL. 2009. The short-rooted phenotype of the brevis radix mutant partly reflects root abscisic acid hypersensitivity. Plant Physiology 149, 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Fluhr R. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiology 141, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR. 1998. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes & Development 12, 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellingen K, Van Der Straeten D, Vandenbussche F, Prinsen E, Remans T, Vangronsveld J, Cuypers A. 2014. Cadmium-induced ethylene production and responses in Arabidopsis thaliana rely on ACS2 and ACS6 gene expression. BMC Plant Biology 14, 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. 2007. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell 19, 2169–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, Millar AH. 2002. The impact of oxidative stress on Arabidopsis mitochondria. The Plant Journal 32, 891–904. [DOI] [PubMed] [Google Scholar]
- Szczerba MW, Britto DT, Balkos KD, Kronzucker HJ. 2008. Alleviation of rapid, futile ammonium cycling at the plasma membrane by potassium reveals K+-sensitive and -insensitive components of NH4+ transport. Journal of Experimental Botany 59, 303–313. [DOI] [PubMed] [Google Scholar]
- Tao JJ, Chen HW, Ma B, Zhang WK, Chen SY, Zhang JS. 2015. The role of ethylene in plants under salinity stress. Frontiers in Plant Science 6, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Noctor G, Foyer CH. 2002. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiology and Biochemistry 40, 501–507. [Google Scholar]
- Wang C, Zhang SH, Wang PF, Hou J, Li W, Zhang WJ. 2008. Metabolic adaptations to ammonia-induced oxidative stress in leaves of the submerged macrophyte Vallisneria natans (Lour.) Hara. Aquatic Toxicology 87, 88–98. [DOI] [PubMed] [Google Scholar]
- Wang C, Zhang SH, Wang PF, Li W, Lu J. 2010. Effects of ammonium on the antioxidative response in Hydrilla verticillata (L.f.) Royle plants. Ecotoxicology and Environmental Safety 73, 189–195. [DOI] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. 2002. How to study gene expression. In: Weigel D, Glazebrook J, eds. Arabidopsis: a laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 243–245. [Google Scholar]
- Xing Y, Jia W, Zhang J. 2008. AtMKK1 mediates ABA-induced CAT1 expression and H2O2 production via AtMPK6-coupled signaling in Arabidopsis. The Plant Journal 54, 440–451. [DOI] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A. 2003. Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. Journal of Biological Chemistry 278, 49102–49112. [DOI] [PubMed] [Google Scholar]
- Yoo SD, Cho Y, Sheen J. 2009. Emerging connections in the ethylene signaling network. Trends in Plant Science 14, 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You W, Barker AV. 2002. Herbicidal actions of root-applied glufosinate-ammonium on tomato plants. Journal of the American Society for Horticultural Science 127, 200–204. [Google Scholar]
- You W, Barker AV. 2005. Ethylene evolution and ammonium accumulation by tomato plants after root-applied glufosinate-ammonium treatment in the presence of ethylene inhibitors. Communications in Soil Science and Plant Analysis 35, 1957–1965. [Google Scholar]
- Zhao B, Tian M, An Q, Ye J, Guo JS. 2017. Characteristics of a heterotrophic nitrogen removal bacterium and its potential application on treatment of ammonium-rich wastewater. Bioresource Technology 226, 46–54. [DOI] [PubMed] [Google Scholar]
- Zou N, Li B, Chen H, Su Y, Kronzucker HJ, Xiong L, Baluška F, Shi W. 2013. GSA-1/ARG1 protects root gravitropism in Arabidopsis under ammonium stress. New Phytologist 200, 97–111. [DOI] [PubMed] [Google Scholar]
- Zou N, Li B, Dong G, Kronzucker HJ, Shi W. 2012. Ammonium-induced loss of root gravitropism is related to auxin distribution and TRH1 function, and is uncoupled from the inhibition of root elongation in Arabidopsis. Journal of Experimental Botany 63, 3777–3788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.