Abstract
Objective
To compare [18F]AV-1451 uptake in the semantic variant of primary progressive aphasia (svPPA) to Alzheimer dementia, and determine whether increased uptake in svPPA is associated with the presence of β-amyloid (Aβ).
Methods
Thirty-one participants with svPPA underwent MRI and Pittsburgh compound B–PET scanning, and 17 of these also underwent [18F]AV-1451 tau-PET. A global Pittsburgh compound B standardized uptake value ratio was calculated for all participants, with a cutoff of 1.42 used to define Aβ(+) participants. We assessed region and voxel-level [18F]AV-1451 uptake in the whole svPPA cohort and separately in Aβ(+) and Aβ(−) svPPA groups, compared to 12 Aβ(+) participants with Alzheimer dementia and 170 cognitively normal, Aβ(−) controls.
Results
Of the entire cohort of participants with svPPA, 26% were Aβ(+). The Aβ(+) participants were older at scan compared to the Aβ(−) participants. svPPA showed elevated [18F]AV-1451 uptake in anteromedial temporal regions but the degree of uptake was lower than in Alzheimer dementia. After controlling for age, Aβ(+) status in svPPA was associated with significantly higher uptake in all anteromedial and inferior/middle lateral temporal regions, but uptake was still lower than in Alzheimer dementia.
Conclusion
Although [18F]AV-1451 uptake is focally elevated in svPPA, the level of uptake is much less than what occurs in Alzheimer dementia and appears to be at least partially related to Aβ. Therefore, it is possible that some of the increased uptake of [18F]AV-1451 in svPPA is related to binding paired helical filament tau.
The semantic variant of primary progressive aphasia (svPPA)1 is characterized by anomia and the loss of conceptual knowledge pertaining to the meaning of words and results from neurodegeneration of the anteromedial temporal lobes. It is most frequently associated with deposition of the TAR DNA binding protein of 43 kDa (TDP-43) at autopsy,2–8 although svPPA cases have been reported with Alzheimer disease3,6,9,10 and definite or possible primary age-related tauopathy (PART).11,12 PET ligands, such as [18F]AV-1451,13 are now available that allow the detection of the paired helical filament 3R + 4R tau associated with Alzheimer disease and show little or no binding to TDP-43.14–16 Despite this, elevated uptake of [18F]AV-1451 has been reported in patients with svPPA, with uptake observed in anteromedial temporal lobes concordant with the topographic distribution of neurodegeneration.17–19 It is, however, unclear whether the degree of uptake observed with [18F]AV-1451 is comparable to the degree of uptake observed in Alzheimer disease. In addition, the biological basis for [18F]AV-1451 uptake in svPPA is currently unknown.17–19
We aimed to determine whether the degree of [18F]AV-1451 uptake in svPPA was similar to that observed in Alzheimer dementia, and whether the pattern or degree of uptake in svPPA differs according to the presence of β-amyloid (Aβ) deposition on PET imaging. The presence of Aβ deposition on PET is typically considered a biomarker for Alzheimer disease, and hence we will be able to evaluate the evidence that [18F]AV-1451 uptake in Aβ(+) participants with svPPA may be related to the presence of paired helical filament tau pathology.
Methods
Participants
Thirty-one participants who met clinical criteria for svPPA1 were recruited from the Department of Neurology, Mayo Clinic, Rochester, MN, into an NIH-funded study (principal investigator K.A.J.) between October 28, 2010, and April 10, 2018. All 31 participants underwent standardized neurologic, speech and language, and neuropsychological assessments, Pittsburgh compound B (PiB)-PET, and a 3-tesla volumetric MRI. Twenty-five of the 31 participants with svPPA underwent APOE genotyping and 17 underwent [18F]AV-1451 PET.
The 17 participants with svPPA who underwent [18F]AV-1451 scans were matched 10:1 by age and sex to 170 Aβ(−) cognitively normal controls (median [interquartile range] age at scan 66 [59–70] years, global PiB standardized uptake value ratio [SUVR] 1.31 [1.27–1.36], and 59% male) recruited into the Mayo Clinic Study of Aging.20 Twelve Aβ(+) participants with typical amnestic Alzheimer dementia (median [interquartile range] age at scan 74 [70–76] years, global PiB SUVR 2.74 [2.57–2.92], Montreal Cognitive Assessment 13 [9–18], and 51% male) were also selected from the Mayo Clinic Alzheimer's Disease Research Center database matched 3:1 by age and sex to the 4 Aβ(+) participants with svPPA who had [18F]AV-1451 scans. All controls and participants with Alzheimer dementia had undergone identical [18F]AV-1451, PiB-PET, and 3-tesla volumetric MRI acquisitions as the participants with svPPA.
All participants consented to having their data utilized for research, and the study was approved by the Mayo Clinic institutional review board.
Clinical test battery
All participants with svPPA were administered identical neurologic, speech and language, and neuropsychological test batteries. The neurologic battery included tests of general cognitive function (Montreal Cognitive Assessment),21 presence of psychiatric features (Neuropsychiatric Inventory–short version),22 behavioral control (Cambridge Behavioral Inventory),23 executive function (Frontal Assessment Battery), praxis (praxis subtest of the Western Aphasia Battery),24 face recognition (10-item facial recognition task),25 and motor parkinsonism (Movement Disorders Society–sponsored revision of the Unified Parkinson's Disease Rating Scale, Part III).26 The speech-language battery included tests of aphasia severity (Western Aphasia Battery Aphasia Quotient), confrontation naming (the Sydney Language Battery for Naming),27 single-word comprehension (Pyramids and Palm Trees Test, word-word version),28 object knowledge (the Sydney Language Battery for Semantic Association Task),27 phonemic fluency (Letter Fluency Test [FAS]), semantic fluency (Animal Fluency Test), syntactic ability (Northwestern Anagram Test),29 sentence repetition (repetition subtest of the Boston Diagnostic Aphasia Examination and the repetition subtest of the Western Aphasia Battery),30 surface vs deep dyslexia (Western Aphasia Battery reading irregular words and reading nonwords), and lexical processing (Peabody Picture Vocabulary Test). The neuropsychological battery included tests of visual perceptual abilities (the Visual Object and Space Perception Battery–fragmented letters),31 visual spatial abilities (Visual Object and Space Perception Battery–cube analysis31 and the Rey-Osterrieth Complex Figure),32 psychomotor speed (Trail Making Test, Part A),32 executive function (Trail Making Test, Part B),32 working memory (Digit Span and Spatial Span),32 verbal and visual episodic memory (the Camden Memory Test–Short Recognition Memory Test for words and faces).33
Image acquisition
