Abstract
Objective
To precisely identify cortical regions that modulate breathing, and delineate a network of cortical structures that underpin ictal central apnea (ICA) during epileptic seizures.
Methods
We electrically stimulated multiple cortical structures in patients undergoing stereotactic EEG (SEEG) evaluation before epilepsy surgery. Structures investigated were orbitofrontal cortex, anterior and posterior cingulate and subcallosal gyri, insula, hippocampus, parahippocampal gyrus, amygdala, temporo-polar cortex, antero-mesial fusiform gyrus, and lateral and basal temporal cortices. Chest/abdominal excursions using thoracic/abdominal belts, peripheral capillary oxygen saturation, end tidal and transcutaneous carbon dioxide, and airflow were continuously monitored.
Results
Nineteen consecutive adult patients (10 female) aged 18–69 years were investigated. Transient central apnea was elicited in 13/19 patients with amygdala, hippocampus head and body, anterior parahippocampal gyrus, and antero-mesial fusiform gyrus. Insula, cingulate, subcallosal, orbitofrontal, lateral, and basal temporal cortices stimulation did not induce apnea. Apnea duration was associated with stimulus duration (p < 0.001) and current intensity (p = 0.004).
Conclusions
These findings suggest a limbic/paralimbic mesial temporal breathing modulation network that includes amygdala, hippocampus, anterior parahippocampal, and antero-mesial fusiform gyri. These structures likely represent anatomical and functional substrates for ICA, a putative sudden unexpected death in epilepsy (SUDEP) breathing biomarker. Damage to such areas is known to occur in high SUDEP risk patients and SUDEP victims, and may underpin the prolonged ICA that is thought to be particularly dangerous. Furthermore, inclusive targeting of apnea-producing structures in SEEG implantations, peri-ictal breathing signal recordings, and stringent analysis of apneic sequences in seizure semiology may enhance accurate identification of symptomatogenic and seizure onset zones for epilepsy surgery.
Ictal central apnea (ICA) appears in 37%–44% of focal seizures,1,2 is usually of temporal lobe origin, and can be both prolonged (≥60 seconds) and associated with profound oxygen desaturation (SpO2 ≤ 75%).2 Prolonged ICA may predispose to fatal outcomes, and has been proposed as a potential biomarker of sudden unexpected death in epilepsy (SUDEP).2,3 Seizure spread into cortical structures involved in breathing regulation may lead to such respiratory dysfunction and SUDEP. Recent evidence from direct electrical cortical stimulation experiments consistently suggests that the human amygdalo-hippocampal complex can exert effects on breathing.4–6 Stereotactic EEG (SEEG) recordings of focal amygdalo-hippocampal seizures confirm that these structures generate ICA.4,5 However, previous animal and human studies have indicated the involvement of multiple structures outside the amygdalo-hippocampal complex in breathing modulation (insula, cingulate and subcallosal gyri, orbitofrontal and temporo-polar cortices) as having major influences on breathing.7–13 Elucidation and confirmation of the extended neural circuitry that influences breathing is essential to determine the mechanisms underlying ictal apneic phenomena and to the understanding of SUDEP pathomechanisms. Modern image coregistration techniques allow precise localization of stimulated electrodes in such circuitry; additional extensive EEG recordings during stimulation sessions monitor afterdischarges and seizures that could otherwise contaminate results. Further, delineation of cortical respiratory modulatory sites should allow accurate interpretation of apneic semiology in epilepsy surgery resections by ensuring that the relevant symptomatogenic zones are implanted. Here, we sought to identify cortical respiratory control networks using electrical brain stimulation in epilepsy patients undergoing SEEG evaluations for epilepsy surgery, investigating all implanted cortical areas, with particular emphasis on sites previously identified in literature as relevant.
Methods
Experimental design
We prospectively studied 19 consecutive patients: 3 were previously reported in a pilot breathing and stimulation project,5 12 were reported in a blood pressure and stimulation project.14 All were medically intractable epilepsy patients undergoing SEEG evaluations for epilepsy surgery in the epilepsy monitoring unit (EMU) at University Hospitals Cleveland Medical Center between June 2015 and June 2018. Inclusion criteria were patients aged ≥18 years, in whom direct cortical electrical stimulation was clinically indicated for mapping of ictal onset or eloquent cortex regions. The number and locations of depth electrodes were tailored according to the putative epileptogenic zone, based on clinical history, semiology, neuroimaging, and scalp EEG. Platinum-iridium depth electrodes (1.1 mm in diameter and 2.5 mm in length), evenly spaced at 5-mm intervals, were implanted stereotactically under general anesthesia. Implantation trajectories were simulated using iplan-stereotaxy 2.6 software (Brainlab, Munich, Germany) based on recent 3T MRI of the brain. Cranial CT was performed within 24 hours postsurgically. Using iPlan software, postsurgical cranial CT and presurgical brain MRI scans were coregistered for precise localization of single electrode contacts within each patient's presurgical MRI. Anatomical electrodes from postoperative CT scans were registered to standard Montreal Neurological Institute template MRI brain using FMRIB's linear image registration tool (FLIRT) available in FSL v5.0.915 (fsl.fmrib.ox.ac.uk/fsl/). MRI cortical reconstruction and volumetric amygdala segmentations were performed using Freesurfer image analysis suite (surfer.nmr.mgh.harvard.edu/),16 and reconstructed using 3D Slicer version 4.8.17
Standard protocol approvals, registrations, and patient consents
The local institutional review board approved the study (IRB UH03-15-05), and all patients signed informed consent.
Stimulation
Respiration, cardiac, blood pressure, and EEG monitoring
Bedside electrical stimulation was carried out using Ojemann (Integra Life Sciences, Plainsboro, NJ), Grass S-88X (Astro-Med, West Warwick, RI), or MS-120BK-EEG (Nihon Kohden, Tokyo, Japan). We carried out bipolar and monopolar biphasic stimulation using the following measures: 0.2 ms pulse width, current intensity from 1 gradually and progressively increased up to 10 mA, and train durations from 2 to 40 seconds. We used low (1 and 5 Hz) and high (50 Hz) stimulation frequencies, similar to brain mapping measures used for clinical purposes. If stimulation-induced seizures occurred, stimulation was discontinued and testing was aborted. Resuscitation equipment and IV lorazepam were always kept in close proximity to the patient in case of need. Stimulation was unilateral except on one occasion (patient 13) where bilateral stimulation of anterior cingulate gyrus was also carried out. Peripheral capillary oxygen saturation (SpO2) and heart rate were monitored using pulse oximetry (Nellcor OxiMax N-600x; Covidien, Dublin, Ireland). Beat-to-beat systolic arterial pressure, diastolic arterial pressure, and mean arterial pressure were continuously recorded using continuous noninvasive arterial pressure monitors (AP Monitor 500; CNSystems, Graz, Austria). Nasal airflow was recorded using nasal thermistors (Thermocouple Airflow Sensor; Pro-Tech, Philadelphia, PA). End-tidal carbon dioxide (ETCO2) was monitored using capnographs (Model 7900; Philips, Best, the Netherlands) and transcutaneous CO2 using digital transcutaneous CO2 sensors (Digital V-Sign; SenTec, Therwil, Switzerland). Chest and abdominal excursions were recorded using inductance plethysmography (Ambu [Ballerup, Denmark] Sleepmate). EEG and ECG were acquired using a diagnostic system (EEG-1200; Nihon Kohden). During stimulation sessions, patients were instructed to not talk or move unless they felt abnormal sensations or discomfort. We defined central apnea as involuntary cessation of breathing (excursions and airflow) where at least one breath was missed, compared to baseline breathing rate, with a drop in peak signal excursion by >90% of pre-event baseline, where stimulation-induced breathing arrest was repeatedly and consistently reproducible using the same or higher stimulating measures. Apnea onset was measured from nadir of the preceding breath (that was clearly reduced) to the beginning of the first inspiratory effort that approximated baseline amplitude. We defined blood pressure response as a decrease or increase by >5 mm of mercury (mm Hg) from the baseline mean during the stimulation period and heart rate change as >20% deviation from baseline.
