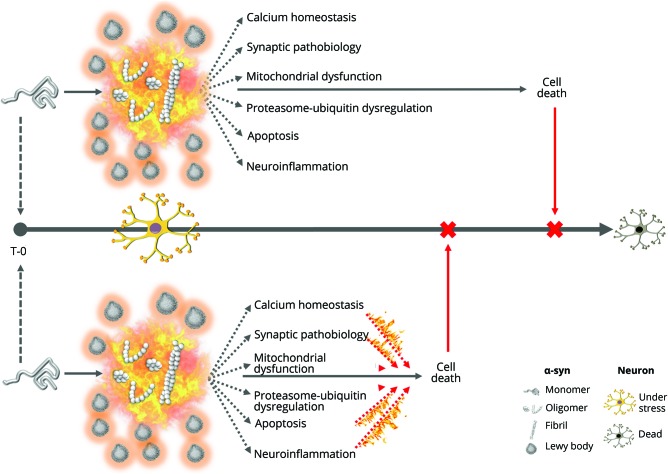
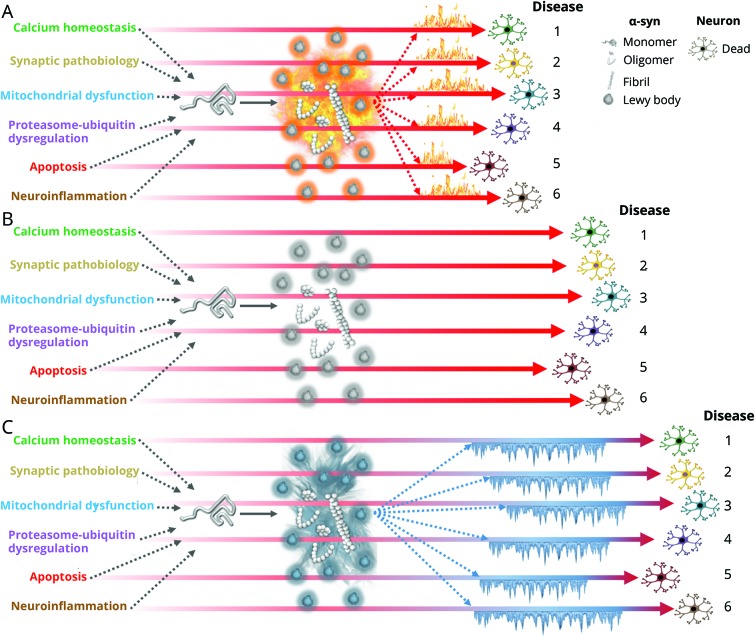
Alberto J Espay
Alberto J Espay, MD, MSc
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,✉,
Joaquin A Vizcarra
Joaquin A Vizcarra, MD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Luca Marsili
Luca Marsili, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Anthony E Lang
Anthony E Lang, MD, FRCPC
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
David K Simon
David K Simon, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Aristide Merola
Aristide Merola, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Keith A Josephs
Keith A Josephs, MD, MST, MSc
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Alfonso Fasano
Alfonso Fasano, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Francesca Morgante
Francesca Morgante, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Rodolfo Savica
Rodolfo Savica, MD, MSc
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
J Timothy Greenamyre
J Timothy Greenamyre, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Franca Cambi
Franca Cambi, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Tritia R Yamasaki
Tritia R Yamasaki, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Caroline M Tanner
Caroline M Tanner, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Ziv Gan-Or
Ziv Gan-Or, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Irene Litvan
Irene Litvan, MD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Ignacio F Mata
Ignacio F Mata, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Cyrus P Zabetian
Cyrus P Zabetian, MD, MS
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Patrik Brundin
Patrik Brundin, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Hubert H Fernandez
Hubert H Fernandez, MD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
David G Standaert
David G Standaert, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Marcelo A Kauffman
Marcelo A Kauffman, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Michael A Schwarzschild
Michael A Schwarzschild, MD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
S Pablo Sardi
S Pablo Sardi, PharmD, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
Todd Sherer
Todd Sherer, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
George Perry
George Perry, PhD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1,
James B Leverenz
James B Leverenz, MD
1From the UC Gardner Neuroscience Institute and Gardner Family Center for Parkinson's Disease and Movement Disorders (A.J.E., J.A.V., L.M., A.M.), Department of Neurology, University of Cincinnati, OH; Edmond J. Safra Program in Parkinson's Disease and the Morton and Gloria Shulman Movement Disorders Clinic (A.E.L., A.F.), Toronto Western Hospital, University of Toronto; Krembil Research Institute (A.E.L., A.F.), Toronto, Canada; Parkinson's Disease and Movement Disorders Center (D.K.S.), Department of Neurology, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, MA; College of Medicine (K.A.J.), Mayo Clinic, Rochester, MN; Institute of Molecular and Clinical Sciences (F.M.), St George's University of London, UK; Division of Movement Disorders (R.S.), Department of Neurology and Department of Health Science Research, Mayo Clinic College of Medicine, Rochester, MN; Department of Neurology and the Pittsburgh Institute for Neurodegenerative Diseases (J.T.G., F.C.), University of Pittsburgh, PA; Department of Neurology (T.R.Y.), University of Kentucky, Lexington; Parkinson's Disease Research, Education and Clinical Center (C.M.T.), Neurology, San Francisco Veterans Affairs Medical Center; Department of Neurology (C.M.T.), University of California–San Francisco; Department of Neurology & Neurosurgery, Montreal Neurological Institute, and Department of Human Genetics (Z.G.-O.), McGill University, Canada; Parkinson & Other Movement Disorders Center UC San Diego (I.L.), Department of Neurosciences, Altman Clinical Translational Research Institute, La Jolla, CA; VA Puget Sound Health Care System and Department of Neurology (I.F.M., CP.Z.), University of Washington, Seattle; Department of Neurology (I.F.M.), University of Washington School of Medicine, Seattle; Center for Neurodegenerative Science (P.B.), Van Andel Research Institute, Grand Rapids, MI; Center for Neurological Restoration (H.H.F.) and Lou Ruvo Center for Brain Health, Neurological Institute (J.B.L.), Cleveland Clinic, OH; Department of Neurology (D.G.S.), University of Alabama at Birmingham; Consultorio y Laboratorio de Neurogenética (M.A.K.), Centro Universitario de Neurología “José María Ramos Mejía” y División Neurología, Hospital JM Ramos Mejía, Facultad de Medicina, UBA; Programa de Medicina de Precision y Genomica Clinica (M.A.K.), Instituto de Investigaciones en Medicina Traslacional, Facultad de Ciencias Biomédicas, Universidad Austral-CONICET, Buenos Aires, Argentina; Department of Neurology (M.A.S.), Massachusetts General Hospital, Boston; Division of Neuroscience (S.P.S.), Sanofi-Genzyme, Framingham, MA; Michael J. Fox Foundation for Parkinson's Research (T.S.), New York, NY; and College of Sciences (G.P.), University of Texas at San Antonio.
1