All PET scans were acquired using a PET/CT scanner (GE Healthcare, Milwaukee, WI) operating in 3-dimensional mode. For tau-PET, an IV bolus injection of approximately 370 MBq (range 333–407 MBq) of [18F]AV-1451 was administered, followed by a 20-minute PET acquisition performed 80 minutes after injection. For [18F]-fluorodeoxyglucose (FDG)-PET, participants were injected with 18F-FDG of approximately 459 MBq (range 367–576 MBq), and after a 30-minute uptake period, an 8-minute 18F-FDG scan was performed. For PiB-PET, participants were injected with PiB of approximately 628 MBq (range 385–723 MBq), and after a 40 minute uptake period, a 20-minute PiB scan was obtained consisting of four 5-minute dynamic frames. Standard corrections were applied. Emission data were reconstructed into a 256 × 256 matrix with a 30-cm field of view (pixel size = 1.0 mm, slice thickness = 1.96 mm). A global PiB SUVR was also generated for each patient in the study using the cerebellar crus gray matter as a reference region, and a cut point of 1.42 was used to establish Aβ positivity, as previously described.34 All participants had a 3-tesla MP-RAGE sequence performed on the same day as the tau-PET, as previously described.34
[18F]AV-1451 analysis
Patterns of [18F]AV-1451 uptake were assessed both at the region and voxel level using SPM12 (fil.ion.ucl.ac.uk/SPM). The [18F]AV-1451 images were each registered to the participant's MP-RAGE using 6 degrees-of-freedom registration. Normalization parameters were computed between each MP-RAGE and the Mayo Clinic Adult Lifespan Template (MCALT) (nitrc.org/projects/mcalt/) using ANTs.35 With these parameters, the MCALT atlases were propagated to native MP-RAGE space and used to output region-level data from the following set of 10 regions of interest (ROIs): temporal pole (middle + superior temporal pole), inferior/middle temporal gyrus, superior temporal gyrus, amygdala, entorhinal cortex, fusiform gyrus, medial frontal lobe (frontal superior medial + anterior cingulate + supplementary motor area), orbitofrontal lobe (inferior, middle, superior, and medial orbitofrontal cortex + rectus gyrus), inferior parietal lobe (inferior parietal + supramarginal + angular gyrus), and precuneus. We specifically analyzed many subregions of the temporal lobe, as well as frontal regions, because of their involvement in svPPA and regions in the parietal lobe since [18F]AV-1451 uptake has been observed in these regions in Alzheimer dementia. Median [18F]AV-1451 was calculated from both gray and white matter voxels in each ROI and divided by median uptake in cerebellar crus gray matter to create SUVRs. For the voxel-level analyses, all voxels in the MP-RAGE-space [18F]AV-1451 images were divided by the median uptake in the cerebellar crus gray matter using the MCALT atlas to create SUVR images. These SUVR images were normalized to the MCALT and smoothed at 6 mm full-width at half maximum. Voxel-level comparisons were performed assessing uptake in the Aβ(−) and Aβ(+) participants with svPPA compared to controls and the participants with Alzheimer dementia. Results were assessed using 2-sided t tests in SPM12, at p < 0.05 after correction for multiple comparisons using the family-wise error correction. Age and sex were included in all analyses as covariates.
Statistical analysis
We fit a Bayesian hierarchical linear regression model using Markov Chain Monte Carlo simulation to estimate mean log-transformed [18F]AV-1451 uptake across the 10 ROIs separately for each of 4 diagnosis groups [Aβ(−) svPPA, Aβ(+) svPPA, Aβ(+) Alzheimer dementia, and controls] while adjusting for age and within-participant correlation. By estimating all regional quantities of interest within a single hierarchical model, we can borrow statistical strength across regions, stabilize our estimates via shrinkage, account for multiple comparisons, and reduce overall mean square error.36–38
Our model specified an overall mean within each group and assumed that regional values for the group were normally distributed with a group-specific SD, a distribution denoted by Normal(μgroup, σgroup). The means of the control and Alzheimer dementia groups were assumed independent of each other and of the 2 svPPA means while the Aβ(−) and Aβ(+) svPPA means were allowed to be correlated. Weakly informative priors were used for the group-wise means by specifying a Normal(0, 2) prior distribution. The group-wise SD for control and Alzheimer dementia groups were modeled as following a half-Normal(0, 2) distribution and the group-wise SD for both svPPA groups as following a half-Normal(1, 3). Our model also included a participant-specific intercept term modeled as Normal(0, σparticipant). A priori, this participant-specific SD was given a weakly informative half-Normal(0, 3) distribution. To account for age, which we centered at 65 and scaled by 10 years, we included an age coefficient for controls, Alzheimer dementia, and svPPA. These 3 parameters were given weakly informative Normal(0, 1) priors.
Results were based on 100 parallel Markov Chain Monte Carlo chains with randomly generated initial values, each of length 200,000 and thinned to every 20th value, resulting in a posterior sample size of 1,000,000. We report interval estimates for regional means and group-wise differences in regional means based on quantiles of the samples. The posterior probability that a regional mean of one group is greater than that of another group is based on the proportion of posterior samples where a positive difference was observed. Analyses were performed with R39 version 3.4.2 using the rjags package40 version 4-6 running JAGS version 4.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Mayo Clinic institutional review board. All participants consented to participate in the research study.
Data availability
All data from the study will be shared in an anonymized format to any qualified investigator on request from the corresponding author.
Results
The cohort of 31 participants with svPPA had a median (25th quartile, 75th quartile) age at scan of 67 (60–72) years and consisted of 52% males (table 1). Eight of the 31 (26%) were Aβ(+) and 11 of 29 (38%) were APOE ε4 carriers. The Aβ(+) participants consisted of 38% male, had median age at scan of 75 (69–77) years, and 63% were APOE ε4 carriers. The Aβ(−) participants consisted of 57% male, had median age at scan of 64 (58–69) years, and 29% were APOE ε4 carriers. Family history of a neurologic disease in immediate family members was present in 4 of 30 participants (13%) (unknown in the last case); all 4 were Aβ(−) and one was an APOE ε4 carrier. In the [18F]AV-1451 cohort, 4 of 17 (24%) were Aβ(+) and 5 of 15 (33%) were APOE ε4 carriers. Demographic and clinical features of the [18F]AV-1451 cohort were no different from the larger cohort and are shown in table 2. The Aβ(+) participants were older at the time of scan and had slightly shorter disease duration compared to the Aβ(−) participants, but performed comparably on clinical testing. The global PiB SUVRs in the Aβ(+) participants ranged from 1.50 to 2.48, and were noticeably lower than the global PiB SUVRs observed in Alzheimer dementia (figure 1).
Table 1.