Statistical analysis
Statistical analyses were performed using Statistical Package for Social Science (SPSS, version 24; IBM, Armonk, NY). Summary statistics were reported as mean ± SD (median, range). Chi-square tests were used to assess association between several stimulation measures and response characteristics. The strength of linear association (correlation) between nonparametric variable apnea duration with other variables was measured using the Spearman rho correlation coefficient r. Continuous variable current intensity was divided into low (<5 mA) and high (≥5). Significance was set at 2-sided p < 0.05.
Data availability statement
The datasets used and analyzed during the current study are available from the corresponding author on request.
Results
Patients and clinical setting
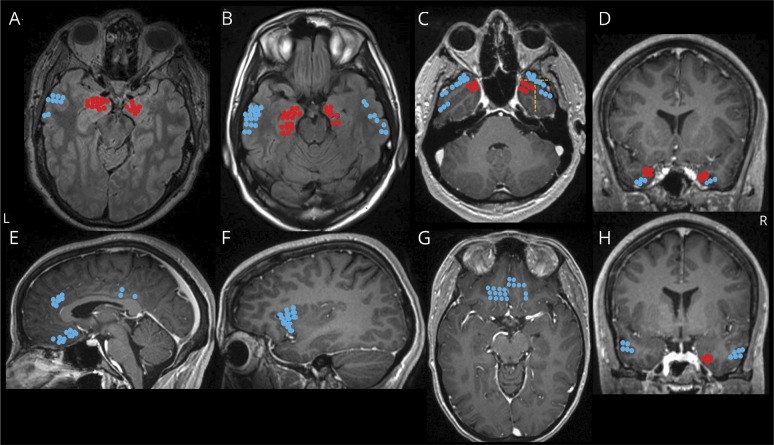
Nineteen patients were recruited into the study (10 female; mean age 41 [18–69] years). Patient demographics and characteristics are shown in table 1. Apart from essential hypertension treated with a calcium channel blocker (5 mg amlodipine) in patient 12, none had cardiorespiratory comorbidity. At the time of stimulation, patient 1 was on habitual doses of lacosamide (200 mg/d) and topiramate (400 mg/d); patients 5 (clonazepam 0.25 mg/d) and 12 (levetiracetam 1,500 mg 3 times a day, lacosamide 200 mg 3 times a day, and clobazam 15 mg twice a day) were on reduced medication. The remaining patients were off seizure medications as a part of the clinical protocol aimed at capturing seizures to localize the epileptogenic focus.
Table 1.
Patient and epilepsy characteristics
Stimulating electrodes
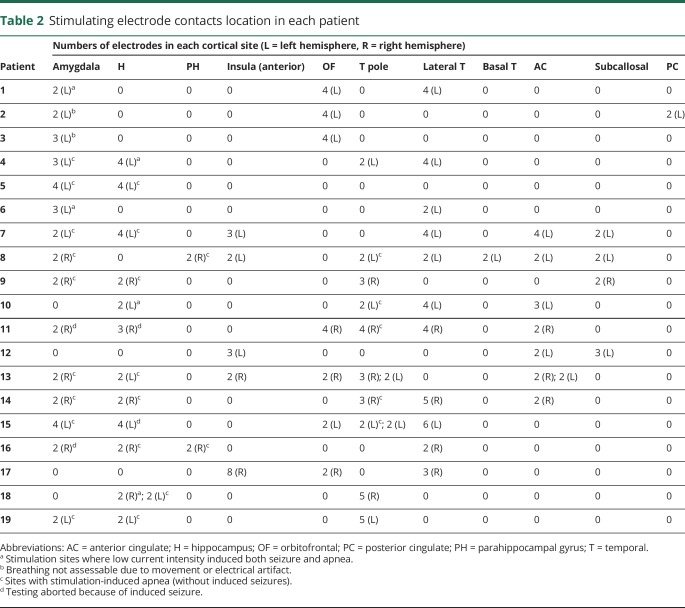
A total of 1,510 electrode contacts were implanted, of which 208 were stimulated according to the study protocol (37 amygdala, 29 hippocampus, 4 parahippocampal gyrus, 18 anterior insula, 22 orbitofrontal, 31 temporopolar, 40 lateral temporal, 2 basal temporal, 19 anterior cingulate, 9 subcallosal and 2 posterior cingulate neocortices [figure 1 and table 2]).
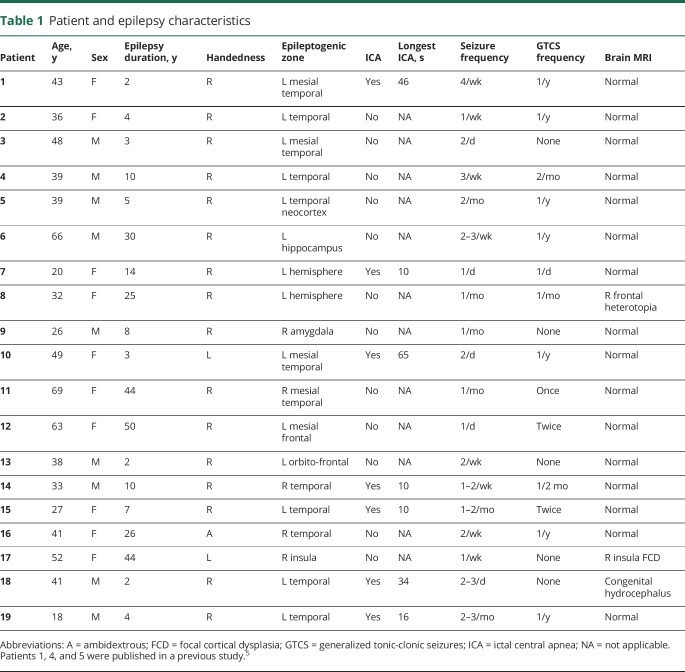
Figure 1. Locations of electrode contacts investigated for structures that produce stimulation-induced apnea.
