Intraspecific cold-adaptation strategies of Rubisco carboxylation kinetics and content, along with a widespread ability for photosynthetic bicarbonate usage, are reported for polar seaweeds.
Keywords: Carbon concentrating mechanisms, carbon fixation, kinetics, macroalgae, photosynthesis, polar, Rubisco, seaweeds
Abstract
Despite the high productivity and ecological importance of seaweeds in polar coastal regions, little is known about their carbon utilization mechanisms, especially the kinetics of the CO2-fixing enzyme Rubisco. We analyzed Rubisco carboxylation kinetics at 4 °C and 25 °C in 12 diverse polar seaweed species (including cold-temperate populations of the same species) and the relationship with their ability to use bicarbonate, by using 13C isotope discrimination and pH drift experiments. We observed a large variation in Rubisco carboxylation kinetics among the selected species, although no correlation was found between either the Michaelis–Menten constant for CO2 (Kc) or Rubisco content per total soluble protein ([Rubisco]/[TSP]) and the ability to use bicarbonate for non-green seaweeds. This study reports intraspecific Rubisco cold adaptation by means of either higher Rubisco carboxylation turnover rate (kcatc) and carboxylase efficiency (kcatc/Kc) at 4 °C or higher [Rubisco]/[TSP] in some of the analyzed species. Our data point to a widespread ability for photosynthetic bicarbonate usage among polar seaweeds, despite the higher affinity of Rubisco for CO2 and higher dissolved CO2 concentration in cold seawater. Moreover, the reported catalytic variation within form ID Rubisco might avert the canonical trade-off previously observed between Kc and kcatc for plant Rubiscos.
Introduction
Littoral and sublittoral hardbottom zones of polar coastal regions are mainly dominated by dense macroalgal communities, which represent a major trophic contribution to these ecosystems (Dunton and Schell, 1987; Amsler et al., 1995; Iken et al., 1998). The productivity of these communities is comparable to that of temperate seaweed forests (Quartino and Boraso de Zaixso, 2008; Hop et al., 2012), despite the low temperatures in polar regions. Thus, polar seaweeds may have developed photosynthetic adaptations to cold waters, although little is known about the specific molecular processes involved in inorganic carbon (Ci) acquisition and assimilation in these organisms.
The first major step of photosynthetic carbon fixation is catalyzed by the enzyme ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco, EC 4.1.1.39). Rubisco is present in most autotrophic organisms from prokaryotes, such as photosynthetic anaerobic bacteria and cyanobacteria, to eukaryotes, such as algae and higher plants (Whitney et al., 2011). Almost all O2 evolvers have a form I Rubisco, which consists of eight large and eight small subunits and is subdivided into forms IA–ID depending on its sequence and lineage (Tabita et al., 2008). Plants, green algae, and most cyanobacteria belong to the ‘green’ chloroplast lineage possessing form IB Rubiscos, while non-green algae from the ‘red’ chloroplast lineage contain form ID Rubiscos (Raven and Beardall, 2003; Falkowski et al., 2004).
In spite of their relevance in global carbon cycling, all Rubiscos have a relatively slow carboxylation turnover rate (kcatc) and low affinity for CO2, along with poor discrimination between CO2 and O2 (Spreitzer and Salvucci, 2002). These catalytic traits, together with the low levels of dissolved CO2 and slow CO2 diffusion rate in water, have led to the evolution of carbon concentrating mechanisms (CCMs) in most aquatic photosynthetic organisms (Giordano et al., 2005). CCMs consist of an active influx of CO2 and/or HCO3− at the plasmalemma and/or a plastid envelope membrane, which act to increase the CO2 concentration around Rubisco (Maberly et al., 1992; Raven and Beardall, 2003; Giordano et al., 2005). Only a few seaweed species appear to lack CCMs (in these species, photosynthesis relies only on passive diffusion of CO2), although in many cases stronger evidence on the presence or absence of CCMs is required (Raven et al., 2005).
Different physiological measurements have been widely used to suggest the presence or absence of CCMs; these include the pH compensation point, the activity of transporters or enzymes that are part of a CCM, and the natural 13C/12C ratio in the algal biomass, among others. The 13C isotope discrimination of the algal biomass (δ13Calga), relative to the isotope composition of the Ci source, is primarily controlled by the interplay between Ci supply and demand, which affects CO2/HCO3− acquisition and accumulation (i.e. CCMs), as well as CO2 fixation and CO2 leakage out of the cell (Sharkey and Berry, 1985). It has been frequently used as a proxy for CCM operation in seaweeds, assuming low leakage, since the increase in the utilization of isotopically heavier HCO3− relative to isotopically lighter CO2 produces a decrease in the 13C isotope discrimination of the algal biomass (higher δ13Calga) with respect to the isotope fractionation of Rubisco (Raven et al., 2002a). The pH compensation point indicates the condition in which the dissolved inorganic carbon (DIC) taken up by the alga equals the CO2 released into the medium by respiration and/or photorespiration. Maberly et al. (1992) reported δ13Calga values more negative than or equal to –30‰ for all seaweeds showing pH compensation points lower than 9.0, that is, the pH at which the dissolved CO2 concentration is nearly zero. Since the ratio of the Rubisco reaction rate with 12CO2 to that with 13CO2 is 1.030 (at least for spinach Rubisco, Roeske and O’Leary, 1984; see Boller et al., 2015 for other Rubisco types), both measurements suggest that photosynthesis in these seaweeds might rely only on diffusive CO2 entry. These species are mainly red algae from the class Florideophyceae inhabiting subtidal habitats, where light is presumed to be the limiting resource (Maberly et al., 1992; Raven et al., 2002a).
While the production and operation of CCMs are energy- and resource-demanding processes, diffusive entry of CO2 for photosynthesis at the current CO2 levels may allow significant oxygenase activity of Rubisco, thereby decreasing the efficiency of photosynthesis; in addition, the photorespiratory metabolism also has an associated energetic cost that depends on the Rubisco CO2/O2 specificity factor (SC/O; Raven et al., 2014). Thus, Rubisco may have evolved towards an increased affinity for CO2, that is, a decreased Michaelis–Menten affinity constant for CO2 (Kc; Nisbet et al., 2007) in those organisms relying only on diffusive entry of CO2. In fact, in the few rhodophyte species analyzed to date, Rubisco has a high SC/O value (Badger et al., 1998). However, most of these rhodophytes are thermo-acidophiles, which are not representative of all species belonging to this phylum. On the other hand, the evolution of CCMs has probably removed some of the pressure to enhance carboxylation efficiency (Meyer and Griffiths, 2013). In this sense, Rubisco from green algae with CCMs and cyanobacteria were found to have high Kc and kcatc (Yeoh et al., 1981; Andrews and Lorimer, 1985; Mueller-Cajar and Whitney, 2008). It is still unknown whether the same applies to ID Rubiscos from non-green algae possessing CCMs. Recently, Young et al. (2016) have reported that Rubisco from diatoms, which have efficient CCMs, exhibits a broad range of Kc values that mostly exceed those of C4 plant Rubisco, but similar and less variable kcatc values, suggesting that the canonical trade-off typically observed between Kc and kcatc for plant form IB Rubisco might not exist for form ID Rubisco. Nevertheless, more data are required to confirm this observation.
Raven et al. (2002b) suggested that the impact of low seawater temperatures on photosynthesis would favor diffusive CO2 entry rather than CCM operation. The affinity for CO2 and SC/O of Rubisco increase at decreasing temperatures, while the carboxylation efficiency notably decreases (kcatc/Kc; Jordan and Ogren, 1984; Perdomo et al., 2015; Galmés et al., 2016). It has been hypothesized that the rate of CO2 supply to Rubisco would not change significantly at lower temperatures, since the elevated concentrations of dissolved CO2 in cold waters mostly compensate for the lower diffusion coefficient of CO2 (Raven et al., 2002b); so, assuming no increase in Rubisco content at low temperatures, the reduced carboxylation efficiency would lead to a greater CO2 concentration around Rubisco (Cc) during steady-state photosynthesis. However, the previous assumption might be altered by other factors, including changes in the activation state of the enzyme, a probable decrease in the contribution of respiratory and photorespiratory CO2, and a larger diffusion boundary layer thickness and changes in transmembrane components affecting the conductance for CO2.
In temperate seaweeds, the acclimation of photosynthesis to low temperature involved the production of high concentrations of Calvin cycle enzymes (Davison, 1987). Similarly, higher plants grown at low temperatures up-regulate their Rubisco content, compensating for the intrinsic decline in kcatc (Holaday et al., 1992; Yamori et al., 2005). This significant increase in Rubisco content might be energetically costly for cold-adapted organisms and requires a high nitrogen (N) investment. Alternatively, photosynthetic adaptation to cold environments by means of higher Rubisco kcatc values, allowing for a partial compensation of the intrinsic decline in enzymatic activity at low temperatures, would represent N and energy savings for the organism. In this regard, Rubiscos from plants belonging to colder climates typically showed a 40% faster kcatc than warm-adapted species when both groups were measured at a standard temperature of 25–30 °C (Sage, 2002; Galmés et al., 2015). Unfortunately, Rubisco kinetics have been barely examined in seaweeds, except for a few species (Yeoh et al., 1981; Johnston, 1991; Whitney et al., 2001; Israel and Hophy, 2002); most of these studies measured Kc at only a single temperature and none of them included polar species. Therefore, more data are needed to explore adaptation patterns in Rubisco investment, kinetics, and Ci acquisition mechanisms in seaweeds from different latitudes. This knowledge would be especially useful for the prediction of the consequences of global change in macroalgal communities at high latitudes. The aim of the present study was to identify the presence of adaptive traits in Rubisco kinetics and Rubisco content of macroalgal species representative of Arctic and Antarctic ecosystems, to compare them with populations of the same species from cold-temperate latitudes when possible, and to relate these data to the presence or absence of CCMs in these species.