Demographics and clinical features of the full svPPA cohort of 31 participants
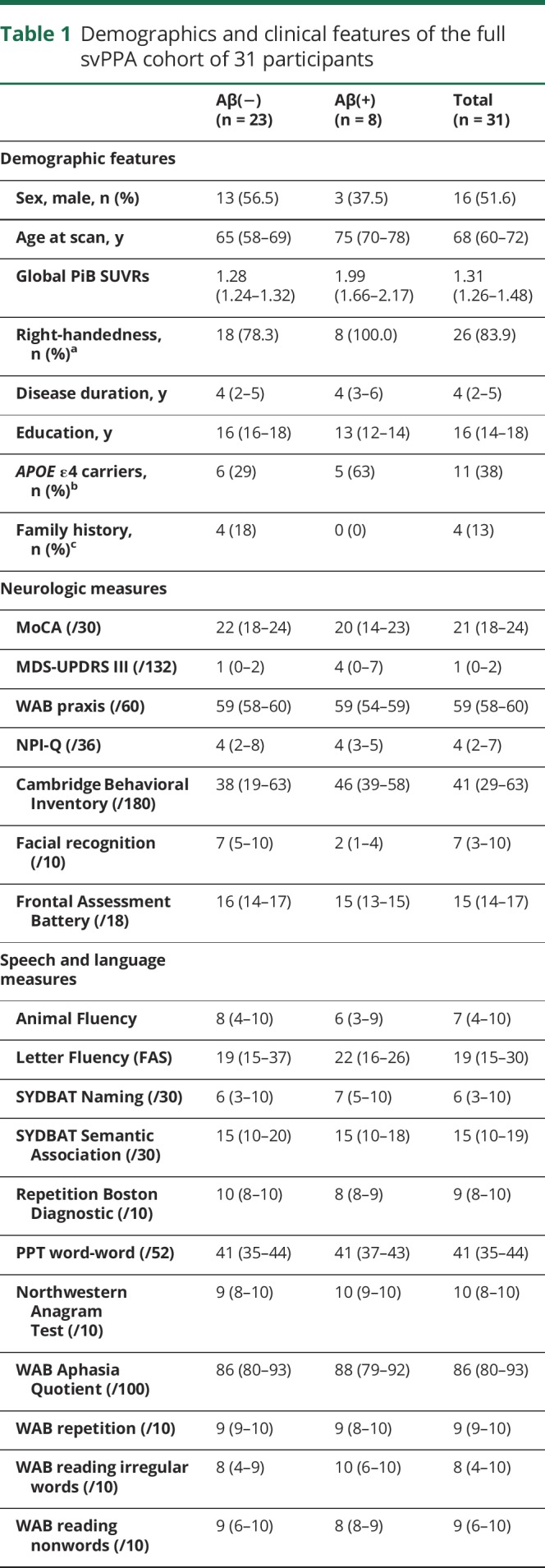
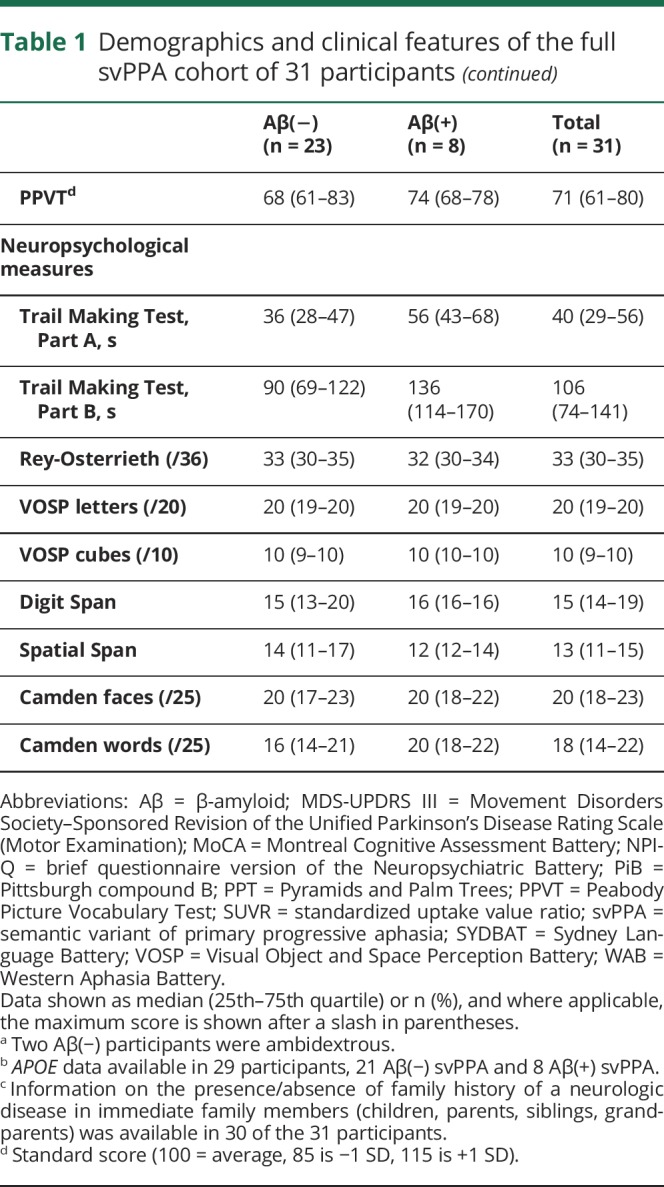
Table 2.
Demographics and clinical features of the [18F]AV-1451 svPPA cohort
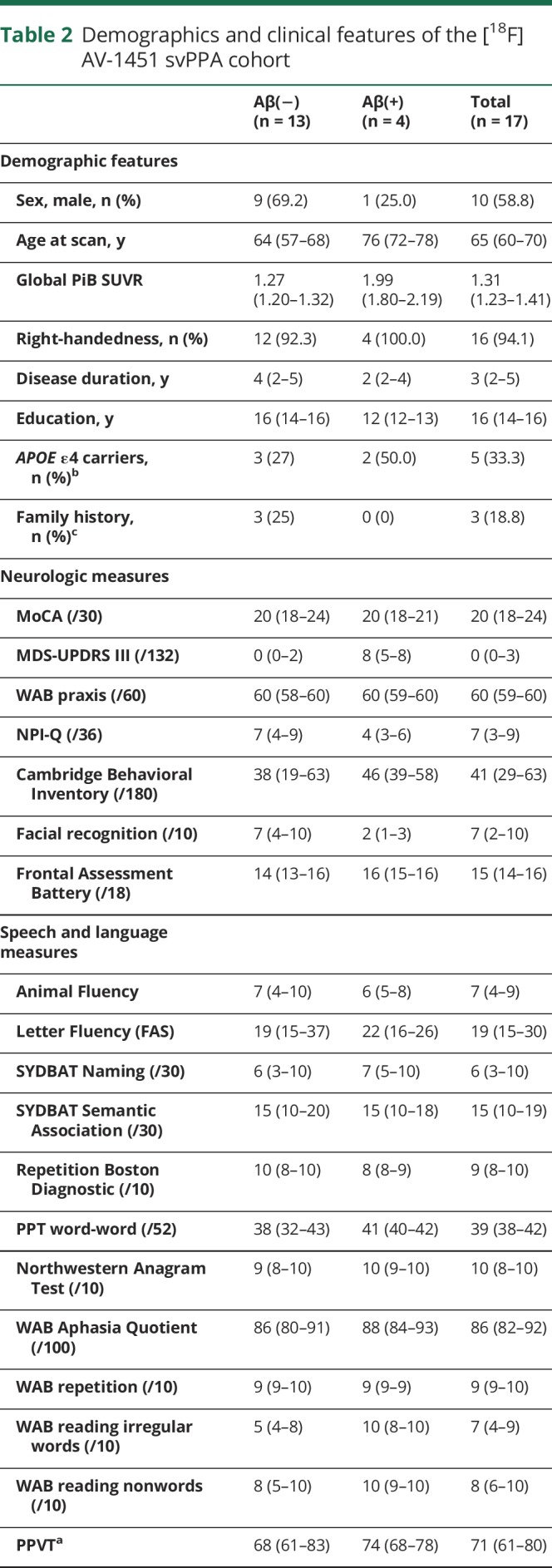
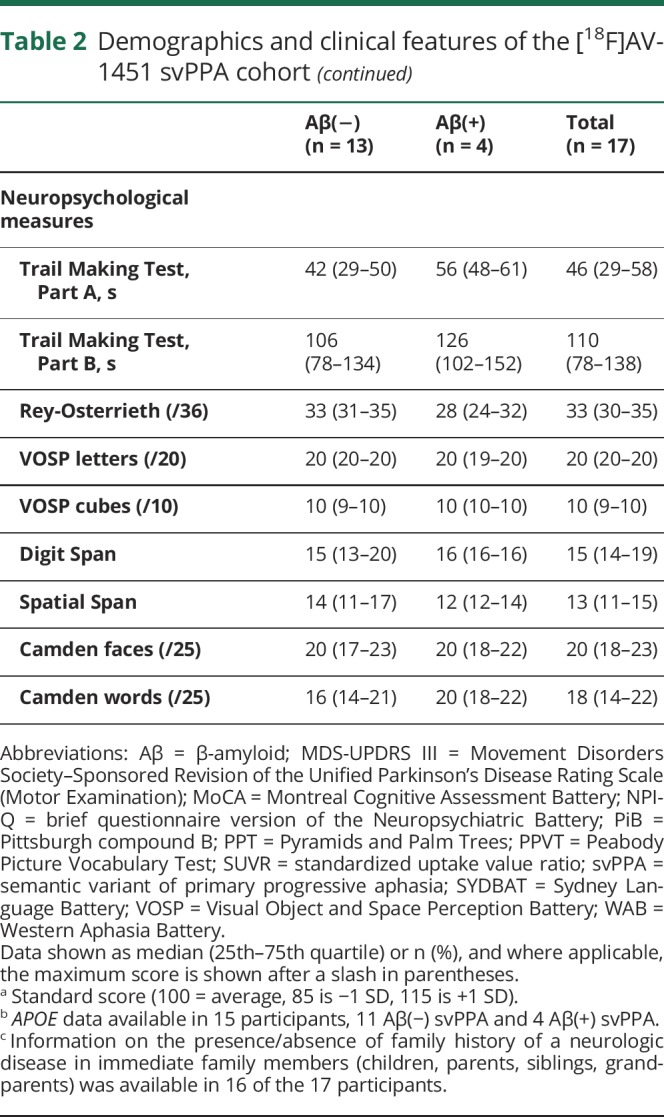
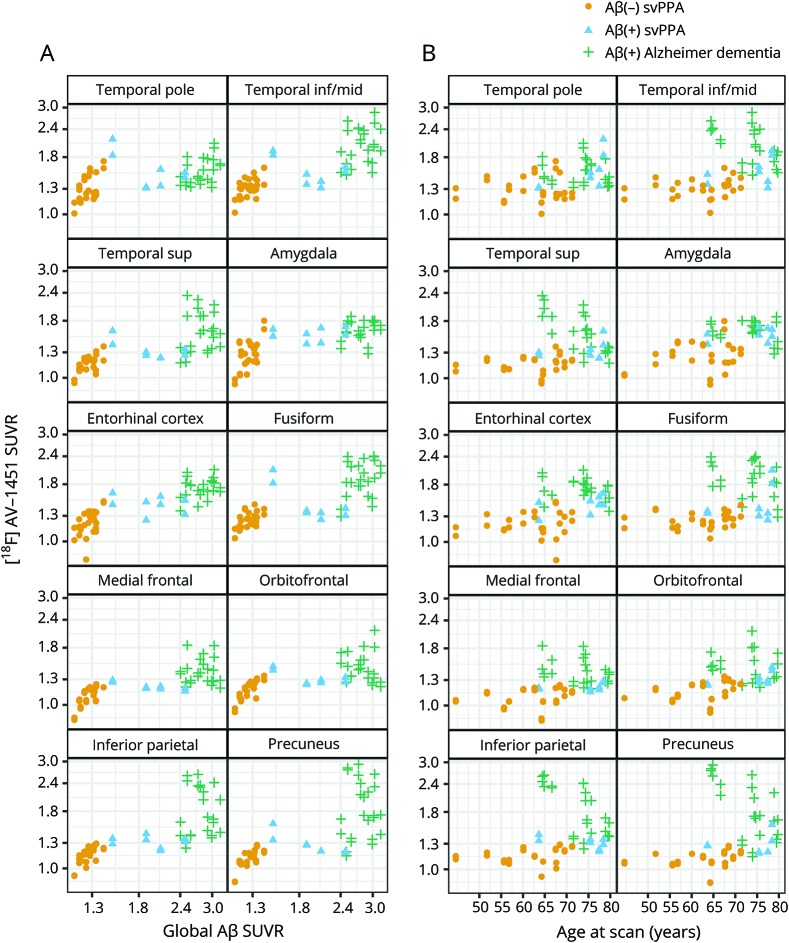
Figure 1. [18F]AV-1451 SUVR by global PiB SUVR (A) and age (B).
[18F]AV-1451 SUVR and global PiB SUVR are shown on a log scale. There are 2 points per patient in each facet, one for each hemisphere. Aβ(−) participants with svPPA are represented by yellow circles, Aβ(+) patients with svPPA by blue triangles, and Aβ(+) participants with Alzheimer dementia by green crosses. Aβ = β-amyloid; inf = inferior; mid = middle; sup = superior; SUVR = standardized uptake value ratio; svPPA = semantic variant of primary progressive aphasia.
[18F]AV-1451 uptake across all ROIs was higher in the Alzheimer dementia group compared to the svPPA group (figure 2). When the participants with svPPA were divided by Aβ status, we found that both the Aβ(−) and Aβ(+) participants showed greater uptake in the temporal pole, inferior/middle temporal gyrus, amygdala, entorhinal cortex, and fusiform