(A–H) Electrode locations of stimulated electrodes, represented in different views of a single MRI brain for convenience. Locations that produced apnea are shown in red (amygdala [A], hippocampus head and body [B], mesial temporo-polar cortex [C, D, and H], and its subregions marked with a yellow dashed line: temporal tip (anterior), lateral and mesial temporo-polar cortices [C], and parahippocampal gyrus [H]). Locations that did not produce apnea are shown in blue (lateral temporal [A, B, C, and H], orbitofrontal cortex [G], anterior insular [F], anterior and posterior cingulate and subcallosal gyri [H]).
Table 2.
Stimulating electrode contacts location in each patient
Apnea responses
Table 2 summarizes stimulated electrode contact locations that induced central apnea. Anatomical locations of electrodes are shown in figure 1. At baseline, all patients had regular breathing signal, without evidence of ≥1 missed breath. Unilateral stimulation of amygdala, hippocampus, anterior parahippocampal, and antero-mesial fusiform gyri in both hemispheres independently elicited central apnea in 13/19 cases. In the remaining 6/19 patients, precise assessments on apnea could not be made because of recording artifact (2 cases); 2 additional patients only had electrodes in the insula, orbitofrontal, lateral temporal, anterior cingulate, and rostral subcallosal gyri, regions that did not elicit apnea with stimulation in any patient, and in 2 cases, stimulation induced both seizure and apnea. During apnea periods, there were no changes in blood pressure or heart rate. Isolated blood pressure responses to stimulation were previously reported on the first 12 patients in this study; none had concurrent breathing and blood pressure changes.14 Similar apnea characteristics were found stimulating nondominant and dominant mesial temporal structures. Infrequently, clinical seizures were induced with stimulation, none requiring IV lorazepam.
Stimulation of temporal structures
Amygdala
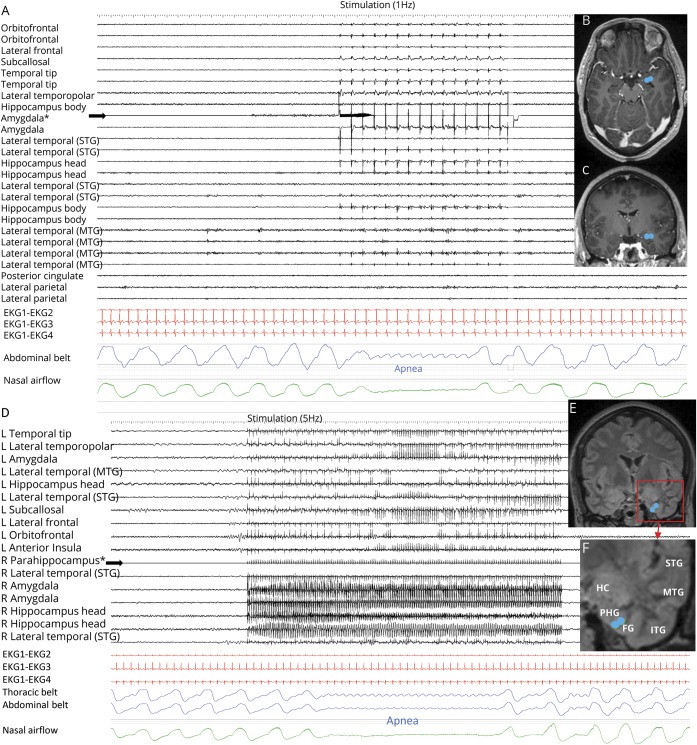
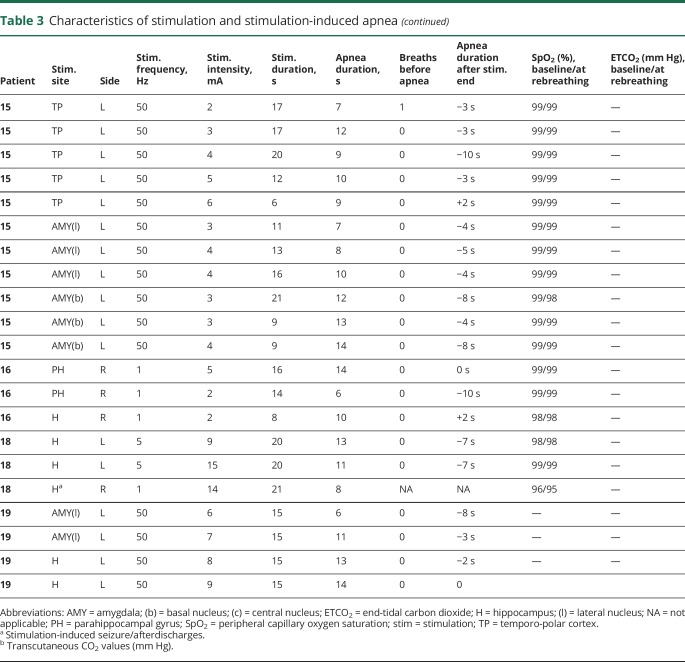
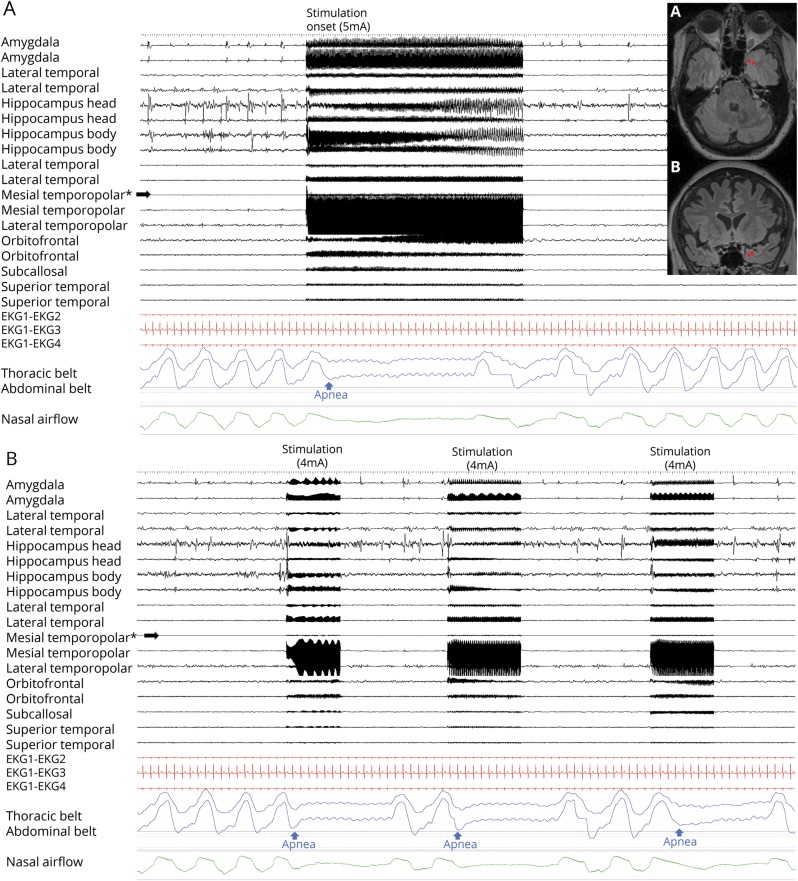
Amygdala stimulation of 28 electrode contacts (20 left, 8 right [figures 1A and 4A]) induced apnea in 11/15 patients. In the remaining 4/15, no apnea was seen, as breathing was either obscured by movement artifact in the plethysmographic breathing belt signal (patients 2 and 3), or testing was aborted because stimulation induced a clinical seizure (patients 11 and 16 [table 2]). In patient 1, amygdala stimulation induced a habitual seizure with ICA at seizure onset that lasted for 56 seconds. The patient had left mesial temporal lobe epilepsy and ICA during habitual spontaneous seizures (figure 2). Patient 6 had an identical amygdala stimulation–induced seizure and ICA lasting 34 seconds. Mean apnea duration during periods uncontaminated by seizures or afterdischarges was 10.5 ± 2.6 seconds (10; 6–16). We found breathing responses in all major amygdalar nuclei, including lateral, basal, and central nuclei. However, there were some differences in stimulus measures used. Low- and high-frequency stimulation of the mesial part of the amygdala (basal and central nuclei) induced apnea; however, only high-frequency and current intensity stimulation of the lateral nucleus induced apnea (table 3).