Materials and methods
Algal material
Twelve different macroalgal species representative of Arctic and Antarctic ecosystems, including some populations of the same species from cold-temperate latitudes, were analyzed. The selected species included taxa with contrasting habitat ecologies and evolutionary histories (see Table 1). The analyzed polar and cold-temperate populations belonging to the same species had previously been identified as different ecotypes (Bischoff and Wiencke, 1995; Van de Poll et al., 2002; Olischläger et al., 2017). For some of the species, samples were collected by divers during July 2013 in Kongsfjorden, Spitsbergen, Svalbard (79°N, 11°E), or May 2015 in Helgoland, Germany (54°N, 7°E), and immediately taken to the laboratory in black plastic bags. Young (when possible) and visually healthy specimens free from macroscopic epibiota were chosen for the analyses. For the remainder of the species studied, young macrothalli were raised from the Alfred Wegener Institute (AWI, Bremerhaven, Germany) stock cultures. Cultivation methods were as described by Wiencke and tom Dieck (1989). Saccharina latissima from Helgoland was grown at 17±1 °C, and Palmaria palmata from Roscoff, Acrosiphonia arcta from Helgoland, and S. latissima from Spitsbergen were grown at 10±1 °C. These species were cultured using a 16:8 h light:dark photoperiod and a constant photon fluence rate (PFR) of 25–30 µmol photons m−2 s−1 provided by white light fluorescent tubes (Osram 58W/965 Biolux). The rest of the polar species were grown at 1±1 °C with a changing photoperiod from 5 to 20 h of light to mimic high-latitude seasonal conditions. PFR was measured by means of a quantum flat-head PAR sensor connected to a radiometer (LI-190 and LI-250A, LI-COR Biosciences). Samples for determination of relative Rubisco content, kinetic characterization, and carbon stable isotope composition were snap frozen in liquid nitrogen and stored at –80 °C until analysis.
Table 1.
Location, origin, evolutionary history, depth zonation, optimum temperature for growth (Tgrowth), and upper survival temperature (UST) of the seaweed populations studied
| Species | Location of sampling | Origin | Depth of collection below sea level (m) | Evolutionary history | Depth zonation | T growth (°C) | UST ( °C) | References for T growth and UST |
| Rhodophyta | ||||||||
| Phycodrys rubens (L.) Batters | Kongsfjord (Spitsbergen) | Natural population | 15 | Non-endemic | Lower sublittoral | 10 | n.d. | Novaczek et al. (1990) |
| Phycodrys rubens (L.) Batters | Helgoland (Germany) | Natural population | 6–8 | Non-endemic | Lower sublittoral | n.d. | 18–20 | Lüning (1984) |
| Ptilota gunneri Silva, Maggs & Irvine | Kongsfjord (Spitsbergen) | Natural population | 10 | Non-endemic | Lower sublittoral | 4–10 | n.d. | Gordillo et al. (2016) |
| Devaleraea ramentacea (L.) Guiry | Kongsfjord (Spitsbergen) | Natural population | 2–3 | Arctic Endemic | Upper sublittoral | 0–10 (0) | 18–20 | Novaczek et al. (1990); Bischoff and Wiencke (1993) |
| Palmaria palmata (L.) Weber & Mohr | Kongsfjord (Spitsbergen) | AWI collection (2265) | – | Non-endemic | Upper sublittoral | 12 | n.d. | Van de Poll et al. (2002) |
| Palmaria palmata (L.) Weber & Mohr | Roscoff (France) | AWI collection | – | Non-endemic | Lower eulittoral/upper sublittoral | 12 | n.d. | Van de Poll et al. (2002) |
| Palmaria decipiens (Reinsch) Ricker | King George Island (Antarctica) | AWI collection (2113) | – | Antarctic Endemic | Mid eulittoral/upper sublittoral | 5 | 15–16 | Wiencke and tom Dieck (1989) |
| Ochrophyta (Phaeophyceae) | ||||||||
| Alaria esculenta (L.) Greville | Kongsfjord (Spitsbergen) | Natural population | 10 | Non-endemic | Mid sublittoral | 4–10 | n.d. | Gordillo et al. (2016) |
| Desmarestia aculeata (L.) Lamouroux | Kongsfjord (Spitsbergen) | Natural population | 5 | Non-endemic | Mid sublittoral | 5 | 20 | Bischoff and Wiencke (1993) |
| Laminaria solidungula J.Agardh | Kongsfjord (Spitsbergen) | Natural population | 4–6 | Arctic Endemic | Mid sublittoral | 5–10 | 16 | tom Dieck (1992) |
| Laminaria digitata (Huds.) Lamouroux | Kongsfjord (Spitsbergen) | Natural population | 5 | Non-endemic | Mid sublittoral | 10 | n.d. | Bolton and Lüning (1982) |
| Saccharina latissima (L.) Lane, Mayes, Druehl, Saunders | Kongsfjord (Spitsbergen) | AWI collection(3123,3124) | – | Non-endemic | Mid sublittoral | 10 | n.d. | Olischläger et al. (2017) |
| Saccharina latissima (L.) Lane, Mayes, Druehl, Saunders | Helgoland (Germany) | AWI collection(3094,3096) | – | Non-endemic | Mid sublittoral | 10–20 (15) | 18–20 | Fortes and Lüning (1980) |
| Himantothallus grandifolius (A. & E.S.Gepp) Zinova | King George Island (Antarctica) | AWI collection(3006,3010) | – | Antarctic Endemic | Mid to lower sublittoral | 0–5 | 11–13 | Wiencke and tom Dieck (1989) |
| Chlorophyta | ||||||||
| Acrosiphonia arcta (Dillwyn) Gain | Disko Island (Greenland) | AWI collection(1120) | – | Non-endemic | Upper sublittoral | 0–10 | 22 | Bischoff and Wiencke (1995) |
| Acrosiphonia arcta (Dillwyn) Gain | Helgoland (Germany) | AWI collection(1083) | – | Non-endemic | Lower eulittoral | 5–15 | 22 | Bischoff and Wiencke (1995) |
| Acrosiphonia arcta (Dillwyn) Gain | King George Island (Antarctica) | AWI collection(1160) | – | Non-endemic | Lower eulittoral | 5 | 22 | Bischoff and Wiencke (1995) |
n.d., Not determined.
Carbon stable isotope composition
The abundance of 13C relative to 12C in the algal samples was determined by mass spectrometry using a DELTA V Advantage (Thermo Electron Corporation) isotope ratio mass spectrometer (IRMS) connected to a Flash EA 1112 CNH analyzer (Thermo Electron Corporation). The calculated δ13Calga was corrected with the isotope composition of DIC found in the medium from which the samples were collected (δ13CDIC), as described by Iñiguez et al. (2016a, b). Measurements of δ13CDIC were done with the same IRMS connected to a GasBench II system (Thermo Electron Corporation).
pH drift
Specimens were placed in 100 ml glass bottles completely filled with 0.2 µm-filtered seawater, with magnetic stirring, and tightly sealed to avoid gas exchange with the air. Assays were performed at saturating PFR (previously determined for each species by chlorophyll a fluorescence rapid light curves by means of a pulse-amplitude-modulated fluorometer; Mini-PAM, Walz) at their respective growth temperatures for specimens from stock cultures, or at 3±1 °C for the species collected in Kongsfjorden. Specimen size was adjusted to prevent self-shading and to ensure effective agitation of the medium. The pH was recorded using a thin glass electrode (CRISON 52 09, pH meter CRISON GLP 22) until it reached a stable reading (after ~24 hours of incubation), which represents the pH compensation point.
Rubisco content and carboxylase kinetics
Rubisco carboxylation kinetics (kcatc and Kc) were determined at 25 °C and 4 °C in rapid crude protein extracts, according to the methods described for plants (Sharwood et al., 2008, 2016) and algae (Heureux et al., 2017; Young et al., 2016). Rapidly prepared fresh extracts instead of purified Rubisco preparations were used to prevent the degradation of Rubisco because the C-terminal loop of the enzyme is often a target for proteases, which results in changes in the catalytic performance (Sharwood et al., 2008). Approximately 0.5 g fresh weight of frozen samples were homogenized in a pre-chilled Mixer Mill (MM 400, Retsch) with 1 ml of ice-cold extraction buffer consisting of 100 mM Bicine-NaOH CO2-free (pH 8.1), 1 mM EDTA, 10 mM DTT, 50 mM β-mercaptoethanol, 20 mM MgCl2, 1 mM benzamidine, 1 mM ε-aminocaproic acid, 1% plant protease inhibitor cocktail (P9599, Sigma-Aldrich), 2 mM phenylmethylsulfonyl fluoride, 0.5% Triton X-100, 25 mg ml−1 polyvinylpolypyrrolidone, and a saturating concentration of NaHCO3 (10 mM, 20 mM, or 40 mM depending on the species). The homogenate was immediately centrifuged for 10 min at 20,000 g and 4 °C. Total soluble protein was determined in the supernatant according to the method of Bradford (1976). Part of the supernatant was then supplemented with sufficient carrier-free NaH14CO3 to adjust the specific radioactivity to 3.7 × 1010 Bq mol−1 (1 Ci mol−1) and incubated for 15–20 min at 25 °C for full activation of Rubisco. Rates of Rubisco 14CO2 fixation were measured in 8 ml septum-sealed glass vials with magnetic stirring, under a 100% N2 atmosphere. The vials contained assay buffer, consisting of 100 mM Bicine-NaOH CO2-free (pH 8.1), 20 mM MgCl2, 1.5 mM ribulose-1,5-bisphosphate (RuBP; R0878, Sigma-Aldrich) and ~100 W-A units of carbonic anhydrase (C3934, Sigma-Aldrich), previously sparged with 100% N2, and one of eight concentrations of NaH14CO3 from 0.2 to 18 mM for the assays at 25 °C and from 0.2 to 6 mM for the assays at 4 °C (except for the green algae, for which NaH14CO3 concentrations from 0.8 to 49 mM for the assays at 25 °C and from 0.8 to 17 mM for the assays at 4 °C were used), each with a specific radioactivity of 3.7 × 1010 Bq mol−1. Assays (0.5 ml final volume) were started by the injection of 10–20 µl of activated algal extract and stopped with the addition of 200 µl 1 M formic acid after 1 min (for the assays at 25 °C) or 2 min (for the assays at 4 °C). The acidified samples were dried at 80 °C and the acid-stable 14C-organic molecules were determined by scintillation counting (Beckman Coulter LS 6500). Values for Kc and maximum carboxylase activity (Vmaxc) were extrapolated from the data fitted to the Michaelis–Menten equation as described by Sharwood et al. (2008) and Whitney et al. (2011). Concentrations of CO2 in solution were calculated assuming an acid dissociation constant (pKa) for carbonic acid of 6.28 at 4 °C and 6.11 at 25 °C (Galmés et al., 2016), a solubility constant for CO2 of 0.0626 mol l−1 atm−1 at 4 °C and 0.034 mol l−1 atm−1 at 25 °C, and using accurate measures of the pH (NBS scale) of each buffer solution at the respective assay temperature. Replicate measurements (n=3–5) were made using independent crude protein extracts from different individuals. A series of assays of Triticum aestivum L. cv. Cajeme was interspersed with those of the algal species analyzed as an external control, yielding values similar to those recently reported in the literature (Hermida-Carrera et al., 2016; Table 2).
Table 2.