cortex compared to controls in the ROI-level analysis, with the Aβ(+) participants also showing greater uptake in the superior temporal gyrus and across the parietal and frontal ROIs compared to controls (figures 2 and 3). The Aβ(+) participants showed generally higher uptake across all ROIs compared to the Aβ(−) participants (figure 2), with the probabilities that Aβ(+) participants had higher uptake ≥0.97 for all temporal and parietal ROIs, with the greatest differences observed for the temporal pole, inferior/middle temporal gyrus, amygdala, entorhinal cortex, and fusiform (figure 3). Uptake in the Aβ(+) participants was lower than uptake observed in the Alzheimer dementia cohort across all ROIs (figures 2 and 3). The tau-PET SUVRs in all 4 of the Aβ(+) participants was generally at the lower range of SUVRs observed in Alzheimer dementia, and did not appear to be associated with PiB-PET SUVR or age (figure 1). The tau-PET SUVRs in the Aβ(−) participants also did not appear to increase with age, but did show a possible relationship with PiB-PET SUVR, with tau-PET SUVRs increasing with increasing PiB-PET SUVRs (figure 1).
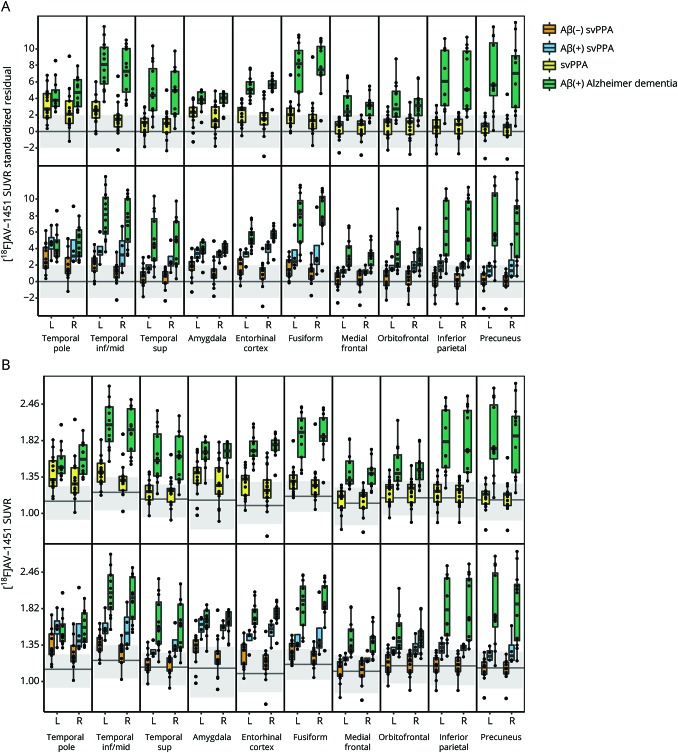
Figure 2. Boxplots of [18F]AV-1451 uptake by region and hemisphere.