Figure 4. Stimulation-induced apnea by low-frequency (1 and 5 Hz) stimulation.
(A) Central apnea appears in abdominal and nasal airflow channels, with 1 Hz right amygdala stimulation in patient 9. All implanted electrodes shown are in the right hemisphere gray matter. Stimulated electrode contacts are seen in axial (B) and coronal (C) MRI sections. No afterdischarges or seizures are seen. (D) Low-frequency parahippocampal stimulation induced central delayed apnea; the patient was able to breathe twice before apnea started. (E, F) Stimulated electrode contacts are shown in coronal cuts.
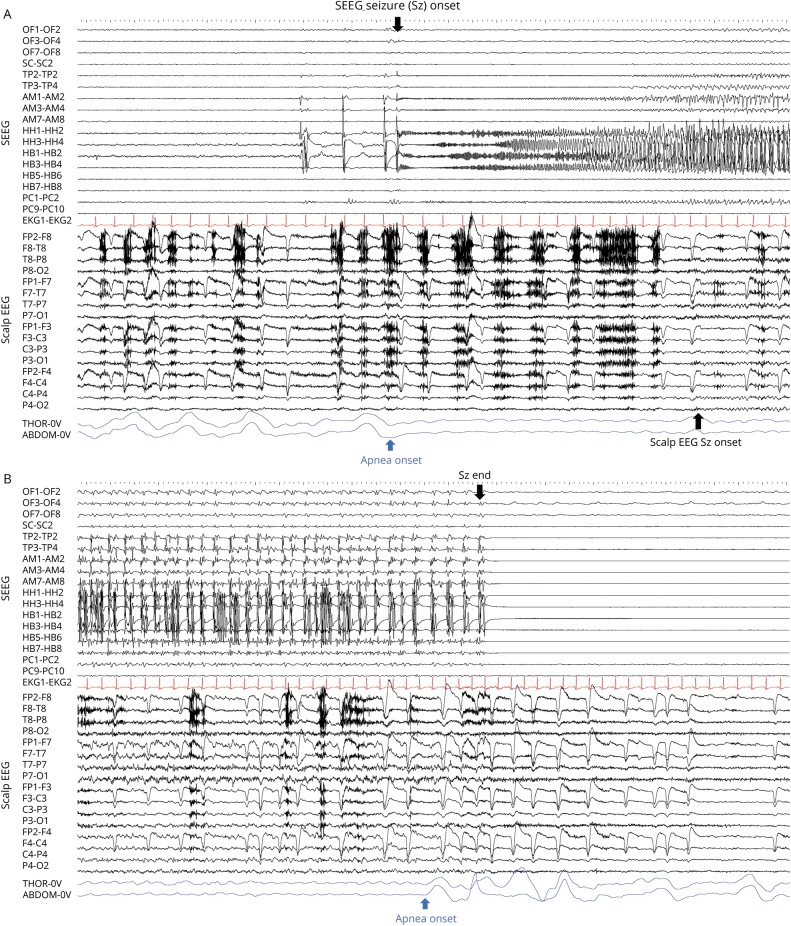
Figure 2. Central apnea in relation to intracranial and scalp EEG seizure onset.
(A, B) Stereotactic EEG (SEEG) recording of spontaneous hippocampal seizure onset in patient 1 after repetitive interictal spiking, with concurrent thoraco-abdominal breathing signal cessation, which is replaced by a pulse artifact, and indicative of ictal central apnea. Twelve seconds later, surface EEG seizure onset appears. OF = orbitofrontal; SC = subcallosal; TP = temporal pole; AM = amygdala; HH = hippocampus head; HB = hippocampus body; PC = posterior cingulate.
Table 3.
Characteristics of stimulation and stimulation-induced apnea
Hippocampus
Hippocampal head and body stimulation induced apnea in 10/12 patients, after stimulation of 28 electrode contacts (20 left, 8 right [figure 1]). In the remaining 2, no apnea comment could be made as testing was aborted because stimulation induced a clinical seizure. In 2 patients, stimulation produced apneic seizures; apnea persisted beyond stimulation end, with no other clinical signs. Mean apnea duration was 10.6 ± 3 seconds (10.5; 5–16). Apnea induced by stimulation of the hippocampus in patient 9 is shown in video 1.
Stimulation session in patient 9 shows apnea induced by right hippocampal stimulation. The patient has no respiratory or cardiovascular comorbidities. Right hippocampal head (electrodes HH1-HH2) stimulation is shown with simultaneous recording of breath excursions and airflow. Stimulation (50 Hz, 0.2 ms pulse width, 4–5 mA) periods evidenced by electrical artifact are clearly seen in the EEG record. Central apnea is seen consistently beginning with stimulation periods. The patient was awake and was instructed to report any abnormal sensations.Download Supplementary Video 1 (26.5MB, mp4) via http://dx.doi.org/10.1212/006920_Video_1
Parahippocampal gyrus (anterior parahippocampal gyrus [Brodmann area 35])
Parahippocampal stimulation was carried out in 2 patients (figures 1E and 4D and video 2). The longest nonseizure, stimulation-induced apnea in our study was seen with anterior parahippocampal stimulation in patient 8 (23 seconds). Mean apnea duration was 17 ± 5.4 seconds (18.5; 6–23).
