Abstract
Objective:
Although implicitly accepted by many that the durability of valve-sparing aortic root replacement (V-SARR) in patients with bicuspid aortic valve (BAV) disease and connective tissue disorders (CTD) will be inferior, this hypothesis has not been rigorously investigated.
Methods:
Between 1993–2009, 233 patients (27% BAV, 40% Marfan syndrome [MFS]) underwent Tirone David V-SARR. Follow-up averaged 4.7± 3.3 years (1,102 patient-years). Freedom from adverse outcome was determined using LogRank calculations.
Results
Survival at 5 and 10 years was 98.7±0.7% and 93.5±5.1%, respectively. Freedom from reoperation (all causes) on the aortic root was 92.2±3.6% at 10 years; three reoperations were aortic valve replacement due to structural valve deterioration (SVD). Freedom from SVD at 10 years was 96.1±2.1%. There were no significant differences in survival (p=0.805, p=0.793), reoperation (p=0.179, p=0.973), SVD (p = 0.639, p = 0.982) or any other functional or clinical endpoints when patients were stratified for valve type (tricuspid aortic valve (TAV) vs. BAV) or associated CTD. At latest echocardiographic follow-up (95% complete), 202 (94.8%) patients had none/trace aortic regurgitation (AR), 10 (4.7%) mild, 0 moderate to severe, and 1 (0.5%) severe AR. Freedom from > 2+ AR at 10 years was 95.3±2.5%. Six patients sustained acute type B aortic dissection (freedom at 10 years= 90.4±5.0%).
Conclusions:
Tirone David reimplantation V-SARR in carefully selected young patients was associated with excellent clinical and echocardiographic outcome in patients with either a TAV or BAV. There was no demonstrable adverse influence of MFS or CTD on durability, clinical outcome, or echocardiographic results.
ULTRAMINI ABSTRACT
The results of Tirone David valve-sparing aortic root replacement (V-SARR) in patients with and without connective tissue disorders or bicuspid aortic valve (BAV) disease were analyzed in 233 patients. V-SARR was associated with excellent clinical and echocardiographic outcome irrespective of whether or not BAV or connective tissue disorder was present.
INTRODUCTION
The surgical management of aortic valve regurgitation and aortic root pathology has evolved over the last three decades.1 The standard of care using composite valve grafts (CVG) with a mechanical or bioprosthetic valve has several important – but different – inherent limitations, e.g., indefinite need for anticoagulation versus limited durability, respectively. Based on the premise that preserving the patient’s native aortic valve would be associated with a substantially lower incidence of all valve-related complications, several surgical techniques have been described and are generically termed “valve-sparing aortic root replacement (V-SARR)”.2, 3
V-SARR has been proposed as a reasonable treatment alternative for patients with connective tissue disorders (CTD) such as the Marfan syndrome (MFS) and bicuspid aortic valve (BAV) disease; however, several groups have observed high reoperation rates in both MFS and BAV patients and raised concern about the use of V-SARR in such patients.4–8 Thus, the widespread use of V-SARR in BAV or CTD patients remains controversial, especially when a reproducible and durable alterative exists such as CVG with a mechanical valve.9, 10 Furthermore, the REDO risks remain undetermined if reoperation after V-SARR should become necessary, but the REDO mortality rate after other types of aortic root replacement procedures has reported to exceed 11%.11
Hence, we compared mid-term survival, risk of reoperation, incidence of structural valve deterioration, and degree of residual aortic regurgitation in patients with either a tricuspid aortic valve (TAV) or BAV with or without CTD using the Tirone David reimplantation technique of V-SARR.