Data are shown as age-corrected residuals (A) and SUVR (B). For the age-corrected residuals, participants with svPPA and Alzheimer dementia were standardized using a simple regression of tau predicted by age at scan within each region in the 170 controls. Within each region, a gray blush is drawn to cover approximately 95% of a cognitively normal, Aβ(−) control population, with the line representing mean values in the controls. In the top part of the plots, the svPPA cohort is compared to the Aβ(+) participants with Alzheimer dementia. In the bottom half of the plots, the Aβ(−) and Aβ(+) participants with svPPA are compared to each other and the Aβ(+) participants with Alzheimer dementia. Aβ = β-amyloid; inf = inferior; mid = middle; sup = superior; SUVR = standardized uptake value ratio; svPPA = semantic variant of primary progressive aphasia.
Figure 3. Posterior densities representing mean differences in [18F]AV-1451 uptake between diagnoses in each region.
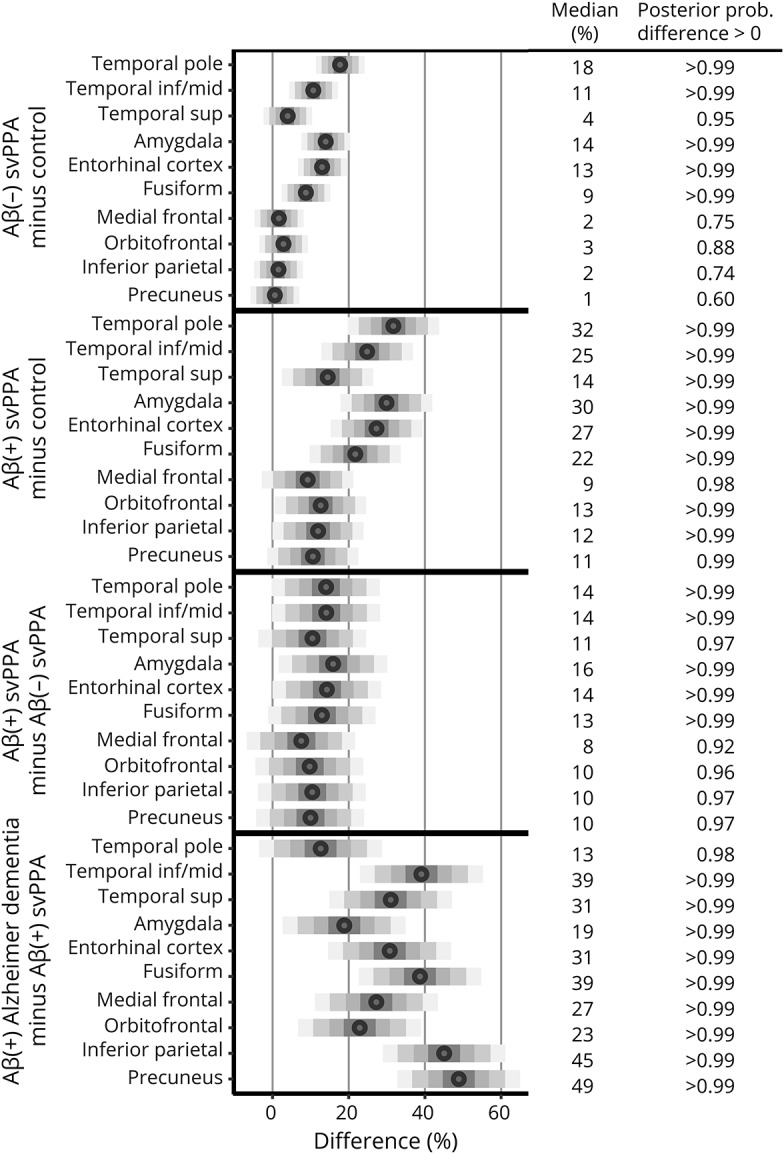
The bars are centered at the median and drawn to cover 50%, 80%, 95%, and 99% of differences from the posterior sample, with the median printed on the right. The posterior probability of a positive difference, i.e., the probability the first diagnosis has higher [18F]AV-1451 uptake than the second diagnosis, is next to the median. Aβ = β-amyloid; inf = inferior; mid = middle; prob. = probability; sup = superior; svPPA = semantic variant of primary progressive aphasia.
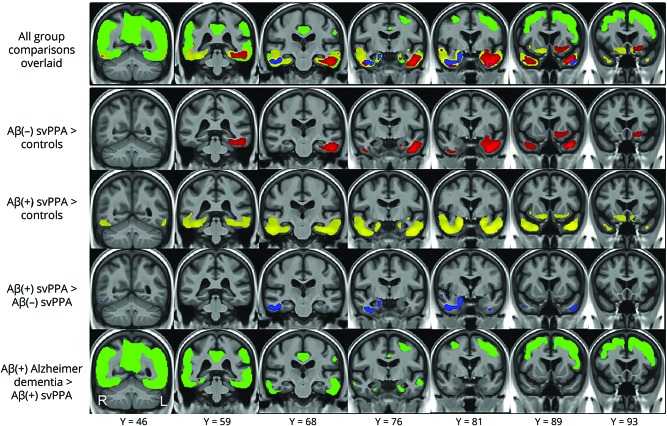
In the voxel-level analysis (figure 4), the Aβ(−) participants with svPPA showed elevated uptake compared to controls in the left inferior and middle temporal gyri, fusiform, amygdala, parahippocampal gyrus, hippocampus, temporal pole, right inferior temporal gyrus, and right temporal pole, with an additional area of increased uptake encompassing the left rectus gyrus, orbitofrontal cortex, anterior putamen, nucleus accumbens, olfactory cortex, and anterior insula. The Aβ(+) participants showed similar, although more bilateral and widespread, patterns of uptake throughout the temporal lobes compared to controls, with greater involvement of the left hemisphere, and uptake also observed in a bilateral area encompassing the anterior insula, anterior cingulum, orbitofrontal cortex, rectus gyrus, nucleus accumbens, and olfactory cortex. On direct comparison between the Aβ(−) and Aβ(+) groups, the Aβ(+) participants showed greater uptake in the right amygdala, hippocampus, entorhinal cortex, fusiform gyrus, inferior, middle, and superior temporal gyri and temporal pole, and left fusiform, inferior, middle, and superior temporal gyri, and temporal pole compared to the Aβ(−) participants. No regions showed greater uptake in the Aβ(−) participants compared to the Aβ(+) participants. The Alzheimer dementia cohort showed greater uptake in bilateral entorhinal cortices, posterior regions of the inferior and middle temporal gyri, and throughout the parietal, occipital, and frontal lobes compared to the Aβ(+) svPPA participants.
Figure 4. Results of the voxel-level comparisons between Aβ(−) svPPA, Aβ(+) svPPA, and Aβ(+) Alzheimer dementia cohorts.
Each row represents results of a different group contrast, with all of these contrasts shown overlaid on the same brain images in the top row of the figure. Red represents regions with greater uptake in Aβ(−) svPPA compared to controls; yellow represents regions with greater uptake in Aβ(+) svPPA compared to controls; blue represents regions with greater uptake in Aβ(+) svPPA compared to Aβ(−) svPPA; green represents regions with greater uptake in Aβ(+) Alzheimer dementia compared to Aβ(+) svPPA. All results are shown corrected for multiple comparisons using the false discovery rate correction at p < 0.05. Aβ = β-amyloid; svPPA = semantic variant of primary progressive aphasia.