Stimulation session in patient 8 shows apnea induced by right parahippocampal gyrus stimulation. The patient has no respiratory or cardiovascular comorbidities. Right parahippocampal gyrus (RT1-RT2) stimulation is shown with simultaneous recording of breath excursions and airflow. Stimulation (5 Hz and 0.2 ms pulse width and 8 mA) periods evidenced by electrical artifact are clearly seen in the EEG record. Central apnea is seen consistently at the beginning of the first stimulation period. However, when the patient was instructed to breathe before the second period, the stimulation failed to elicit apnea.Download Supplementary Video 2 (37.6MB, mp4) via http://dx.doi.org/10.1212/006920_Video_2
Temporo-polar cortex (Brodmann area 38)
Temporo-polar stimulation induced apnea in 5 of 10 patients with electrodes in this structure during stimulation of 13 electrode contacts (7 right, 6 left [table 2]). All stimulated electrode contacts that induced apnea were located in the mesial part of the temporo-polar region (antero-mesial part of the fusiform gyrus) (figure 1C). An example of mesial temporo-polar stimulation–induced apnea is shown in figure 3. Temporal tip and lateral temporo-polar electrodes in 5 patients (4, 9, 13, 15, 18, and 19) failed to induce breathing responses. Mean apnea duration was 8.8 ± 2.4 seconds (8; 4–13).
Figure 3. Mesial temporal pole stimulation-induced apnea.
Right mesial temporo-polar stimulation in patient 11. (A) 19-second stimulation train (50 Hz, 0.2 ms pulse width, 5 mA), which induced immediate cessation of thoracic and abdominal excursions following the end of expiration (blue signal) in addition to airflow cessation (green). The apnea period lasted 13 seconds. SpO2 and transcutaneous CO2 were 94% and 39.2 mm Hg, respectively, and remained steady during the apnea period. The locations of stimulation electrodes are shown in axial (A) and coronal (B) fluid-attenuated inversion recovery MRI sections. (B) Short stimulation sessions inducing immediate apnea. In these cases, the patient restored breathing a few seconds after the stimulation end. *Stimulated electrodes.
Lateral temporal and basal temporal neocortices
A total of 40 contacts were stimulated in the superior and middle temporal gyri and 2 in the basal temporal; none produced breathing changes.
Stimulation of extratemporal structures
Anterior, posterior cingulate, and rostral subcallosal gyri stimulation (Brodmann areas 32, 24, and 25, respectively) was investigated in 30 sites in 9 patients (figure 1). In patient 13, additional bilateral anterior cingulate stimulation was carried out. Neither unilateral nor bilateral stimulation (patient 13) induced breathing changes. Orbitofrontal (22) and anterior insula (18) gyri were also investigated in both hemispheres, without breathing changes. Stimulation of these areas also did not induce any seizures during stimulation.
Characteristics of stimulation-induced central apnea
Effect of stimulation on breathing cycle
Apnea always occurred following completion of an expiratory cycle. Stimulation in the inspiratory phase of the breathing cycle produced inhibition of inspiratory effort at any point of that phase, evident on respiratory belt signal and video, with the thorax then assuming a resting position (i.e., no active inspiration or expiration). If stimulation was initiated at any point of the passive, expiratory phase of the breathing cycle, expiration was continued to completion (videos 1 and 2).
Apnea awareness during stimulation
Patients were always agnostic of apnea and thus upon questioning, none was aware of any breathing difficulties or dyspnea during apnea periods. When reiteratively asked “Did you feel anything?” the answer was always “no.” Processes for voluntary air movement involving vocalization superseded stimulation effects; when patients were instructed to voluntarily hyperventilate, and stimulation initiated during hyperventilation, we were unable to produce apnea, despite using identical stimulation parameters (video 2).
Effect of stimulus current intensity and frequency on breathing
High current intensity (≥5 mA) was associated with longer apnea duration (p = 0.03) than low intensity (<5 mA). On the other hand, high- vs low-stimulation frequency (50 Hz vs 1 or 5 Hz) did not affect apnea duration (p = 0.6). High-frequency stimulation (50 Hz) was followed by immediate apneic responses, whereas low-frequency stimulations (1 or 5 Hz) resulted in delayed apnea onset (apnea began 1–2 breaths after stimulation onset) (p < 0.001). An example of delayed apnea after low-frequency stimulation (at 5 Hz) is shown in figure 4D. Although breathing responses were more easily elicitable using high current intensity and high stimulus frequency, we found that doing so with amygdala and hippocampus stimulations often resulted in electroclinical seizures, where seizure manifestations prevented conclusions on the pure effect of stimulations.
Effect of stimulation duration on respiration
Stimulation duration was associated with apnea duration (p < 0.01). Mean apnea duration was 11 ± 3.7 seconds (median 10; range 4–23) without induced seizures or afterdischarges. When an apneic seizure was induced, the apnea period lasted longer (up to 56 seconds) (table 3). Little or no change in SpO2 and CO2 was noted when patients began rebreathing. We found no causal relationship between apnea duration and low SpO2/high CO2 levels as possible apnea overriding mechanisms. Resumption of breathing usually occurred before any changes in SpO2 or CO2 (table 3). However, there were 2 notable exceptions in patient 8 during parahippocampal gyrus stimulation. Apnea durations were the longest noted in our series, 21 and 23 seconds, respectively, and associated with increases in ETCO2 (from 43 to 48 mm Hg) and drops in SpO2 (from 99% to 94%). In 51/66 (78%) stimulations, patients resumed breathing after the apnea period, but before or at stimulation end. In 15/66 (22%), apnea continued for 1–10 seconds after stimulation was discontinued. An example of apnea continuing after stimulation was discontinued is shown in figure 3 during short (5–6 seconds) stimulation durations.