Rubisco carboxylation kinetics of the analyzed polar populations measured at 25 °C and 4 °C, the ratio between 25 °C and 4 °C measurements for each kinetic parameter, and Rubisco content at the growth conditions
| Species | K c (µM) | k cat c (s −1) | k cat c /K c (s −1 mM −1) | [Rubisco]/ [TSP] (%) | ||||||
| 25 °C | 4 °C | 25 °C/4 °C | 25 °C | 4 °C | 25 °C/4 °C | 25 °C | 4 °C | 25 °C/4 °C | ||
| Rhodophyta | ||||||||||
| Phycodrys rubens | 18.9±0.8 | 4.8±0.7 | 3.94±0.40 | 1.76±0.03 | 0.14±0.01 | 12.2±0.9 | 93.5±3.8 | 30.1±2.0 | 3.11±0.10 | 8.0±0.7 |
| Ptilota gunneri | 14.4±1.1 | 5.1±0.6 | 2.83±0.12 | 1.60±0.23 | 0.17±0.03 | 9.3±0.5 | 110.7±7.2 | 33.8±2.4 | 3.27±0.05 | 6.6±0.9 |
| Devaleraea ramentacea | 17.5±1.0 | 5.6±0.8 | 3.13±0.26 | 2.59±0.16 | 0.34±0.04 | 7.6±0.6 | 148.1±8.3 | 60.9±0.9 | 2.43±0.14 | 7.4±1.7 |
| Palmaria palmata | 15.9±0.6 | 4.9±0.2 | 3.24±0.01 | 2.08±0.05 | 0.27±0.01 | 7.8±0.2 | 131.3±8.1 | 54.6±2.1 | 2.40±0.07 | 9.9±0.6 |
| Palmaria decipiens | 17.4±1.2 | 5.0±0.2 | 3.50±0.12 | 2.43±0.16 | 0.31±0.01 | 7.8±0.2 | 139.7±3.3 | 62.9±0.3 | 2.22±0.05 | 7.8±0.1 |
| Ochrophyta (Phaeophyceae) | ||||||||||
| Alaria esculenta | 23.6±1.0 | 4.1±0.5 | 5.85±1.01 | 2.13±0.14 | 0.23±0.02 | 9.5±1.3 | 90.3±4.3 | 55.5±1.9 | 1.63±0.06 | 17.4±1.8 |
| Desmarestia aculeata | 13.3±0.6 | 2.1±0.3 | 6.44±0.89 | 1.37±0.12 | 0.12±0.02 | 11.3 ±1.3 | 103.3±12 | 61.0±22 | 1.79±0.41 | 17.7±4.7 |
| Laminaria solidungula | 18.5±1.3 | 3.9±0.8 | 4.96±1.23 | 1.60±0.12 | 0.17±0.02 | 9.2±0.7 | 86.6±6.2 | 47.1±14 | 1.92±0.41 | 30.1±1.4 |
| Laminaria digitata | 17.0±1.0 | 4.0±0.3 | 4.25±0.40 | 1.42±0.28 | 0.10±0.02 | 16.4±2.9 | 84.3±20 | 26.1±2.8 | 3.84±0.36 | 24.7±7.5 |
| Saccharina latissima | 19.4±1.2 | 3.8±0.4 | 4.99±0.29 | 1.79±0.18 | 0.16±0.03 | 10.8±0.6 | 92.5±7.5 | 43.3±8.4 | 2.17±0.23 | 37.3±6.1 |
| Himantothallus grandifolius | 18.1±1.1 | 4.4±0.8 | 4.14±0.49 | 2.08±0.02 | 0.23±0.01 | 9.2±0.6 | 115±7.4 | 51.7±6.5 | 2.23±0.16 | n.d. |
| Chlorophyta | ||||||||||
| Acrosiphonia arcta (Arctic) | 52.8±1.6 | 17.7±2.6 | 3.01±0.33 | 5.08±0.15 | 0.82±0.03 | 6.2±0.1 | 96.2±2.7 | 46.7±5.7 | 2.07±0.22 | 7.6±0.1 |
| Control | ||||||||||
| Triticum aestivum | 9.6±0.4 | 3.1±0.1 | 3.06±0.14 | 2.20±0.23 | 0.20±0.01 | 11.2±0.6 | 230.1±28 | 62.8±4.1 | 3.66±0.28 | 42.3±2.5 |
k cat c, Rubisco carboxylase turnover rate; Kc, Michaelis–Menten affinity constant for CO2; kcatc/Kc, carboxylation efficiency; [Rubisco]/[TSP], percentage of Rubisco in the total soluble protein. Data are means ±SD (n=3–5 independent thalli). n.d., Not determined.
k cat c was calculated by dividing Vmax by the concentration of Rubisco active sites, which was quantified from the same crude protein extracts by 2ʹ-carboxyarabinitol-1,5-bisphosphate (14C-CABP) binding (Ruuska et al., 1998), assuming eight binding sites per Rubisco (Blayney et al., 2011). Our preliminary assays revealed that analyzed red and brown seaweed Rubiscos need an increased CABP concentration (up to 1.2 mM) for saturating Rubisco active sites than that previously used for form IB Rubiscos (29–80 µM; Ruuska et al., 1998; Kubien et al., 2011). These results agree well with those from Pearce (2006) showing a 100-fold higher semi-saturation constant for CABP inhibition and 2-fold slower binding of form ID than form IB Rubisco active sites. A 15 µl aliquot of activated extract was incubated with 14C-CABP for 30 min at room temperature before chromatographic separation of Rubisco-bound and free 14C-CABP. Previous incubation of the mixture for up to 24 h at 4 °C followed by 30 min at room temperature before chromatographic separation did not significantly increase 14C-CABP binding. Immunoblotting of the crude protein extracts using a Rubisco large subunit antibody and purified spinach Rubisco standard (AS03 037 and AS01 017S, Agrisera) gave similar values of Rubisco concentration to those obtained by 14C-CABP binding (data not shown).
Carboxylation assay controls at saturating NaH14CO3 concentrations either without RuBP addition or with activated algal extract pre-incubated for 30 min with non-radioactive CABP (up to 1.2 mM) were carried out in order to confirm that the observed acid-stable 14C was only the result of Rubisco catalytic activity. Both controls gave values lower than 5% of the maximum activity in all analyzed species. Saturating concentrations of RuBP, 14C-CABP, and H14CO3−, as well as the optimum incubation time and temperature for full Rubisco activation, were determined in preliminary assays. RuBP of ≥90% purity (R0878, Sigma-Aldrich) and of ≥99% purity (83895, Sigma-Aldrich) were also compared, obtaining no significant differences in Rubisco kinetics at saturating conditions.
Data analysis
Significance of differences between populations of the same species (n=3–5) were tested using one-way analysis of variance, after normality (Shapiro–Wilk test) and homogeneity of variances were confirmed. Post hoc comparisons were performed using Fisher’s least significant difference test. Pearson correlation coefficients were obtained for the significance of the association between different variables. The confidence interval for all these tests was set at 95% (P≤0.05). All statistical analyses were performed using SigmaPlot 12.0 statistical software (Systat Software Inc.).
Results
Variability in Rubisco kinetics and its thermal response among polar seaweeds
At 25 °C, when considering only form ID Rubiscos (i.e. rhodophytes and ochrophytes), Kc and kcatc varied ~2-fold among species (Table 2). The lowest values corresponded to Desmarestia aculeata (Kc=13.3 μM, kcatc=1.37 s−1) and the highest values were found in Alaria esculenta for Kc (23.6 µM) and in Devaleraea ramentacea for kcatc (2.59 s−1). Rubisco from A. arcta, the only chlorophyte included in the study, had the highest values for Kc and kcatc, which were two to four times higher than those found in the other species.
The highest values of kcatc/Kc were found for the polar endemic species Palmaria decipiens (140 s−1 mM−1) and D. ramentacea (148 s−1 mM−1), while the lowest value was obtained for Laminaria digitata (84.3 s−1 mM−1; Table 2). The Antarctic endemic species Himantothallus grandifolius presented the highest value among the ochrophytes (115 s−1 mM−1). The chlorophyte A. arcta had a kcatc/Kc in the range of the other species.
The percentage of Rubisco in the total soluble protein ([Rubisco]/[TSP]) was highly variable among the species, ranging from 6.6% in Ptilota gunneri to 37.3% in S. latissima (Table 2).
The range of variation for Kc and kcatc was smaller at 4 °C compared with 25 °C (Table 2). Among form ID Rubiscos, at 4 °C, Kc and kcatc ranged from 2.1 µM (D. aculeata) and 0.1 s−1 (L. digitata) to 5.6 µM and 0.34 s−1 (both for D. ramentacea), respectively. The chlorophyte A. arcta again showed the highest values for Kc and kcatc at 4 °C, with values being three to eight times higher than those in the remaining species. At 4 °C, the species showing maximum and minimum values of kcatc/Kc were the same as those identified at 25 °C, with the exception of D. aculeata, which presented one of the highest values (61 s−1 mM−1).
Regarding thermal dependencies of the different kinetics parameters, the ratio (Kc)25 °C/(Kc)4 °C varied between 2.83 for Ptilota gunneri and 6.44 for D. aculeata, while (kcatc)25 °C/(kcatc)4 °C ranged from 6.2 in A. arcta to 16.4 in L. digitata (Table 2). The lowest value of (kcatc)25 °C/(kcatc)4 °C within the Rhodophyta was obtained for the Arctic endemic species D. ramentacea (7.6), and within the Ochrophyta, for the two polar endemic species Laminaria solidungula and H. grandifolius (9.2), whereas (kcatc/Kc)25 °C/(kcatc/Kc)4 °C varied from 1.63 in A. esculenta to 3.84 in L. digitata.
Intraspecific differences in Rubisco kinetics and its thermal response between polar and cold-temperate populations
For Phycodrys rubens, the only statistically significant difference between the Arctic and cold-temperate populations was found in Kc and kcatc/Kc at 25 °C, consisting of a lower affinity for CO2 and a lower carboxylation efficiency in the polar population compared with the Atlantic population (Table 3). These differences at 25 °C were not found at 4 °C, and, furthermore, [Rubisco]/[TSP], kcatc, and the ratio 25 °C/4 °C for the different kinetic parameters were not significantly different between the two populations.
Table 3.