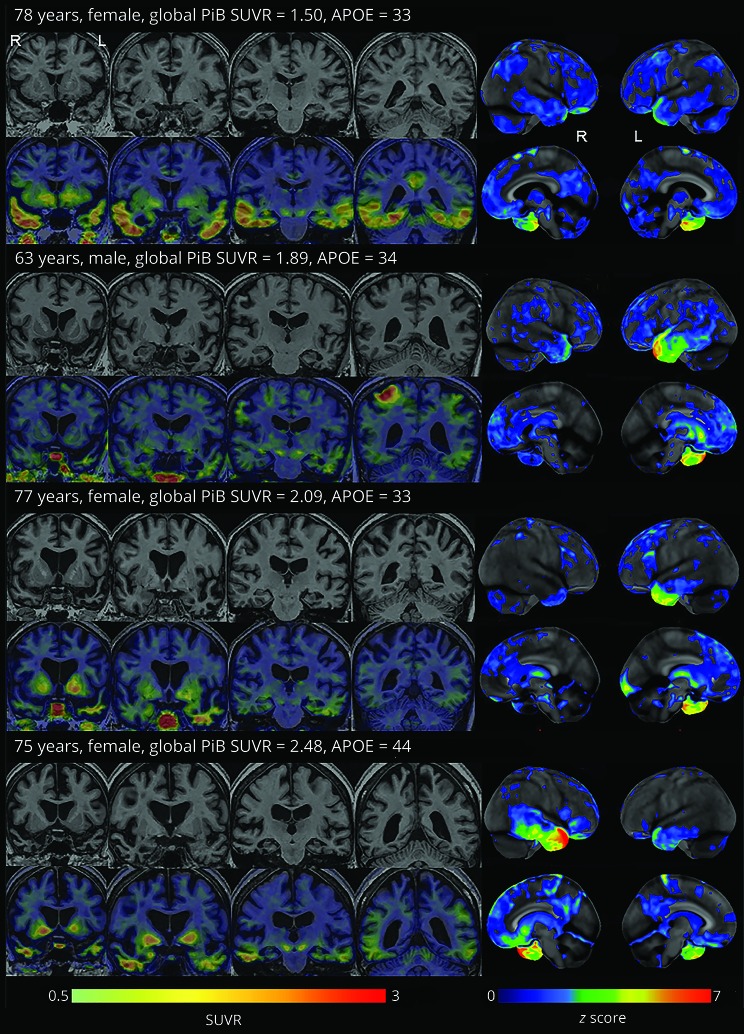
Figure 5 shows individual [18F]AV-1451, MRI, and FDG-PET images for the 4 Aβ(+) participants with svPPA. All 4 participants showed predominant anteromedial temporal hypometabolism and atrophy on FDG-PET and MRI, respectively, consistent with the diagnosis of svPPA. Elevated [18F]AV-1451 uptake was observed in the temporal lobes of all 4 participants, with additional involvement of the precuneus in participant 1 and lateral parietal lobe in participant 2.
Figure 5. MRI, [18F]AV-1451, and FDG-PET images for the 4 Aβ(+) participants with svPPA.
FDG-PET images were normalized to the pons and are shown as surface projection z score maps representing regions of abnormalities compared to controls (right side of the figure). [18F]AV-1451 standardized uptake value ratio images are shown overlaid on coronal MRI. Aβ = β-amyloid; FDG = [18F]-fluorodeoxyglucose; svPPA = semantic variant of primary progressive aphasia.
Discussion
This study demonstrates that the degree of uptake of [18F]AV-1451 in svPPA is lower than that observed in Alzheimer dementia, a disease that has paired helical filament tau deposition at autopsy. However, the degree of uptake was greater in participants with svPPA who were Aβ(+), suggesting some contribution of paired helical filament tau in these participants.
A high proportion of our svPPA cohort showed Aβ deposition on PET, with a frequency of 26% Aβ(+) observed in our larger cohort of 31 participants. This is on the higher end of the frequencies previously published, which range from 0% to 25%.12,41–43 Differences across studies could be attributable to different techniques used to determine Aβ status. We used a quantitative derived cut point while visual ratings were used in other studies. Another explanation could be attributable to different tracers as we used PiB-PET while florbetapir has been used in other studies. Differences in the age distribution of svPPA cohorts across studies could also be contributing. All studies have found a decade difference in age between those who are Aβ(+) and those who are Aβ(−), and hence studies with more older participants will likely get a higher proportion of Aβ(+) cases.
We and others have previously reported patterns of [18F]AV-1451 in svPPA compared to controls,17–19 but now we expand on our previous study by reporting findings according to Aβ status and comparing findings in svPPA to Alzheimer dementia. We confirm that elevated [18F]AV-1451 uptake is observed in Aβ(−) participants with svPPA. These participants showed mild patterns of uptake that was highly asymmetric involving the anteromedial temporal lobe, as well as regions in the basal frontal lobes, insula, and putamen. It remains unclear what the ligand is binding to in these cases. It has been hypothesized that it may represent spill out from increased binding in white matter due to the expression of monoamine oxidase B by reactive astrocytes,18 may bind to low levels of abnormal tau that coexists with TDP-43,17,18 to some cellular marker of neurodegeneration,17–19 or may even represent low-level binding to TDP-43.18,19 However, when Aβ deposition was present in svPPA, we observed more striking uptake with higher SUVRs particularly in medial and lateral temporal regions, but also in parietal regions, compared to the Aβ(−) participants. These differences were observed despite the fact that the sample size was smaller in the Aβ(+) group and remained after correction for age differences between the groups; hence, in a more highly powered study, the differences could have been more pronounced. These results could be interpreted to suggest that paired helical filament tau may be present in at least some of the Aβ(+) participants, contributing to the higher [18F]AV-1451 uptake. It is possible that Alzheimer disease is the only pathology in these cases, but it is also possible that Alzheimer disease pathology may coexist with TDP-43 pathology. One autopsy study reported 2 Aβ(+) svPPA patients with TDP-43 pathology as the primary pathology, with concomitant Alzheimer disease pathology, although one of these cases had a Braak neurofibrillary tangle stage of only II.12 Clinicopathologic studies on svPPA have, however, very rarely reported any other pathology except TDP-43. Of 55 cases of svPPA reported with autopsy from 4 centers,3,4,6,9 3 (5%) had Alzheimer disease pathology and 3 (5%) had TDP-43 pathology with coexistent Alzheimer disease. In this study, we found that 31% of our participants with svPPA showed Aβ deposition on PET. Given the general rarity of Alzheimer disease in clinicopathologic series of svPPA, it seems unlikely that all of our Aβ(+) cases will have underlying Alzheimer disease pathology at autopsy. In other words, there could be alternative explanations for the elevated Aβ and elevated [18F]AV-1451 observed on PET imaging and it may not be correct to diagnose these participants as having Alzheimer disease based on PET findings. One possibility is that the PET scans are correctly binding to their identified targets, but that some of these participants have PART that is being detected by [18F]AV-1451. Possible PART can be diagnosed in the presence of Aβ deposition, as long as the Aβ Thal phase is low (1–2).11 Consistent with our findings, PART occurs most frequently in older people and typically targets the temporal lobes, and an autoradiographic study has shown [18F]AV-1451 binding to tau proteins in brain tissue in PART.14 The older age could also be the explanation for the presence of Aβ deposition; the fact that the global PiB SUVRs were lower in the participants with svPPA compared to those with Alzheimer dementia could support this theory. Another possibility is that [18F]AV-1451 is binding to another unexpected target. It could be binding in low levels to neuritic tau present in the Aβ plaques in the Aβ(+) participants.14,15 Other tauopathies have, however, also been observed in svPPA at autopsy that could also be contributing to our findings, such as Pick disease,3,8 globular glial tauopathies,3,44 and argyrophilic grain disease.45
The distribution of [18F]AV-1451 uptake in the Aβ(+) participants was relatively typical for svPPA, involving the anteromedial temporal lobes, left and/or right, and did not show a widespread distribution through the brain that has been observed in Alzheimer dementia.46–48 In addition, the degree of [18F]AV-1451 uptake across all regions was lower than that observed in Alzheimer dementia. It is certainly possible that Alzheimer disease pathology could have an atypical medial temporal distribution48 or have a low Braak stage,49 although if the cases had a pure Alzheimer disease pathology, one might expect the [18F]AV-1451 uptake values to be higher and more in the range observed in Alzheimer dementia. The Aβ(+) participants did show a more bilateral pattern of uptake compared to the more asymmetric pattern observed in the Aβ(−) participants—perhaps a feature that could help predict Aβ deposition. Little is known about [18F]AV-1451 in vivo in PART, but the temporal predominance would fit with the possible explanation that these participants have TDP-43 pathology with PART. The Aβ(+) participants also had a higher APOE ε4 frequency compared to the Aβ(−) participants (50% vs 14%), which suggests APOE ε4 may have a role in driving the presence of Aβ deposition and could possibly support an underlying Alzheimer disease mechanism, although one study has suggested that APOE is not a risk factor for Alzheimer disease across all variants of PPA.4 Ultimately, however, we can only hypothesize about the underlying pathology, and autopsy confirmation will be needed on these participants in order to determine the underlying pathologic substrate for the increased [18F]AV-1451 uptake. It is quite possible of course that the underlying pathology will differ across the 4 Aβ(+) participants. In fact, 2 of the Aβ(+) participants showed quite striking and focal regions of elevated [18F]AV-1451 uptake in the parietal lobe while the other 2 did not, perhaps pointing to an underlying Alzheimer disease pathology in the 2 with parietal uptake.