Discussion
In this study, we found evidence of a network of breathing modulatory cortical structures including the anterior parahippocampal gyrus, antero-mesial fusiform gyrus, amygdala, and hippocampus, and were able to reasonably exclude lateral temporo-polar, lateral temporal, orbitofrontal, anterior insular, cingulate, and subcallosal neocortices as modulatory sites. We carefully assessed for seizure and afterdischarge influence (to prevent false-positive results from seizure spread to other apnea-producing structures), and confirmed the role of the amygdala and hippocampus in influencing breathing, consistent with other recent studies.4–6 Within the amygdala, an earlier study reported breathing responses after stimulation of only lateral and basal nuclei,4 whereas a more recent study found breathing responses exclusively from the central amygdalar nucleus.6 Our findings demonstrate that apnea can be elicited with stimulation of the more medial part, including basal (patient 8) and central (patient 13) nuclei. The lateral amygdala also produced apnea, albeit with higher frequency and higher current intensity stimulation (table 3). This may therefore represent stimulation effect spread to nearby structures (basal or central nuclei) rather than symptomatogenicity in the stimulated lateral nucleus.18 Amygdala nuclei projections may underlie these findings; the central nucleus of the amygdala has major projections to areas of the parabrachial pons19 as well as brainstem respiratory areas implicated in resting respiratory rhythm.20 However, the lateral nucleus does not directly project to the brainstem, but sends substantial projections to the hippocampus, thalamus,21 mesial temporo-polar cortex,13 and other cortical areas. All amygdaloid nuclei are intrinsically connected,22 and lateral amygdala apnea influences may be exerted through those intrinsic connections to medial amygdala nuclei or through hippocampal and thalamic projections. Mesial temporal modulation of breathing is evidenced by hippocampal activity increases before apnea termination in cats.23 Some hippocampal and amygdalar neurons phase-lock with the respiratory cycle in humans, suggesting that these structures are involved in breathing regulation.24,25 Single-pulse stimulation of the amygdala central nucleus in cats paces the inspiratory cycle.26
In addition to the amygdala and hippocampus, we found that stimulation of the anterior parahippocampal gyrus and antero-mesial fusiform gyrus in the mesial temporo-polar cortex also induced apnea. These findings are consistent with Kaada's animal and human observations of central apnea with human temporo-polar and hippocampal gyri stimulation.7,10 Of interest, in our study, temporo-polar apneic responses were exclusive to the paralimbic, antero-mesial extent of the fusiform gyrus; lateral temporo-polar stimulation did not produce apnea. Cytoarchitectural differences may explain this observation,27 since this area is considered a paralimbic cortical region that consists of agranular cortex, is distinct from the lateral temporo-polar (dysgranular) regions, and is phylogenetically related to hippocampus and parahippocampal allocortical regions we also found to respond with apnea on stimulation.28 Our results point to a network of mesial temporal structures, which can influence breathing. Electrical stimulation of these sites induces central apnea. This network likely represents the anatomical substrate of a limbic/paralimbic breathing modulation system.29,30 The amygdala also has rich internodal connectivity with the temporo-polar cortex through the fasciculus amygdalo-temporalis, which connects the lateral amygdala to mesial temporo-polar cortex.13 The temporo-polar cortex has heavy projection to the hippocampus; its afferents synapse with entorhinal cortex to project to the subiculum and the hippocampus. Conversely, the temporal pole receives hippocampal afferents via the subiculum.13 Thus, there is rich connectivity between all the mesial temporal network nodes identified through stimulation.
These network nodes, through the amygdala and temporo-polar cortex, are connected with the midbrain tegmentum via the temporopontine tract.7 The central nucleus of the amygdala, together with the lateral bed nucleus of the stria terminalis, has strong projections to the periaqueductal gray (PAG). The PAG modulates preinspiratory neurons in the pre-Bötzinger complex region, the kernel for eupneic rhythm generation. Perturbation of neural function in and around this area severely disrupts breathing rhythm.31 Stimulation in the most caudal portions of the ventrolateral PAG generates apnea.20 Although various nuclei in the pons and medulla contribute to normal, eupneic respiratory rhythm,32–34 eupnea is continuously adjusted by several rostral brain structures, to suit changing environmental circumstances, including emotional reactions (laughter, crying, or fear), but also other basic behaviors such as coughing, vomiting, or voluntary vocalization.30 The amygdala (involved in various aspects of emotional processing) maintains extensive efferent and afferents pathways with the hypothalamus and bed nucleus of the stria terminalis.13
This abundant anatomical and functional connectivity among temporo-limbic, paralimbic, and brainstem structures is likely to explain the functional role of these nodes in a respiratory network, mainly archicortex and paleocortex, that have a primitive phylogenetic hierarchy. However, in the limbic and paralimbic network, what mesial temporal nodes assume preeminence cannot be determined from our study. Apart from patient 8, in whom the longest apnea periods were seen after parahippocampal gyrus stimulation, there were no significant differences in apnea durations produced by individual structures, nor in the stimulus intensities that produced these apneas. In all patients, low current intensity stimulation (<5 mA) elicited apnea. For example, in patient 13, low current intensity and low frequency stimulation in either parahippocampal gyrus or amygdala induced similar apnea characteristics. Hence, network preeminence was not determinable.
Further to identification of breathing influences from the above noted structures, anterior insula, orbitofrontal, and anterior and posterior cingulate regions were also examined. Kaada and Jasper,7 Pool and Ransohoff,8 and Chapman et al.9 previously posited their role in breathing and stimulation experiments. In our study, exploration of these regions did not produce any change in breathing patterns. There are 2 possible explanations for this discrepancy. First, previous studies did not distinguish between stimulation-induced apnea and stimulation-induced seizures producing apnea. For example, one patient in the study by Kaada and Jasper7 reported a “funny feeling like before an attack” during apnea.7 Second, stimulated electrodes may have been placed in locations close to but not in the regions we studied, or different measures were used during their experiments. Though our anterior cingulate electrode positions were similar to those that produced apnea in the study by Kaada and Jasper,7 a notable exception was coverage of the anterior most region, just in front of the callosal genu, which none of our patients had. Pool and Ransohoff8 also used 120-Hz stimulation, a frequency beyond that permitted by our study protocol.8 Because apnea occurred with cessation of inspiratory efforts rather than termination of expiration in all stimulation sessions, it is likely that the “next” inspiration was immediately inhibited by stimulation. This observation suggests that the most likely downstream driver for apnea is stimulus-induced inhibition or disruption of brainstem inspiratory neuronal action. Similar findings have been reported in previous simulation studies and also during ictal central apnea in focal seizures.4–6
The observation of apnea agnosia, also made by previous studies, is consistent with functional imaging studies aiming to delineate the time course of air hunger or dyspnea perception.35 Prominent activation of the amygdala and the peri-amygdaloid striae terminalis occurs with air hunger,35–37 and stimulation-induced, functional deafferentation of these structures may block brainstem inputs. Apnea agnosia (during stimulation or seizures) may also explain why ICA has largely gone unrecognized until most recently, because thoracic and abdominal belts are not commonly used in the EMU.