Rubisco carboxylation kinetics of the polar and cold-temperate populations of the same species measured at 25 °C and 4ºC, the ratio between 25 °C and 4 °C measurements for each kinetic parameter, and Rubisco content at the growth conditions (data corresponding to the polar populations are also shown in Table 2)
| Species | K c (µM) | k cat c (s −1) | k cat c /K c (s −1 mM −1) | [Rubisco]/ [TSP] (%) | ||||||
| 25 °C | 4 °C | 25 °C/4 °C | 25 °C | 4 °C | 25 °C/4 °C | 25 °C | 4 °C | 25 °C/4 °C | ||
| Rhodophyta | ||||||||||
| Phycodrys rubens (Arctic) | 18.9±0.8 b | 4.8±0.7 a | 3.94±0.40 a | 1.76±0.03 a | 0.14±0.01 a | 12.2±0.9 a | 93.5±3.8 a | 30.1±2.0 a | 3.11±0.10 a | 8.0±0.7 a |
| Phycodrys rubens (Helgoland) | 15.7±0.7 a | 5.3±1.9 a | 3.24±1.14 a | 1.92±0.19 a | 0.16±0.03 a | 12.1±1.3 a | 122±10.6 b | 31.9±6.8 a | 3.95±0.90 a | 8.5±0.3 a |
| Palmaria palmata (Arctic) | 15.9±0.6 a | 4.9±0.2 a | 3.24±0.01 a | 2.08±0.05 b | 0.27±0.01 b | 7.8±0.2 a | 131.3±8.1 a | 54.6±2.1 b | 2.40±0.07 a | 9.9±0.6 a |
| Palmaria palmata (Roscoff) | 14.9±0.8 a | 4.6±0.1 a | 3.22±0.22 a | 1.85±0.04 a | 0.23±0.01 a | 8.2±0.3 a | 124.8±9.4 a | 49.1±2.6 a | 2.54±0.09 a | 9.4±0.2 a |
| Ochrophyta (Phaeophyceae) | ||||||||||
| Saccharina latissima (Arctic) | 19.4±1.2 a | 3.8±0.4 a | 4.99±0.29 a | 1.79±0.18 a | 0.16±0.03 b | 10.8±0.6 a | 92.5±7.5 b | 43.3±8.4 b | 2.17±0.23 a | 37.3±6.1 a |
| Saccharina latissima (Helgoland) | 19.6±0.5 a | 4.2±0.6 a | 4.75±0.61 a | 1.34±0.44 a | 0.11±0.04 a | 12.6±0.7 b | 68.1±21.5 a | 26.3±10 a | 2.69±0.38 b | 36.6±6.4 a |
| Chlorophyta | ||||||||||
| Acrosiphonia arcta (Arctic) | 52.8±1.6 b | 17.7±2.6 a | 3.01±0.33 a | 5.08±0.15 a | 0.82±0.03 a | 6.19±0.07 c | 96.2±2.7 b | 46.7±5.7 a | 2.07±0.22 b | 7.6±0.1 b |
| Acrosiphonia arcta (Helgoland) | 57.4±1.6 c | 16.4±0.6 a | 3.49±0.16 a | 4.89±0.06 a | 0.82±0.02 a | 5.94±0.11 b | 85.3±2.9 a | 50.1±0.9 a | 1.70±0.09 a | 2.9±0.4 a |
| Acrosiphonia arcta (Antarctic) | 48.2±0.9 a | 15.6±1.5 a | 3.10±0.25 a | 4.99±0.02 a | 0.86±0.01 b | 5.78±0.06 a | 103.5±2.0 c | 55.0±6.0 a | 1.87±0.16 ab | 6.9±1.4 b |
k cat c, Rubisco carboxylase turnover rate; Kc, Michaelis–Menten affinity constant for CO2; kcatc/Kc, carboxylation efficiency; [Rubisco]/[TSP], percentage of Rubisco in the total soluble protein. Different letters indicate statistically significant differences (P<0.05) between populations of the same species. Data are means ±SD (n=3–5 independent thalli).
For Palmaria palmata, the Arctic population displayed ~15% higher kcatc and kcatc/Kc at 4 °C compared with the Atlantic population (Table 3). Non-significant differences were observed between the two populations in Kc, [Rubisco]/[TSP], and the ratio 25 °C/4 °C for the different kinetic parameters.
The Arctic population of S. latissima had a higher kcatc at 4 °C compared with the cold-temperate population, together with a higher kcatc/Kc at both assayed temperatures (65% higher at 4 °C; see Table 3). As a consequence, the ratios (kcatc)25 °C/(kcatc)4 °C and (kcatc/Kc)25 °C/(kcatc/Kc)4 °C were lower in polar S. latissima relative to the Atlantic population, while [Rubisco]/[TSP] was similar in the two populations.
The Antarctic population of A. arcta showed the lowest Kc at 25 °C, followed by the Arctic population, in comparison to the cold-temperate population; this difference was reflected in a higher kcatc/Kc for the Antarctic population (Table 3). At 4 °C, non-significant differences in kcatc/Kc were found among the three populations, despite the higher kcatc for the Antarctic population. Most noticeably, [Rubisco]/[TSP] was ~150% higher in the two polar populations compared with the cold-temperate population.
Carbon utilization of polar versus cold-temperate populations
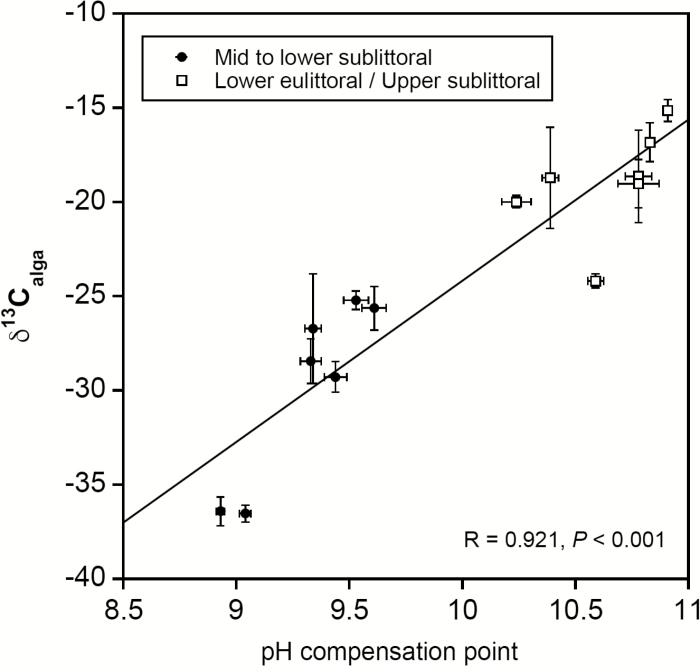
Among all the species, only two of them (the lower sublittoral rhodophytes Phycodrys rubens and Ptilota gunneri) displayed values of δ13Calga <–30‰ and pH compensation point ≤9 (Table 4). A positive correlation was found between δ13Calga and pH compensation point among all seaweed species studied (R=0.921, P<0.001, Fig. 1) and also when considering species with form ID Rubisco exclusively (R=0.891, P<0.001). As shown in Fig. 1, there was a striking separation into two groups, one for the species growing in the mid to lower sublittoral, with δ13Calga <–25‰ and pH compensation point <10, and the other for the species growing in the upper sublittoral to lower eulittoral, with δ13Calga >–25‰ and pH compensation point >10.
Table 4.
Stable carbon isotope discrimination values (δ13Calga) and pH compensation points of the analyzed seaweed populations
| Species | δ13Calga(‰) | pH compensation point |
| Rhodophyta | ||
| Phycodrys rubens (Arctic) | –36.5±0.4 a | 9.04±0.03 |
| Phycodrys rubens (Helgoland) | –37.4±0.5 b | n.d. |
| Ptilota gunneri | –36.4±0.8 | 8.93±0.02 |
| Devaleraea ramentacea | –24.2±0.4 | 10.59±0.04 |
| Palmaria palmata (Arctic) | –18.6±2.4 a | 10.78±0.06 b |
| Palmaria palmata (Roscoff) | –18.7±2.7 a | 10.39±0.04 a |
| Palmaria decipiens | –19±1.3 | 10.78±0.09 |
| Ochrophyta (Phaeophyceae) | ||
| Alaria esculenta | –28.4±1.2 | 9.33±0.05 |
| Desmarestia aculeata | –26.7±2.9 | 9.34±0.04 |
| Laminaria solidungula | –29.3±0.8 | 9.44±0.05 |
| Laminaria digitata | –25.6±1.2 | 9.61±0.05 |
| Saccharina latissima (Arctic) | –21.8±0.8 a | n.d. |
| Saccharina latissima (Helgoland) | –23.7±1.4 b | n.d. |
| Himantothallus grandifolius | –25.2±0.5 | 9.53±0.06 |
| Chlorophyta | ||
| Acrosiphonia arcta (Arctic) | –15.1±0.6 a | 10.91±0.01 b |
| Acrosiphonia arcta (Helgoland) | –20±0.3 c | 10.37±0.03 a |
| Acrosiphonia arcta (Antarctic) | –16.8±1 b | 10.83±0.01 b |
Different letters indicate statistically significant differences (P<0.05) between populations of the same species. Data are means ±SD (n=4–5 independent thalli). n.d., Not determined.
Fig. 1.
Relationship between the pH compensation point and carbon isotope discrimination (δ13Calga) from all analyzed seaweed populations. Filled circles represent populations distributed in the mid to lower sublittoral and open squares represent populations distributed in the lower sublittoral and/or upper sublittoral. Data are presented as mean ±SD (n=4–5).
All polar populations showed a significantly higher pH compensation point and/or less negative δ13Calga values than their cold-temperate counterparts (Table 4). The Atlantic population of Phycodrys rubens had a slightly more negative δ13Calga value than its polar counterpart, although both values were lower than –30‰. The pH compensation point found in the Palmaria palmata population from Roscoff was lower than that from the polar population of the same species, whereas the δ13Calga values were similar. For S. latissima, the δ13Calga value was lower in the Helgoland population relative to the polar population. The Atlantic A. arcta showed lower δ13Calga and pH compensation point values than the Arctic and Antarctic populations of this species.
Trade-off among Rubisco carboxylation kinetics and their relationship with carbon utilization
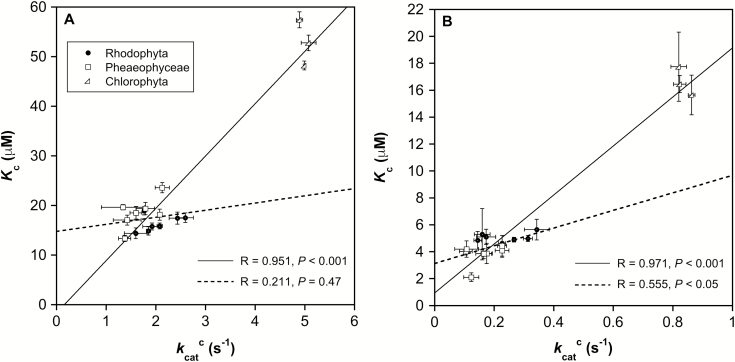
When analyzing the data from all species assayed at both 4 °C and 25 °C, kcatc and Kc correlated positively (R=0.958, P<0.001). The same correlation was also found when considering the species with form ID Rubisco exclusively (R=0.935, P<0.001). At each assay temperature, the correlation between kcatc and Kc was significant when all species were analyzed together (R=0.951, P<0.001 at 25 °C and R=0.971, P<0.001 at 4 °C; Fig. 2). However, the analysis of form ID Rubiscos alone revealed a significant correlation only between kcatc and Kc at 4 °C (R=0.555, P<0.05), and not at 25 °C (R=0.211, P=0.47, Fig. 2).