Of note, all 4 of the Aβ(+) participants fulfilled diagnostic criteria for svPPA and performed comparably to the Aβ(−) participants with svPPA on tests of naming and semantic association. All 4 also performed within the normal range on tests of sentence repetition, and none met clinical criteria for the logopenic variant of PPA.1 Findings on FDG-PET also supported a diagnosis of svPPA in all cases.
Although not an aim of our study, we also observed some interesting relationships of [18F]AV-1451 uptake in the Aβ(−) participants with svPPA. [18F]AV-1451 uptake may be associated with global PiB SUVR, rather than age, in these participants, with [18F]AV-1451 uptake across all ROIs tending to increase with global PiB SUVR, albeit below our cutoff of abnormality. A relationship between [18F]AV-1451 uptake and global PiB SUVR was not observed in the Aβ(+) participants, although this could have been because of the small number of Aβ(+) participants. It is currently unclear why this relationship was observed in the Aβ(−) participants, although it mirrors relationships observed between increasing [18F]AV-1451 and global PiB SUVR that have been observed in Aβ(−) cognitively normal people.50 Further work is needed to assess this relationship in larger numbers of participants in order to determine a potential biological explanation.
Strengths of our study were in our well-characterized cohort, which all underwent extensive clinical testing, including an assessment with a speech/language pathologist, as well as scanning with multiple brain imaging modalities. Our single Bayesian model increased our statistical strength in this small sample by simultaneously estimating all regional quantities of interest and accounting for age, resulting in more stable estimates while accounting for multiple comparisons. A limitation of the study was the small number of participants with svPPA in our [18F]AV-1451 cohort. The lack of autopsy confirmation, which will be critical to determine the pathologic basis of the PET findings, was also a limitation.
The findings from this study add to our limited understanding of [18F]AV-1451 uptake in svPPA. We show strong evidence using Bayesian modeling that [18F]AV-1451 uptake is greater in Aβ(+) compared to Aβ(−) svPPA, although not to the degree observed in Alzheimer dementia. While more work is needed to confirm the underlying pathology in these cases, our findings do suggest that strong bilateral anteromedial temporal [18F]AV-1451 signal, perhaps also with involvement of the parietal lobes, could help clinically to increase suspicion that a person with svPPA may have Aβ deposition and, hence, influence patient prognosis and potential treatment.
Acknowledgment
The authors acknowledge AVID Radiopharmaceuticals for provision of AV-1451 precursor, chemistry production advice and oversight, and FDA regulatory cross-filing permission and documentation needed for this work.
Glossary
- Aβ
β-amyloid
- FDG
[18F]-fluorodeoxyglucose
- MCALT
Mayo Clinic Adult Lifespan Template
- MP-RAGE
magnetization-prepared rapid-acquisition gradient echo
- PART
primary age-related tauopathy
- PiB
Pittsburgh compound B
- ROI
region of interest
- SUVR
standardized uptake value ratio
- svPPA
semantic variant of primary progressive aphasia
- TDP-43
TAR DNA binding protein of 43 kDa
Appendix 1. Author contributions
Study funding
This study was funded by NIH grants R21-NS94684, P50 AG16574, U01 AG006786, R01 AG11378, and R01 AG041851, Mayo Clinic Radiology Research, and support from the Elsie and Marvin Dekelboum Family Foundation.
Disclosure
J. Whitwell receives research support from the NIH. P. Martin reports no disclosures relevant to the manuscript. J. Duffy receives research support from the NIH. H. Clark receives research support from the NIH. M. Machulda receives research support from the NIH. C. Schwarz receives research support from the NIH. S. Weigand reports no disclosures relevant to the manuscript. I. Sintini receives research support from the Department of Radiology, Mayo Clinic. M. Senjem owns stock in Align Technology, Inc., Gilead Sciences, Inc., Globus Medical Inc., Inovio Biomedical Corp., Johnson & Johnson, LHC Group, Inc., 2017, Medtronic, Inc., Mesa Laboratories, Inc., Natus Medical Incorporated, Parexel International Corporation, and Varex Imaging Corporation. N. Ertekin-Taner has a patent “Human Monoclonal Antibodies Against Amyloid β Protein” issued and a patent “Their Use as Therapeutic Agents Application” pending, and receives research support from the NIH. H. Botha reports no disclosures relevant to the manuscript. R. Utianski receives research support from the NIH. J. Graff-Radford receives research support from the NIH. D. Jones receives research support from the NIH and the MN Partnership for Biotechnology and Medical Genomics. B. Boeve has served as an investigator for clinical trials sponsored by GE Healthcare and FORUM Pharmaceuticals. He receives royalties from the publication Behavioral Neurology of Dementia (Cambridge Medicine, 2009). He serves on the Scientific Advisory Board of the Tau Consortium. He has consulted for Isis Pharmaceuticals. He receives research support from the NIH, the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation, and the Mangurian Foundation. D. Knopman served as deputy editor for Neurology®; served on a data safety monitoring board for Lilly Pharmaceuticals; serves on a data safety monitoring board for Lundbeck Pharmaceuticals and for the DIAN study; served as a consultant to TauRx Pharmaceuticals ending in November 2012; was an investigator in clinical trials sponsored by Baxter and Elan Pharmaceuticals in the past 2 years; is currently an investigator in a clinical trial sponsored by TauRx; and receives research support from the NIH. R. Petersen serves as a consultant for Roche, Inc., Merck, Inc., Genentech, Inc., Biogen, Inc., and Eli Lilly, serves on data monitoring committees for Pfizer, Inc. and Janssen Alzheimer Immunotherapy, receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the NIH. C. Jack serves as a consultant for Janssen, Bristol-Myers Squibb, General Electric, and Johnson & Johnson; is involved in clinical trials sponsored by Allon and Baxter, Inc.; owns stock in Johnson & Johnson, and receives research support from Pfizer, Inc., the NIH, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation. V. Lowe is a consultant for Bayer Schering Pharma, Merck Research, and Piramal Imaging Inc., and receives research support from GE Healthcare, Siemens Molecular Imaging, Avid Radiopharmaceuticals, the NIH (NIA, National Cancer Institute), the Elsie and Marvin Dekelboum Family Foundation, the Liston Family Foundation, and the MN Partnership for Biotechnology and Medical Genomics. K. Josephs receives research support from the NIH. Go to Neurology.org/N for full disclosures.