Efficient apnea induction depended on stimulus settings that provided higher current and frequency measures; 50 Hz stimulation was more likely to produce immediate apnea, whereas lower frequency measures were significantly associated with apnea onset delay by 1 or 2 breaths. That higher current intensity (≥5 mA) was significantly accompanied by apneas is not surprising, since it is the most influential measure in electrical stimulation.38 Apnea duration positively correlated with stimulation duration, suggesting that both apnea onset and termination are stimulus-related. As in previous studies,5 patients resumed breathing before termination of stimulation in the majority of the trials (78%). Adaptation of neural processes may underlie these phenomena, rather than increased pCO2 as an override mechanism that compels resumption of breathing.4 Indeed, we found no relationship between CO2 or SpO2 changes and apnea termination. On 2 occasions, apnea lasted beyond 20 seconds with CO2 and SpO2 changes when breathing was restored, although it is unclear that these findings represent a causal relationship. In animal models, for example, ICAs were not reversed by increasing CO2 as an impetus for ventilatory drive.39 In 22% of our stimulations trials, apnea persisted beyond stimulation termination. This finding was typically with short stimulation periods (always ≤10 seconds), and produced apnea periods 1–10 seconds after stimulation cessation. Such poststimulation apneas may indicate stimulation-induced refractoriness in the respiration control in the brainstem that prevents resumption of breathing. Patient 8 was an exception, where apnea persisted 1–7 seconds beyond prolonged (>10 seconds) amygdala and parahippocampal gyrus stimulations (table 3).
These findings provide robust evidence that sites within limbic/paralimbic (amygdala, hippocampus, anterior parahippocampal gyrus, and antero-mesial fusiform gyrus [mesial temporo-polar region]) structures have the potential to suppress breathing and may provide the symptomatogenic substrate for ICA. Direct electrical stimulation of mesial temporal structures may simulate focal seizures in these structures; hence, apnea thus produced may represent ICA.3 Accurate identification of apnea-inducing sites has important implications in the unraveling of the semiologic value of ICA. ICA is a frequent seizure sign, seen in 36.5%–44% of focal epileptic seizures1,2 (54% of temporal lobe seizures), and can occur as long as 29 seconds before unequivocal scalp EEG onset (8 ± 4.9 [1–29] seconds) in 54% of focal seizures, and up to 50 seconds (12.3 ± 9.7 [1–50] seconds) before any other clinical signs in 69%.2 The apnea-inducing phenomenon can indicate a highly focal mesial temporal lobe seizure intracranially, sufficient to inhibit breathing rhythm or inspiration, and drive ICA, but so exquisitely localized as to cause no surface EEG change. An example of an SEEG recording of spontaneous hippocampal seizure onset in patient 1 and ICA is shown in figure 2 preceding a surface EEG seizure onset 12 seconds later. Our findings, if replicated in a larger study designed to specifically address this issue, may have value in the epilepsy surgery domain. Precise localization and depth electrode targeting of apnea-producing structures may help improve SEEG anatomo-electro-clinical correlations through additional analysis of ictal onset zones, based on ICA relationship to EEG onsets. ICA's potential value was evident in the post hoc analysis of patient 18 with suspected mesial temporal lobe epilepsy, whose hippocampal head, body, amygdala, and temporal tip were implanted bilaterally, but without coverage of the anterior parahippocampal and fusiform gyri in the mesial temporo-polar cortex. ICA was the first clinical sign (similar to habitual seizures) and preceded intracranial left hippocampal seizure onset by up to 21 seconds. In this case, the unequivocal demonstration of ICA as clinical seizure onset, in the absence of seizure discharge on invasive electrodes, indicated that important extra-amygdalo-hippocampal cortical structures critical to generation of ICA were not covered in the invasive evaluation. The patient continued to have seizures after left hippocampal transections as a memory-sparing procedure, and re-evaluation is planned. Similarly, unequivocal identification of ICA as the initial semiologic sign may help avoid extensive extratemporal implantations. In this study, we delineate the symptomatogenic zones for ICA; however, further studies assessing ICA in SEEG evaluations are necessary to confirm the possible localizing value (temporal vs extratemporal; mesial vs lateral temporal) of ICA as a semiologic sign in epilepsy surgery assessments.
Video-EEG monitored SUDEP deaths have established the role of central apnea in the fatal cascade of cardiorespiratory phenomena, a phenomenon that may be driven by the structures identified in this study. Recent imaging evidence suggesting damage to the anterior hippocampus, amygdala, and parahippocampus in SUDEP and high SUDEP risk patients40 supports speculation that such damage may predispose to death through exaggerated apneic mechanisms in susceptible patients. Whereas the majority of patients stimulated had a brief, mean apnea duration of 11 seconds with no or small changes in CO2 or SpO2 after the apnea period, such susceptibility is indicated by patient 8, who provokes major interest. He had longstanding epilepsy (25 years), with onset at age 7 years, and had high generalized tonic-clonic seizure frequency (1/month), all characteristics that are associated with substantially increased risk of SUDEP. Stimulation-induced apnea periods were consistently longest in this patient (parahippocampal gyrus stimulation), with persistence of apnea beyond termination of stimulation (parahippocampal gyrus and amygdalar stimulation) (table 3).
Some limitations of our study need to be considered. Although this study is the largest case series of breathing responses to human direct cortical electrical stimulation, our conclusions are based on a relatively small number of patients. All patients had refractory epilepsy, and sampling of stimulation areas was dependent on clinical diagnosis and the surgical hypothesis for implantation. These data (both positive and negative findings) should be reproduced in a larger cohort of patients, with particular emphasis on extratemporal limbic structures.
Acknowledgment
The authors thank Norma J. Hupp for technical help during the stimulation sessions.
Glossary
- EMU
epilepsy monitoring unit
- ICA
ictal central apnea
- PAG
periaqueductal gray
- SEEG
stereotactic EEG
- SUDEP
sudden unexpected death in epilepsy
Appendix 1. Authors
Study funding
Samden Lhatoo and Ronald Harper are funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407.
Disclosure
N. Lacuey and J.P. Hampson report no disclosures relevant to the manuscript. R.M. Harper is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407. J.P. Miller reports no disclosures relevant to the manuscript. S. Lhatoo is funded by the Center for SUDEP Research: NIH/NINDS U01-NS090405 and NIH/NINDS U01-NS090407. Go to Neurology.org/N for full disclosures.