Fig. 2.
Trade-off between the maximum carboxylation rate (kcatc) and the Michaelis–Menten affinity constant for CO2 (Kc) for the analyzed seaweed Rubiscos at (A) 25 °C and (B) 4 ° C. The solid line represents the correlation of all populations together (including chlorophytes); the dashed line represents the correlation of form ID Rubiscos alone. Data are presented as mean ±SD (n=3–5).
Generally, a larger number of significant correlations was obtained between Rubisco kinetics and either δ13Calga values, pH compensation points, or the ratio [Rubisco]/[TSP] when all species were analyzed together than when the three populations of the chlorophyte A. arcta were excluded from the analysis (Table 5). [Rubisco]/[TSP] correlated negatively with kcatc/Kc at 25 °C. The significant negative correlation between [Rubisco]/[TSP] and kcatc at both 4 °C and 25 °C was lost when only form ID Rubiscos were analyzed. A positive relationship was found between kcatc measured at both temperatures and the pH compensation point. The ratio kcatc/Kc at 25 °C correlated positively with the pH compensation point only for form ID Rubiscos. Conversely, all significant correlations between δ13Calga and Rubisco kinetics were lost when only form ID Rubiscos were analyzed (Table 5).
Table 5.
Pearson’s correlation coefficients between the Rubisco kinetic parameters at 25 °C and 4 °C and either the percentage of Rubisco in the total soluble protein, 13C isotope discrimination, or pH compensation point, considering (A) all 17 seaweed populations together, and (B) the 14 red and brown seaweed populations (all possessing form ID Rubiscos) alone
| (A) Data from all populations analyzed together | ||||||
| 25 °C | 4 °C | |||||
| K c | k cat c | k cat c /K c | K c | k cat c | k cat c /K c | |
| δ 13 C alga | 0.505* | 0.57* | 0.1 | 0.502* | 0.601* | 0.514* |
| pH compensation point | 0.455 | 0.637* | 0.488 | 0.554* | 0.65* | 0.519 |
| [Rubisco]/ [TSP] | –0.34 | –0.519* | –0.575* | –0.476 | –0.513* | –0.339 |
| (B) Data from red and brown algal species (Form ID Rubiscos) | ||||||
| 25 °C | 4 °C | |||||
| K c | k cat c | k cat c /K c | K c | k cat c | k cat c /K c | |
| δ 13 C alga | 0.01 | 0.27 | 0.26 | 0.13 | 0.44 | 0.518 |
| pH compensation point | –0.15 | 0.669* | 0.806** | 0.41 | 0.777** | 0.573 |
| [Rubisco]/ [TSP] | 0.42 | –0.545 | –0.768** | –0.549 | –0.536 | –0.29 |
k cat c/Kc, carboxylation efficiency; kcatc, carboxylase turnover rate; Kc, Michaelis–Menten affinity constant for CO2; [Rubisco]/[TSP], percentage of Rubisco in the total soluble protein; δ13Calga, 13C isotope discrimination. *P<0.05, **P<0.01, ***P<0.001.
Discussion
Variability in Rubisco carboxylation kinetics
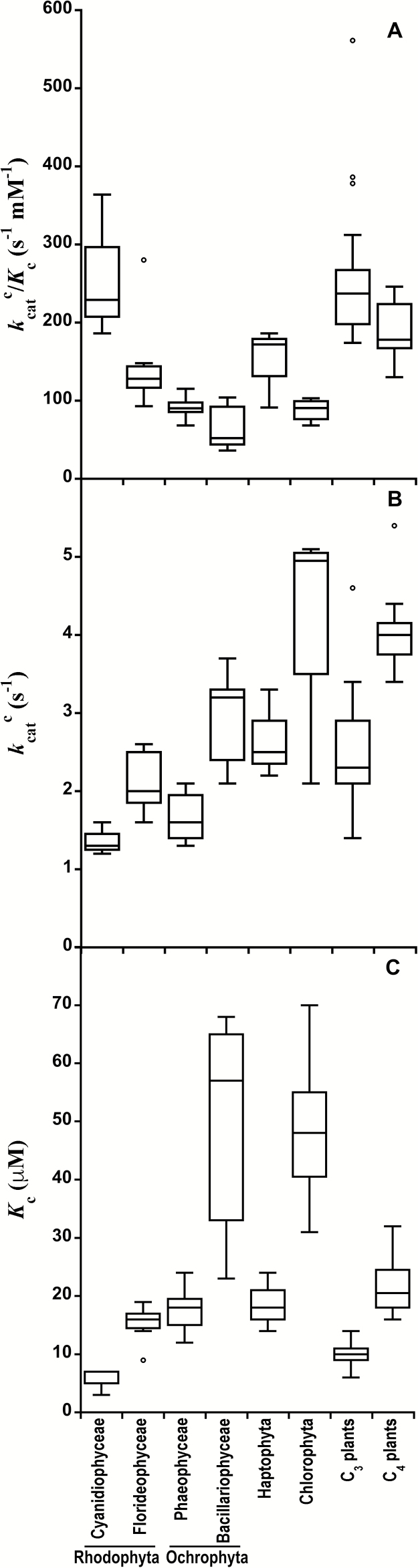
The Kc values obtained in the present study at 25 °C (Tables 2 and 3) fell within the wide range reported for the few seaweed species analyzed in previous studies, between 9.3 µM in Griffithsia monilis (Whitney et al., 2001) and 70 µM in Ulva sp. (Yeoh et al., 1981). Fig. 3 represents a compilation of our results and previously published carboxylation kinetics measured at 25 °C for other phylogenetic groups possessing form I Rubisco (see Supplementary Table S1 at JXB online), showing that brown seaweeds (Phaeophyceae) display lower Kc and kcatc values at 25 °C than diatoms (Bacillariophyceae), and lower kcatc but similar Kc to haptophytes (which also possess form ID Rubisco). On the other hand, red seaweeds (Florideophyceae) present higher Kc and kcatc values and lower kcatc/Kc than red algae belonging to the family Cyanidiophyceae. These differences reveal a large variation within form ID Rubisco kinetics.
Fig. 3.
Box plots depicting Rubisco carboxylation kinetics parameters at 25 °C for different taxonomic groups, including the species analyzed in this study (see Tables 2 and 4) and previous published values for others species (see Supplementary Table S1). (A) Carboxylation efficiency (kcatc/Kc); (B) carboxylase turnover rate (kcatc); (C) Michaelis–Menten affinity constant for CO2 (Kc). For each plot, the horizontal line represents the median, the box and whiskers represent the 25th to 75th percentile and the minimum to maximum distributions of the data, respectively, and any value outside this range is displayed as an individual point.
Rubisco Kc values from brown and red seaweeds, along with those from haptophytes, were similar to those of C4 plants and higher than those of C3 plants, whereas chlorophytes and diatoms displayed the highest Kc values within the eukaryotes (Fig. 3C). These differences in Kc might be related to the presence and strength of CCMs. However, the same differences were not observed in kcatc values (Fig. 3B), leading to lower carboxylation efficiencies (kcatc/Kc) in all seaweeds analyzed in the present study relative to C3 vascular plants; this seems to be a general pattern within marine algae (Fig. 3A). Moreover, Ochrophyta and Chlorophyta also showed significantly lower kcatc/Kc than C4 vascular plants.
The absence of a significant correlation between Kc and kcatc at 25 °C when only red and brown seaweed Rubiscos were analyzed is in agreement with the results of Young et al. (2016) and Heureux et al. (2017) for other form ID Rubiscos. These results suggest that the canonical trade-off typically observed between Kc and kcatc for plants, which was thought to be due to a fundamental mechanistic constraint of their interrelated rate constants (Tcherkez et al., 2006), might not be universal for all Rubiscos. Differences in the relationship between kcatc and Kc may arise from differences in the intrinsic equilibrium of the RuBP enolization reaction (Tcherkez, 2013).
Co-evolution of Rubisco and CCMs in seaweeds
It has previously been demonstrated that Rubisco kinetics of organisms from other phylogenetic groups have a strong correlation with CCMs, showing higher values of kcatc and Kc for Rubiscos adapted to higher [CO2]:[O2] ratios (Tcherkez et al., 2006; Savir et al., 2010; Whitney et al., 2011). The positive correlation observed in the present study between Rubisco kinetic parameters and the CCM proxies, δ13Calga and pH compensation point, for all species analyzed together (Table 5) is in agreement with these previous reports. By contrast, when only form ID Rubiscos were analyzed, Kc was correlated with neither δ13Calga nor the pH compensation point, although kcatc was positively correlated with the pH compensation point at both assay temperatures studied. These results suggest a positive selection in form ID Rubisco kcatc from macroalgae possessing CCMs without a significant concomitant increase in Kc, diverging from the canonical trade-off between Kc and kcatc.
It is important to consider that, unlike in C4 plants, the expression of CCMs in algae is facultative, and is regulated by a large number of environmental factors (Giordano et al., 2005). Thus, pH drift experiments must indicate the presence of a potential CCM capacity, since long-term exposure under constant and saturating irradiance to decreasing CO2 concentrations as the pH increases may lead to the expression of CCM components (Raven et al., 2005). In contrast, δ13Calga values might reflect CCM operation during the growth of the thalli, which could be down-regulated due to energetic constraints (Hepburn et al., 2011). Despite the different timescales of both measurements, a positive correlation between δ13Calga and pH compensation point was found, along with a striking separation between intertidal and subtidal species for these measurements (see Fig. 1); similar observations were previously reported in a global meta-analysis including 141 marine macrophyte species (Stepien, 2015).
Since δ13Calga values could be influenced not only by the isotopic composition of the Ci source but also by CO2 leakage (Sharkey and Berry, 1985), these data should be treated with caution when used as a proxy for CCM operation. High CO2 leakage prevents the accumulation of δ13Calga within the intracellular carbon pool, thereby decreasing δ13Calga, which must approach the Rubisco isotope fractionation. Moreover, Rubisco isotope fractionation of the analyzed red and brown seaweeds, as previously shown for other ID Rubiscos (Boller et al., 2015), might be different from that of spinach Rubisco (–30‰; Roeske and O’Leary, 1984), even though this value has been widely used as a cutoff for excluding HCO3– use in marine seaweeds (Maberly et al., 1992; Raven et al., 2002a). pH drift experiments could also be affected by CO2 leakage and proton extrusion, leading to a lower pH compensation point than the one corresponding to the species’ capacity for HCO3− use. However, these interferences might be negligible in our study because total alkalinity was not significantly altered after pH drift experiments (data not shown). Despite the possible effect of CO2 leakage, most of the analyzed species showed pH compensation points significantly higher than 9, indicating that these species must possess the ability to use HCO3− for photosynthesis.