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephs KA, Hodges JR, Snowden JS, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 2011;122:137–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol 2017;81:430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 2014;137:1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyton CE, Britton AK, Hodges JR, Halliday GM, Kril JJ. Distinctive pathological mechanisms involved in primary progressive aphasias. Neurobiol Aging 2016;38:82–92. [DOI] [PubMed] [Google Scholar]
- 6.Davies RR, Hodges JR, Kril JJ, Patterson K, Halliday GM, Xuereb JH. The pathological basis of semantic dementia. Brain 2005;128:1984–1995. [DOI] [PubMed] [Google Scholar]
- 7.Snowden J, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol 2007;114:31–38. [DOI] [PubMed] [Google Scholar]
- 8.Hodges JR, Mitchell J, Dawson K, et al. Semantic dementia: demography, familial factors and survival in a consecutive series of 100 cases. Brain 2010;133:300–306. [DOI] [PubMed] [Google Scholar]
- 9.Harris JM, Gall C, Thompson JC, et al. Classification and pathology of primary progressive aphasia. Neurology 2013;81:1832–1839. [DOI] [PubMed] [Google Scholar]
- 10.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol 2008;63:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crary JF, Trojanowski JQ, Schneider JA, et al. Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 2014;128:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santos-Santos MA, Rabinovici GD, Iaccarino L, et al. Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol 2018;75:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia CF, Arteaga J, Chen G, et al. [(18)F]T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement 2013;9:666–676. [DOI] [PubMed] [Google Scholar]
- 14.Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquie M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sander K, Lashley T, Gami P, et al. Characterization of tau positron emission tomography tracer [18F]AV-1451 binding to postmortem tissue in Alzheimer's disease, primary tauopathies, and other dementias. Alzheimers Dement 2016;12:1116–1124. [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Martin PR, Botha H, et al. [18F]AV-1451 tau-PET and primary progressive aphasia. Ann Neurol 2018;83:599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bevan-Jones WR, Cope TE, Jones PS, et al. [18F]AV-1451 binding in vivo mirrors the expected distribution of TDP-43 pathology in the semantic variant of primary progressive aphasia. J Neurol Neurosurg Psychiatry 2018;89:1032–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makaretz SJ, Quimby M, Collins J, et al. Flortaucipir tau PET imaging in semantic variant primary progressive aphasia. J Neurol Neurosurg Psychiatry 2018;89:1024–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 22.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000;12:233–239. [DOI] [PubMed] [Google Scholar]
- 23.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? J Neurol Neurosurg Psychiatry 2000;69:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kertesz A. Western Aphasia Battery (Revised). San Antonio: PsychCorp; 2007. [Google Scholar]
- 25.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135:1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 27.Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR. Distinguishing subtypes in primary progressive aphasia: application of the Sydney Language Battery. Dement Geriatr Cogn Disord 2013;35:208–218. [DOI] [PubMed] [Google Scholar]
- 28.Howard D, Patterson K. Pyramids and Palm Trees: A Test of Semantic Access from Pictures and Words. Bury St. Edmunds: Thames Valley Publishing Company; 1992. [Google Scholar]
- 29.Weintraub S, Mesulam MM, Wieneke C, Rademaker A, Rogalski EJ, Thompson CK. The Northwestern Anagram Test: measuring sentence production in primary progressive aphasia. Am J Alzheimers Dis Other Demen 2009;24:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodglass H, Kaplan E, Barresi B. The Boston Diagnostic Aphasia Examination (BDAE). Baltimore: Lippincott, Williams and Wilkins; 2001. [Google Scholar]
- 31.Warrington EK, James M. The Visual Object and Space Perception Battery. Bury St. Edmunds: Thames Valley Test Company; 1991. [Google Scholar]
- 32.Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment, 5th ed. New York: Oxford University Press; 2012. [Google Scholar]
- 33.Clegg F, Warrington EK. Four easy memory tests for older adults. Memory 1994;2:167–182. [DOI] [PubMed] [Google Scholar]
- 34.Jack CR Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 2008;12:26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenland S. Principles of multilevel modelling. Int J Epidemiol 2000;29:158–167. [DOI] [PubMed] [Google Scholar]
- 37.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian Data Analysis, 3rd ed. Boca Raton, FL: Chapman & Hall/CRC, 2013. [Google Scholar]
- 38.Gelman A, Hill JL. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge, UK: Cambridge University Press; 2006. [Google Scholar]
- 39.R Core team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 40.Plummer M. rjags: Bayesian Graphical Models using MCMC. R package version 4-6. 2016. CRAN.R-project.org/package=rjags. [Google Scholar]
- 41.Brown EE, Graff-Guerrero A, Houle S, et al. Amyloid deposition in semantic dementia: a positron emission tomography study. Int J Geriatr Psychiatry 2016;31:1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Villarejo-Galende A, Llamas-Velasco S, Gomez-Grande A, et al. Amyloid PET in primary progressive aphasia: case series and systematic review of the literature. J Neurol 2017;264:121–130. [DOI] [PubMed] [Google Scholar]
- 43.Josephs KA, Duffy JR, Strand EA, et al. APOE4 influences β-amyloid burden in primary progressive aphasia and speech apraxia. Alzheimers Dement 2014;10:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Graff-Radford J, Josephs KA, Parisi JE, Dickson DW, Giannini C, Boeve BF. Globular glial tauopathy presenting as semantic variant primary progressive aphasia. JAMA Neurol 2016;73:123–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deramecourt V, Lebert F, Debachy B, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology 2010;74:42–49. [DOI] [PubMed] [Google Scholar]
- 46.Cho H, Choi JY, Hwang MS, et al. Tau PET in Alzheimer disease and mild cognitive impairment. Neurology 2016;87:375–383. [DOI] [PubMed] [Google Scholar]
- 47.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitwell JL, Graff-Radford J, Tosakulwong N, et al. [(18) F]AV-1451 clustering of entorhinal and cortical uptake in Alzheimer's disease. Ann Neurol 2018;83:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259. [DOI] [PubMed] [Google Scholar]
- 50.Lockhart SN, Scholl M, Baker SL, et al. Amyloid and tau PET demonstrate region-specific associations in normal older people. Neuroimage 2017;150:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data from the study will be shared in an anonymized format to any qualified investigator on request from the corresponding author.