References
- 1.Bateman LM, Li CS, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain 2008;131:3239–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacuey N, Zonjy B, Hampson JP, et al. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 2018;59:573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuele SU, Afshari M, Afshari ZS, et al. Ictal central apnea as a predictor for sudden unexpected death in epilepsy. Epilepsy Behav 2011;22:401–403. [DOI] [PubMed] [Google Scholar]
- 4.Dlouhy BJ, Gehlbach BK, Kreple CJ, et al. Breathing inhibited when seizures spread to the amygdala and upon amygdala stimulation. J Neurosci 2015;35:10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacuey N, Zonjy B, Londono L, Lhatoo SD. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 2017;88:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobis WP, Schuele S, Templer JW, et al. Amygdala stimulation-induced apnea is attention and nasal-breathing dependent. Ann Neurol 2018;83:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaada BR, Jasper H. Respiratory responses to stimulation of temporal pole, insula, and hippocampal and limbic gyri in man. AMA Arch Neurol Psychiatry 1952;68:609–619. [DOI] [PubMed] [Google Scholar]
- 8.Pool JL, Ransohoff J. Autonomic effects on stimulating rostral portion of cingulate gyri in man. J Neurophysiol 1949;12:385–392. [DOI] [PubMed] [Google Scholar]
- 9.Chapman WP, Livingston RB, Livingston KE. Frontal lobotomy and electrical stimulation of orbital surface of frontal lobes; effect on respiration and on blood pressure in man. Arch Neurol Psychiatry 1949;62:701–716. [DOI] [PubMed] [Google Scholar]
- 10.Kaada BR, Pribram KH, Epstein JA. Respiratory and vascular responses in monkeys from temporal pole, insula, orbital surface and cingulate gyrus; a preliminary report. J Neurophysiol 1949;12:347–356. [DOI] [PubMed] [Google Scholar]
- 11.Kremer WF. Autonomic and somatic reactions induced by stimulation of the cingular gyrus in dogs. J Neurophysiol 1947;10:371–379. [DOI] [PubMed] [Google Scholar]
- 12.Smith W. The functional significance of the rostral cingular cortex as revealed by its responses to electrical excitation. J Neurophysiol 1945;8:241–255. [Google Scholar]
- 13.Klingler J, Gloor P. The connections of the amygdala and of the anterior temporal cortex in the human brain. J Comp Neurol 1960;115:333–369. [DOI] [PubMed] [Google Scholar]
- 14.Lacuey N, Hampson JP, Theeranaew W, et al. Cortical structures associated with human blood pressure control. JAMA Neurol 2018;75:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage 2012;62:782–790. [DOI] [PubMed] [Google Scholar]
- 16.Saygin ZM, Kliemann D, Iglesias JE, et al. High-resolution magnetic resonance imaging reveals nuclei of the human amygdala: manual segmentation to automatic atlas. Neuroimage 2017;155:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D slicer as an image computing platform for the quantitative imaging network. Magn Reson Imaging 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephani C, Luders HO. Electrical stimulation of invasive electrodes in extratemporal lobe epilepsy. In: Koubeissi MZ, ed. Extratemporal Lobe Epilepsy Surgery. Montrouge: John Libbey Eurotext; 2011:261–313. [Google Scholar]
- 19.Hopkins DA, Holstege G. Amygdaloid projections to the mesencephalon, pons and medulla oblongata in the cat. Exp Brain Res 1978;32:529–547. [DOI] [PubMed] [Google Scholar]
- 20.Subramanian HH, Holstege G. The midbrain periaqueductal gray changes the eupneic respiratory rhythm into a breathing pattern necessary for survival of the individual and of the species. Prog Brain Res 2014;212:351–384. [DOI] [PubMed] [Google Scholar]
- 21.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci 2002;3:563–573. [DOI] [PubMed] [Google Scholar]
- 22.Savander V, Miettinen R, Ledoux JE, Pitkanen A. Lateral nucleus of the rat amygdala is reciprocally connected with basal and accessory basal nuclei: a light and electron microscopic study. Neuroscience 1997;77:767–781. [DOI] [PubMed] [Google Scholar]
- 23.Poe GR, Rector DM, Harper RM. Hippocampal reflected optical patterns during sleep and waking states in the freely behaving cat. J Neurosci 1994;14:2933–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelano C, Jiang H, Zhou G, et al. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci 2016;36:12448–12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frysinger RC, Harper RM. Cardiac and respiratory correlations with unit discharge in epileptic human temporal lobe. Epilepsia 1990;31:162–171. [DOI] [PubMed] [Google Scholar]
- 26.Harper RM, Frysinger RC, Trelease RB, Marks JD. State-dependent alteration of respiratory cycle timing by stimulation of the central nucleus of the amygdala. Brain Res 1984;306:1–8. [DOI] [PubMed] [Google Scholar]
- 27.Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol 2009;514:595–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chabardes S, Kahane P, Minotti L, Hoffmann D, Benabid AL. Anatomy of the temporal pole region. Epileptic Disord 2002;4(suppl 1):S9–S15. [PubMed] [Google Scholar]
- 29.Duffin J, Hockman CH, Rupert AH, Vachon BR. Limbic system modulation of respiratory neurones. Bull Physiopathol Respir 1974;10:255–260. [PubMed] [Google Scholar]
- 30.Holstege G. The emotional motor system. Eur J Morphol 1992;30:67–79. [PubMed] [Google Scholar]
- 31.Feldman JL, Mitchell GS, Nattie EE. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 2003;26:239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 1995;75:1–45. [DOI] [PubMed] [Google Scholar]
- 33.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol 2013;75:423–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 2013;36:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binks AP, Evans KC, Reed JD, Moosavi SH, Banzett RB. The time-course of cortico-limbic neural responses to air hunger. Respir Physiol Neurobiol 2014;204:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 2002;88:1500–1511. [DOI] [PubMed] [Google Scholar]
- 37.Evans KC. Cortico-limbic circuitry and the airways: insights from functional neuroimaging of respiratory afferents and efferents. Biol Psychol 2010;84:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranck JB Jr. Which elements are excited in electrical stimulation of mammalian central nervous system: a review. Brain Res 1975;98:417–440. [DOI] [PubMed] [Google Scholar]
- 39.St-John WM, Rudkin AH, Homes GL, Leiter JC. Changes in respiratory-modulated neural activities, consistent with obstructive and central apnea, during fictive seizures in an in situ anaesthetized rat preparation. Epilepsy Res 2006;70:218–228. [DOI] [PubMed] [Google Scholar]
- 40.Wandschneider B, Koepp M, Scott C, et al. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain 2015;138:2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stimulation session in patient 9 shows apnea induced by right hippocampal stimulation. The patient has no respiratory or cardiovascular comorbidities. Right hippocampal head (electrodes HH1-HH2) stimulation is shown with simultaneous recording of breath excursions and airflow. Stimulation (50 Hz, 0.2 ms pulse width, 4–5 mA) periods evidenced by electrical artifact are clearly seen in the EEG record. Central apnea is seen consistently beginning with stimulation periods. The patient was awake and was instructed to report any abnormal sensations.Download Supplementary Video 1 (26.5MB, mp4) via http://dx.doi.org/10.1212/006920_Video_1
Stimulation session in patient 8 shows apnea induced by right parahippocampal gyrus stimulation. The patient has no respiratory or cardiovascular comorbidities. Right parahippocampal gyrus (RT1-RT2) stimulation is shown with simultaneous recording of breath excursions and airflow. Stimulation (5 Hz and 0.2 ms pulse width and 8 mA) periods evidenced by electrical artifact are clearly seen in the EEG record. Central apnea is seen consistently at the beginning of the first stimulation period. However, when the patient was instructed to breathe before the second period, the stimulation failed to elicit apnea.Download Supplementary Video 2 (37.6MB, mp4) via http://dx.doi.org/10.1212/006920_Video_2
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on request.