Only the lower sublittoral rhodophytes Phycodrys rubens and Ptilota gunneri showed a pH compensation point ≤9; these species were also the only ones with a δ13Calga value more negative than –30‰. Therefore, assuming the limitations explained above, these findings might suggest that photosynthesis in these species relies only on diffusive CO2 entry. Our results are in agreement with previous studies suggesting the presence or absence of CCMs in the same species as were analyzed in the present study (Surif and Raven, 1989; Johnston et al., 1991; Maberly et al., 1992; Beardall and Roberts, 1999; Sherlock and Raven, 2001; Raven et al., 2002a, 2005; Klenell et al., 2004; Gordillo et al., 2006; Iñiguez et al., 2016b; Olischläger et al., 2017).
All polar populations had higher or similar pH compensation point and δ13Calga values than their cold-temperate counterparts (Table 4), even though the dissolved CO2 concentration of cold air-equilibrated seawater is significantly higher than at warmer temperatures (Skirrow, 1975). This fact, together with the observed strong decrease in Kc and kcatc at 4 °C, would lead to closer CO2 saturation conditions of the analyzed form ID Rubiscos in polar air-equilibrated seawater, although Rubisco oxygenation kinetics must be analyzed in these species to further corroborate this assumption. Therefore, the maintenance or even increase of active HCO3− use at low temperatures might be related to the fact that equilibrium conditions are not frequently met in cold oceans owing to the thermohaline circulation, biological activity, and slow equilibration of CO2 between the surface of the oceans and the atmosphere relative to that between CO2 and the other DIC species (Raven and Falkowski, 1999). The solubility of O2 also increases at low temperatures (Skirrow, 1975), and there is a considerable reduction in the uncatalyzed rate of CO2 supply from bicarbonate (Egleston et al., 2010) and the diffusion rate of CO2 (Boudreau, 1997) in cold waters. Furthermore, the exposure to continuous light during the summer months at polar latitudes in combination with low temperatures results in the activation of photoprotection mechanisms for dissipation of excess energy; one example is the relevant level of cyclic electron flow reported for Antarctic diatoms (Goldman et al., 2015), so the energetically costly CCMs might be part of these photoprotection mechanisms at low temperatures (Gordillo et al., 2016). It should be taken into account that the ability to use HCO3− for photosynthesis does not necessarily mean that Cc in steady-state photosynthesis is higher than [CO2] in the external medium. Ci uptake can operate at a lower rate than that of Rubisco carboxylation, yet still improve CO2 fixation by lessening the degree to which the [CO2] limits Rubisco carboxylation (Koch et al., 2013).
The negative correlation between [Rubisco]/[TSP] and kcatc that was obtained for all the species analyzed together (Table 5) agrees well with previous studies indicating that faster carboxylation rates of Rubisco enable these seaweeds to invest less in Rubisco relative to the total soluble protein fraction (Seemann et al., 1984; Ghannoum et al., 2005; Galmés et al., 2014). The same trend was observed when only form ID Rubiscos were considered, although it was not statistically significant (P=0.054 for 25 °C, P=0.059 for 4 °C). Nevertheless, [Rubisco]/[TSP] must be higher in actively growing thalli than in old specimens, whereas Rubisco kinetics are constant for a particular organism, which might alter the previous correlation. In the present study, the growth rate of field samples was unknown, although young thalli were selected for the analyses when possible.
The absence of correlation between the two CCM proxies, δ13Calga and pH compensation point, and [Rubisco]/[TSP] (R=–0.037, P=0.893 and R=–0.444, P=0.128, respectively) contrasts with previous results found in comparisons of C3 and C4 plants that indicated a lower [Rubisco]/[TSP] in C4 plants owing to the contribution of proteins involved in CCMs to total soluble protein and less investment in Rubisco (Ghannoum et al., 2005). However, in algae, many CCM proteins are insoluble membrane transporters, and soluble carbonic anhydrases might be also present in organisms without CCMs (Beer et al., 2014). Remarkably, brown seaweeds were found to have 3-fold to 4-fold higher [Rubisco]/[TSP] than red and green macroalgae (Tables 2 and 3), which could be related to the high productivity of Laminariales and Desmarestiales underwater forests in cold-temperate to polar waters (Wiencke et al., 2007). Alternatively, differences in [Rubisco]/[TSP] between groups could be partly related to a lower efficiency in the extraction of proteins other than Rubisco in the studied brown seaweeds, due to the presence of high contents of secondary metabolites and polysaccharides in these species that might interfere with protein solubilization. Very efficient total protein extraction protocols have been developed for Laminariales (Olischläger et al., 2014), but these protocols unavoidably involve protein denaturation.
Intraspecific adaptation of seaweed Rubiscos to low temperatures
The Arctic populations of Palmaria palmata and S. latissima presented significantly higher kcatc/Kc at 4 °C than their temperate counterparts, driven by an increase in kcatc (Table 3). This would lead to higher CO2-saturated photosynthetic rates at low temperatures in the polar populations compared with the cold-temperate populations. Similarly, when comparing populations belonging to the genus Palmaria, it was observed that the endemic Antarctic species Palmaria decipiens showed significantly higher kcatc and kcatc/Kc at 4 °C than the Arctic Palmaria palmata (Table 2), which might be related to the much longer cold-water history of Antarctica relative to the Arctic Ocean (Zacher et al., 2011).
Despite these differences in Rubisco kinetics at 4 °C between polar and temperate populations, and considering their similar [Rubisco]/[TSP] values (Table 3), we suspect the existence of a higher Rubisco activation state in polar red and brown seaweeds in order to achieve the photosynthetic rates that have been measured in previous studies of the same species and locations (Thomas and Wiencke, 1991; Iñiguez et al., 2016a, b; Olischläger et al., 2017). Young et al. (2015) also suggested that Rubisco must be almost fully active in Antarctic diatoms, after comparison of its photosynthetic carbon fixation rates with Rubisco kcatc and quantity at low temperatures.
In contrast, the polar populations of the chlorophyte A. arcta showed no significant differences in kcatc/Kc at 4 °C compared with the temperate population, although a more than 2-fold increase in the [Rubisco]/[TSP] of both polar populations was observed (Table 3). These results are in agreement with those reported by Devos et al. (1998), who observed a similar 2-fold increase in the relative Rubisco content of two psychrophilic strains of the chlorophyte genus Chloromonas compared with their mesophilic counterparts, which might suggest different photosynthetic cold-adaptation responses between form IB and form ID Rubiscos.
The Arctic population of Phycodrys rubens did not show cold-adaptive traits compared with the cold-temperate population of this species, in terms of either Rubisco kinetics or [Rubisco]/[TSP]. As this species grows in the lower sublittoral, its photosynthetic rates should be constrained by the low irradiance reaching these depths and not by the maximum Rubisco carboxylation rate, probably leading to a lack of energetically expensive CCMs (Raven et al., 2002a, b, 2014), as suggested by the CCM proxies (Table 4).
It is unsurprising that the (Kc)25 °C/(Kc)4 °C ratio of polar Rubiscos was not lower (i.e. Kc less temperature dependent) than that of their cold-temperate counterparts (Table 3), as all Rubiscos, from either polar or temperate populations, showed increasing affinity for CO2 at decreasing temperatures at the expense of a reduced catalytic activity, which facilitates the CO2 saturation of the enzyme. Thus, assuming that carbon fixation might constrain photosynthesis in cold waters, polar environments should lead to a selection for higher kcatc Rubiscos instead of lower Kc. However, this assumption does not take into account biochemical processes other than Rubisco activity that can be affected by temperature, such as RuBP regeneration (Bernacchi et al., 2003). Our results showed that the ratios (kcatc)25 °C/(kcatc)4 °C and (kcatc/Kc)25 °C/(kcatc/Kc)4 °C were significantly lower in the Arctic S. latissima compared with its cold-temperate counterpart, but not in the other polar versus temperate population comparisons, while the lowest values of (kcatc)25 °C/(kcatc)4 °C within the Rhodophyta and Ochrophyta were obtained for the polar endemic species analyzed from each phylum (Table 2). This might reflect adaptation of the enzyme’s temperature sensitivity according to its environment, as previously described (Sage, 2002; Galmés et al., 2005, 2015, 2016; Young et al., 2015).
Conclusions
Our results provide novel data on Rubisco kinetics from ecologically relevant polar and cold-temperate seaweeds belonging to different taxonomic groups. In contrast to previous findings in other photosynthetic groups, there was no correlation between either Kc or [Rubisco]/[TSP] and the CCM proxies for the red and brown seaweeds analyzed in the present study. Moreover, most of the analyzed polar populations showed signs of CCMs despite the lower Kc in form ID seaweed Rubiscos and the higher dissolved [CO2] in air-equilibrated seawater at 4 °C. We also report evidence of cold adaptation in Rubisco carboxylation kinetics or [Rubisco]/[TSP] of polar macroalgae likely possessing CCMs. In spite of this, photosynthesis at low temperatures and saturating irradiance conditions in red and brown seaweeds must be constrained by carbon fixation rates, and must require a high Rubisco activation state. Further studies of the regulation of form ID Rubiscos (see Mueller-Cajar et al., 2011; Loganathan et al., 2016) in seaweeds, about which data are currently scarce, are needed in order to make accurate predictions of productivity and ecosystem functioning in near-future scenarios.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Rubisco carboxylation kinetics at 25ºC taken from other datasets and used in Fig. 3.
Supplementary Material
Acknowledgements
This work was financed by projects CTM2011-24007/ANT and CGL2015-67014R, and also partially financed by projects AGL2009–07999 and AGL2013–42364R, from the Spanish Ministry for Science and Innovation and the Spanish Ministry for Economy and Competitiveness. CI was supported by an FPU grant from the Spanish Ministry for Education. Part of this work was performed at the International Arctic Environmental Research and Monitoring Facility, Ny-Ålesund, Spitsbergen, Norway. We thank Raquel Carmona and M. Rosario Lorenzo for their help taking the samples during the stay in Ny-Ålesund. We also thank the AWI diving team, Claudia Daniel, Andreas Wagner, and Inka Bartsch for providing us with the algal material. Furthermore, we thank Arantxa Molins for her help with the training for the Rubisco kinetic measurements, and Sergio Cañete and Elisa Gordo for supervising radioactivity handling.
References
- Amsler CD, Rowley RJ, Laur DR, Quetin LB, Ross RM. 1995. Vertical distribution of Antarctic Peninsular macroalgae: cover, biomass, and species composition. Phycologia 34, 424–430. [Google Scholar]
- Andrews TJ, Lorimer GH. 1985. Catalytic properties of a hybrid between cyanobacterial large subunits and higher plant small subunits of ribulose bisphosphate carboxylase-oxygenase. Journal of Biological Chemistry 260, 4632–4636. [PubMed] [Google Scholar]
- Assali NE, Mache R, Loiseaux-de Goër S. 1990. Evidence for a composite phylogenetic origin of the plastid genome of the brown alga Pylaiella littoralis (L.) Kjellm. Plant Molecular Biology 15, 307–315. [DOI] [PubMed] [Google Scholar]
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GE. 1998. The diversity and co-evolution of Rubisco, plastids, pyrenoids, and chloroplast- based CO2-concentrating mechanisms in algae. Canadian Journal of Botany 76, 1052–1071. [Google Scholar]
- Beardall J, Roberts S. 1999. Inorganic carbon acquisition by two Antarctic macroalgae, Porphyra endivifolia (Rhodophyta: Bangiales) and Palmaria decipiens (Rhodophyta: Palmariales). Polar Biology 21, 310–315. [Google Scholar]
- Beer S, Björk M, Beardall J. 2014. Photosynthesis in the marine environment. Oxford: Wiley-Blackwell. [Google Scholar]
- Bernacchi CJ, Pimentel C, Long SP. 2003. In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell and Environment 26, 1419–1430. [Google Scholar]
- Bischoff B, Wiencke C. 1993. Temperature requirements for growth and survival of macroalgae from Disko Island (Greenland). Helgoländer Meeresunters 47, 167–191. [Google Scholar]
- Bischoff B, Wiencke C. 1995. Temperature ecotypes and biogeography of Acrosiphoniales (Chlorophyta) with Arctic–Antarctic disjunct and Arctic/cold-temperate distributions. European Journal of Phycology 30, 19–27. [Google Scholar]
- Blayney MJ, Whitney SM, Beck JL. 2011. NanoESI mass spectrometry of Rubisco and Rubisco activase structures and their interactions with nucleotides and sugar phosphates. Journal of the American Society for Mass Spectrometry 22, 1588–1601. [DOI] [PubMed] [Google Scholar]
- Boller AJ, Thomas PJ, Cavanaugh CM, Scott KM. 2015. Isotopic discrimination and kinetic parameters of RubisCO from the marine bloom-forming diatom, Skeletonema costatum. Geobiology 13, 33–43. [DOI] [PubMed] [Google Scholar]
- Bolton JJ, Lüning K. 1982. Optimal growth and maximal survival temperatures of Atlantic Laminaria species (Phaeophyta) in culture. Marine Biology 66, 89–94. [Google Scholar]
- Boudreau BP. 1997. Diagenetic models and their implementation modelling transport and reactions in aquatic sediments. Berlin: Springer. [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Cavanagh AP, Kubien DS. 2014. Can phenotypic plasticity in Rubisco performance contribute to photosynthetic acclimation? Photosynthesis Research 119, 203–214. [DOI] [PubMed] [Google Scholar]
- Davison IR. 1987. Adaptation of photosynthesis in Laminaria saccharina (Phaeophyta) to changes in growth temperature. Journal of Phycology 23, 27. [Google Scholar]
- Devos N, Ingouff M, Loppes R, Matagne R. 1998. RUBISCO adaptation to low temperatures: a comparative study in psychrophilic and mesophilic unicellular algae. Journal of Phycology 34, 665–669. [Google Scholar]
- Dunton KH, Schell DM. 1987. Dependence of consumers on macroalgal (Laminaria solidungula) carbon in an Arctic kelp community: δ13C evidence. Marine Biology 93, 615–625. [Google Scholar]
- Egleston ES, Sabine CL, Morel FMM. 2010. Revelle revisited: buffer factors that quantify the response of ocean chemistry to changes in DIC and alkalinity. Global Biogeochemical Cycles 24, GB1002. [Google Scholar]
- Falkowski P, Schofield O, Katz M, Van de Schootbrugge B, Knoll A. 2004. Why is the land green and the ocean red? In: Thierstein H, Young J, eds. Coccolithophores. Berlin: Springer, 429–453. [Google Scholar]
- Fortes MD, Lüning K. 1980. Growth rates of North Sea macroalgae in relation to temperature, irradiance and photoperiod. Helgoländer Meeresunters 34, 15–29. [Google Scholar]
- Galmés J, Flexas J, Keys AJ, Cifre J, Mitchell R, Madgwick P, Haslem R, Medrano H, Parry MA. 2005. Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant, Cell and Environment 28, 571–579. [Google Scholar]
- Galmés J, Hermida-Carrera C, Laanisto L, Niinemets Ü. 2016. A compendium of temperature responses of Rubisco kinetic traits: variability among and within photosynthetic groups and impacts on photosynthesis modeling. Journal of Experimental Botany 67, 5067–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Andralojc PJ, Conesa MÀ, Keys AJ, Parry MA, Flexas J. 2014. Expanding knowledge of the Rubisco kinetics variability in plant species: environmental and evolutionary trends. Plant, Cell & Environment 37, 1989–2001. [DOI] [PubMed] [Google Scholar]
- Galmés J, Kapralov MV, Copolovici LO, Hermida-Carrera C, Niinemets Ü. 2015. Temperature responses of the Rubisco maximum carboxylase activity across domains of life: phylogenetic signals, trade-offs, and importance for carbon gain. Photosynthesis Research 123, 183–201. [DOI] [PubMed] [Google Scholar]
- Ghannoum O, Evans JR, Chow WS, Andrews TJ, Conroy JP, von Caemmerer S. 2005. Faster Rubisco is the key to superior nitrogen-use efficiency in NADP-malic enzyme relative to NAD-malic enzyme C4 grasses. Plant Physiology 137, 638–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano M, Beardall J, Raven JA. 2005. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annual Review of Plant Biology 56, 99–131. [DOI] [PubMed] [Google Scholar]
- Goldman JA, Kranz SA, Young JN, Tortell PD, Stanley RH, Bender ML, Morel FM. 2015. Gross and net production during the spring bloom along the Western Antarctic Peninsula. New Phytologist 205, 182–191. [DOI] [PubMed] [Google Scholar]
- Gordillo FJ, Aguilera J, Jiménez C. 2006. The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. Journal of Experimental Botany 57, 2661–2671. [DOI] [PubMed] [Google Scholar]
- Gordillo FJL, Carmona R, Viñegla B, Wiencke C, Jiménez C. 2016. Effects of simultaneous increase in temperature and ocean acidification on biochemical composition and photosynthetic performance of common macroalgae from Kongsfjorden (Svalbard). Polar Biology 39, 1993–2007. [Google Scholar]
- Hepburn CD, Pritchard DW, Cornwall CE, McLeod RJ, Beardall J, Raven JA, Hurd CL. 2011. Diversity of carbon use strategies in a kelp forest community: implications for a high CO2 ocean. Global Change Biology 17, 2488–2497. [Google Scholar]
- Hermida-Carrera C, Kapralov MV, Galmés J. 2016. Rubisco catalytic properties and temperature response in crops. Plant Physiology 171, 2549–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heureux AMC, Young JN, Whitney SM, Eason-Hubbard MR, Lee RBY, Sharwood RE, Rickaby REM. 2017. The role of Rubisco kinetics and pyrenoid morphology in shaping the CCM of haptophyte microalgae. Journal of Experimental Botany 68, 3959–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holaday AS, Martindale W, Alred R, Brooks AL, Leegood RC. 1992. Changes in activities of enzymes of carbon metabolism in leaves during exposure of plants to low temperature. Plant Physiology 98, 1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hop H, Wiencke C, Vögele B, Kovaltchouk NA. 2012. Species composition, zonation, and biomass of marine benthic macroalgae in Kongsfjorden, Svalbard. Botanica Marina 55, 399–414. [Google Scholar]
- Iken K, Quartino M, Barrera Oro E, Palermo J, Wiencke C, Brey T. 1998. Trophic relations between macroalgae and herbivores. Reports on Polar and Marine Research 299, 258–262. [Google Scholar]
- Iñiguez C, Carmona R, Lorenzo MR, Niell FX, Wiencke C, Gordillo FJL. 2016a Increased CO2 modifies the carbon balance and the photosynthetic yield of two common Arctic brown seaweeds: Desmarestia aculeata and Alaria esculenta. Polar Biology 39, 1979–1991. [Google Scholar]
- Iñiguez C, Carmona R, Lorenzo MR, Niell FX, Wiencke C, Gordillo FJL. 2016b Increased temperature, rather than elevated CO2, modulates the carbon assimilation of the Arctic kelps Saccharina latissima and Laminaria solidungula. Marine biology 163, 248. [Google Scholar]
- Israel A, Hophy M. 2002. Growth, photosynthetic properties and Rubisco activities and amounts of marine macroalgae grown under current and elevated seawater CO2 concentrations. Global Change Biology 8, 831–840. [Google Scholar]
- Johnston AM. 1991. The acquisition of inorganic carbon by marine macroalgae. Canadian Journal of Botany 69, 1123–1132. [Google Scholar]
- Jordan DB, Ogren WL. 1984. The CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Planta 161, 308–313. [DOI] [PubMed] [Google Scholar]
- Kapralov MV, Kubien DS, Andersson I, Filatov DA. 2011. Changes in Rubisco kinetics during the evolution of C4 photosynthesis in Flaveria (Asteraceae) are associated with positive selection on genes encoding the enzyme. Molecular Biology and Evolution 28, 1491–1503. [DOI] [PubMed] [Google Scholar]
- Klenell M, Snoeijs P, Pedersen M. 2004. Active carbon in Laminaria digitata and L. saccharina (Phaeophyta) is driven by a proton pump in the plasma membrane. Hydrobiologia 514, 41–53. [Google Scholar]
- Koch M, Bowes G, Ross C, Zhang XH. 2013. Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology 19, 103–132. [DOI] [PubMed] [Google Scholar]
- Kubien DS, Brown C, Kane H. 2011. Quantifying the amount and activity of Rubisco in leaves. Methods in Molecular Biology 684, 349–362. [DOI] [PubMed] [Google Scholar]
- Le Corguillé G, Pearson G, Valente M, et al. 2009. Plastid genomes of two brown algae, Ectocarpus siliculosus and Fucus vesiculosus: further insights on the evolution of red-algal derived plastids. BMC Evolutionary Biology 9, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan N, Tsaia YC, Mueller-Cajar O. 2016. Characterization of the heterooligomeric red-type rubisco activase from red algae. Proceedings of the National Academy of Sciences 113, 14019–14024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüning K. 1984. Temperature tolerance and biogeography of seaweeds: the marine algal flora of Helgoland (North Sea) as an example. Helgoländer Meeresunters 38, 305–317. [Google Scholar]
- Maberly SC, Raven JA, Johnston AM. 1992. Discrimination between 12C and 13C by marine plants. Oecologia 91, 481–492. [DOI] [PubMed] [Google Scholar]
- Meyer M, Griffiths H. 2013. Origins and diversity of eukaryotic CO2-concentrating mechanisms: lessons for the future. Journal of Experimental Botany 64, 769–786. [DOI] [PubMed] [Google Scholar]
- Morita K, Hatanaka T, Misoo S, Fukayama H. 2014. Unusual small subunit that is not expressed in photosynthetic cells alters the catalytic properties of rubisco in rice. Plant Physiology 164, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller-Cajar O, Whitney SM. 2008. Evolving improved Synechococcus Rubisco functional expression in Escherichia coli. The Biochemical Journal 414, 205–214. [DOI] [PubMed] [Google Scholar]
- Mueller-Cajar O, Stotz M, Wendler P, Hartl FU, Bracher A, Hayer-Hartl M. 2011. Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase. Nature 479, 194–199. [DOI] [PubMed] [Google Scholar]
- Nisbet EG, Grassineau NV, Howe CJ, Abell PI, Regelous M, Nisbet RER. 2007. The age of RubisCO: the evolution of oxygenic photosynthesis. Geobiology 5, 311–355. [Google Scholar]
- Novaczek I, Lubbers GW, Breeman AM. 1990. Thermal ecotypes in amphi-Atlantic algae. I. Algae of Arctic to cold-temperate distribution (Chaetomorpha melagonium, Devaleraea ramentacea and Phycodrys rubens). Helgoländer Meeresunters 44, 459–474. [Google Scholar]
- Olischläger M, Iñiguez C, Gordillo FJ, Wiencke C. 2014. Biochemical composition of temperate and Arctic populations of Saccharina latissima after exposure to increased pCO2 and temperature reveals ecotypic variation. Planta 240, 1213–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olischläger M, Iñiguez C, Koch K, Wiencke C, Gordillo FJ. 2017. Increased pCO2 and temperature reveal ecotypic differences in growth and photosynthetic performance of temperate and Arctic populations of Saccharina latissima. Planta 245, 119–136. [DOI] [PubMed] [Google Scholar]
- Pearce FG. 2006. Catalytic by-product formation and ligand binding by ribulose bisphosphate carboxylases from different phylogenies. The Biochemical Journal 399, 525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdomo JA, Cavanagh AP, Kubien DS, Galmés J. 2015. Temperature dependence of in vitro Rubisco kinetics in species of Flaveria with different photosynthetic mechanisms. Photosynthesis Research 124, 67–75. [DOI] [PubMed] [Google Scholar]
- Quartino ML, Boraso de Zaixso AL. 2008. Summer macroalgal biomass in Potter Cove, South Shetland Islands. Antarctica: its production and flux to the ecosystem. Polar Biology 31, 281–294. [Google Scholar]
- Raven JA, Ball LA, Beardall J, Giordano M, Maberly SC. 2005. Algae lacking carbon-concentrating mechanisms. Canadian Journal of Botany 83, 879–890. [Google Scholar]
- Raven JA, Beardall J. 2003. Carbon acquisition mechanisms of algae: carbon dioxide diffusion and carbon dioxide concentrating mechanisms. In: Larkum AW, Douglas SE, Raven JA, eds. Photosynthesis in algae. Advances in Photosynthesis and Respiration, Vol. 14 Dordrecht: Kluwer Academic Publishers, 225–244. [Google Scholar]
- Raven JA, Beardall J, Giordano M. 2014. Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynthesis Research 121, 111–124. [DOI] [PubMed] [Google Scholar]
- Raven JA, Falkowski PG. 1999. Oceanic sinks for CO2. Plant, Cell and Environment 22, 275–278. [Google Scholar]
- Raven JA, Johnston AM, Kübler JE, et al. 2002. a Mechanistic interpretation of carbon isotope discrimination by marine macroalgae and seagrasses. Functional Plant Biology 29, 335–78. [DOI] [PubMed] [Google Scholar]
- Raven JA, Johnston AM, Kübler JE, et al. 2002b. Seaweeds in cold seas: evolution and carbon acquisition. Annals of Botany 90, 525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeske CA, O’Leary MH. 1984. Carbon isotope effects on the enzyme-catalyzed carboxylation of ribulose bisphosphate. Biochemistry 23, 6275–6284. [DOI] [PubMed] [Google Scholar]
- Ruuska S, Andrews T, Badger M, Hudson G, Laisk A, Price GD, von Caemmerer S. 1998. The interplay between limiting processes in C3 photosynthesis studied by rapid-response gas exchange using transgenic tobacco impaired in photosynthesis. Australian Journal of Plant Physiology 25, 859–870. [Google Scholar]
- Sage RF. 2002. Variation in the kcat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. Journal of Experimental Botany 53, 609–620. [DOI] [PubMed] [Google Scholar]
- Savir Y, Noor E, Milo R, Tlusty T. 2010. Cross-species analysis traces adaptation of Rubisco toward optimality in a low-dimensional landscape. Proceedings of the National Academy of Sciences, USA 107, 3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seemann JR, Badger MR, Berry JA. 1984. Variations in the specific activity of ribulose-1,5-bisphosphate carboxylase between species utilizing differing photosynthetic pathways. Plant Physiology 74, 791–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Berry JA. 1985. Carbon isotope fractionation of algae influenced by an inducible CO2-concentrating mechanism. In: Lucas WJ, Berry JA, eds. Inorganic carbon uptake by aquatic photosynthetic organisms. Rockville: American Society of Plant Physiologists, 389–401. [Google Scholar]
- Sharwood RE, Ghannoum O, Kapralov MV, Gunn LH, Whitney SM. 2016. Temperature responses of Rubisco from Paniceae grasses provide opportunities for improving C3 photosynthesis. Nature Plants 2, 16186. [DOI] [PubMed] [Google Scholar]
- Sharwood RE, von Caemmerer S, Maliga P, Whitney SM. 2008. The catalytic properties of hybrid Rubisco comprising tobacco small and sunflower large subunits mirror the kinetically equivalent source Rubiscos and can support tobacco growth. Plant Physiology 146, 83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock JA, Raven JA. 2001. Interactions between carbon dioxide and oxygen in the photosynthesis of three species of marine red algae. Botanical Journal of Scotland 113, 301–307. [Google Scholar]
- Skirrow G. 1975. The dissolved gases-carbon dioxide. In: Wiley JP, Skirrow G, eds. Chemical oceanography, Vol. 2 New York: Academic Press, 1–192. [Google Scholar]
- Spreitzer RJ, Salvucci ME. 2002. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Annual Review of Plant Biology 53, 449–475. [DOI] [PubMed] [Google Scholar]
- Stepien CC. 2015. Impacts of geography, taxonomy and functional group on inorganic carbon use patterns in marine macrophytes. Journal of Ecology 103, 1372–1383. [Google Scholar]
- Surif MB, Raven JA. 1989. Exogenous inorganic carbon sources for photosynthesis in seawater of the Fucales and Laminariales (Phaeophyta): ecological and taxonomic implications. Oecologia 78, 97–105. [DOI] [PubMed] [Google Scholar]
- Tabita FR, Satagopan S, Hanson TE, Kreel NE, Scott SS. 2008. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. Journal of Experimental Botany 59, 1515–1524. [DOI] [PubMed] [Google Scholar]
- Tcherkez G. 2013. Modelling the reaction mechanism of ribulose-1,5-bisphosphate carboxylase/oxygenase and consequences for kinetic parameters. Plant, Cell & Environment 36, 1586–1596. [DOI] [PubMed] [Google Scholar]
- Tcherkez GGB, Farquhar GD, Andrews TJ. 2006. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proceedings of the National Academy of Sciences, USA 103, 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DN, Wiencke C. 1991. Photosynthesis, dark respiration and light independent carbon fixation of endemic Antarctic macroalgae. Polar Biology 11, 329 –337. [Google Scholar]
- tom Dieck I. 1992. North Pacific and North Atlantic digitate Laminaria species (Phaeophyta): hybridization experiments and temperature responses. Phycologia 31, 147–163. [Google Scholar]
- Valentin K, Zetsche K. 1989. The genes of both subunits of ribulose-1,5-bisphosphate carboxylase constitute an operon on the plastome of a red alga. Current Genetics 16, 203–209. [DOI] [PubMed] [Google Scholar]
- van de Poll WH, Eggert E, Buma AGJ, Breeman AM. 2002. Temperature dependence of UV radiation effects in arctic and temperate isolates of three red macrophytes. European Journal of Phycology 37, 59–68. [Google Scholar]
- Wiencke C, Clayton MN, Gómez I, Iken K, Lüder UH, Amsler CD, Karsten U, Hanelt D, Bischof K, Dunton K. 2007. Life strategy, ecophysiology and ecology of seaweeds in polar waters. Reviews in Environmental Science and Biotechnology 6, 95–126. [Google Scholar]
- Wiencke C, tom Dieck I. 1989. Temperature requirements for growth and temperature tolerance of macroalgae endemic to the Antarctic region. Marine Ecology Progress Series 54, 189–197. [Google Scholar]
- Whitney SM, Baldet P, Hudson GS, Andrews TJ. 2001. Form I Rubiscos from non-green algae are expressed abundantly but not assembled in tobacco chloroplasts. The Plant Journal 26, 535–547. [DOI] [PubMed] [Google Scholar]
- Whitney SM, Houtz RL, Alonso H. 2011. Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme, Rubisco. Plant Physiology 155, 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamori W, Noguchi K, Terashima I. 2005. Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant, Cell and Environment 28, 536–547. [Google Scholar]
- Yeoh HH, Badger MR, Watson L. 1981. Variations in kinetic properties of ribulose-1,5-bisphosphate carboxylases among plants. Plant Physiology 67, 1151–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JN, Goldman JA, Kranz SA, Tortell PD, Morel FM. 2015. Slow carboxylation of Rubisco constrains the rate of carbon fixation during Antarctic phytoplankton blooms. New Phytologist 205, 172–181. [DOI] [PubMed] [Google Scholar]
- Young JN, Heureux AM, Sharwood RE, Rickaby RE, Morel FM, Whitney SM. 2016. Large variation in the Rubisco kinetics of diatoms reveals diversity among their carbon-concentrating mechanisms. Journal of Experimental Botany 67, 3445–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacher K, Rautenberger R, Hanelt D, Wulff A, Wiencke C. 2011. The abiotic environment of polar benthic algae. In: Wiencke C, ed. Biology of polar benthic algae. Berlin: De Gruyter, 9–22. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.