MATERIALS AND METHODS
Patients
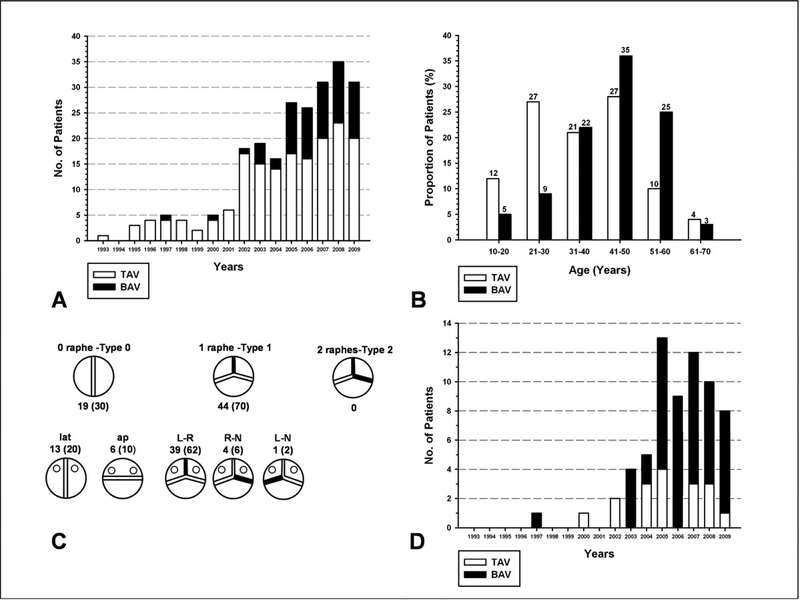
Either a T. David-I, T. David-V, or T. David-V-Stanford modification V-SARR was performed in 233 patients at Stanford from July 1993 to December 2009 (total number done at Stanford now exceeds 300) with the follow-up window closing in June 2010. One REDO patient undergoing root re-replacement after a previous Yacoub remodeling procedure for acute aortic dissection was excluded. One hundred and seventy (73%) patients had a TAV and 63 (27%) a BAV. Mean age was 36±13 (range 11–68) years for the TAV group and 43±12 (range 19–64) years for the BAV group; 115 (67%) and 50 (80%) patients were men in the TAV and BAV groups, respectively. No patient had an emergency procedure or acute type A aortic dissection. Additional demographic variables according to valve type are listed in TABLE 1. The distribution of patients by valve type over time is illustrated in FIGURE 1A. Age distribution of TAV and BAV patients and BAV subtypes according to Sievers’ classification12 are shown in FIGURE 1B and 1C, respectively. The patients in the TAV group were significantly younger, taller, and slimmer compared to the BAV group (TABLE 1). While the TAV group had larger aortic root dimensions, the BAV patients had larger ascending aortic diameters.
Table 1.
Baseline clinical characteristics of patients undergoing Tirone David valve-sparing aortic root replacement categorized by tricuspid aortic valve (TAV) versus bicuspid aortic valve (BAV).
| TAV (n=170) | BAV (n=63) | p-value | |
|---|---|---|---|
| Age, y, mean ± SD (range) | 36±13 (11–68) | 43±12 (19–64) | p<0.001 |
| Gender | |||
| Male (%) | 115 (67%) | 50 (80%) | p=0.08 |
| Height, cm, mean±SD (range) | 184±12 (148–214) | 175±24 (155–198) | p=0.003 |
| Weight, kg, mean±SD (range) | 81±19 (30–152) | 84±19 (42–127) | p=0.002 |
| BMI, kg/m2 mean±SD (range) | 24±5 (11–48) | 26±16 (13–45) | p<0.001 |
| BSA, m2, mean±SD (range) | 2.0±0.3 (1.1–2.7) | 2.0±0.3 (1.4–2.6) | p=0.798 |
| Hypertension | 44 (26%) | 23 (37%) | |
| Diabetes (oral treatment or insulin) | 4 (2%) | 2 (3%) | |
| Marfan syndrome | 91 (54%) | 3 (5%) | p<0.001 |
| Loeys-Dietz syndrome | 8 (5%) | 0 | |
| Acute aortic dissection | 0 | 0 | |
| Sinus rhythm | 170 (100%) | 63 (100%) | |
| Left Ventricular EF, %, median (IQR) | 61 (57–65) | 62 (60–65) | p=0.1 |
| Aortic root dia, cm, median (IQR) | 5.0 (4.6–5.4) | 4.3 (3.9–4.8) | p<0.001 |
| Aortic asc dia, cm, median (IQR) | 3.8 (3.0–4.8) | 4.9 (4.4–5.3) | p<0.001 |
| Aortic regurgitation | |||
| None/Trivial | 66 | 23 | |
| Mild | 43 | 21 | |
| Moderate-Severe | 43 | 9 | |
| Severe | 18 | 10 | |
| Average | 1.9±1.3 | 1.7±1.5 | |
| Previous CV surgery | 8 (5%) | 4 (6%) |
BMI= body mass index; BSA= body surface area; NYHA= New York Heart Association; EF= ejection fraction; IQR= interquartile range; TAV= tricuspid aortic valve; BAV= bicuspid aortic valve; CV= cardiovascular.
Figure 1:
[A] Distribution of patients undergoing valve-sparing aortic root replacement according to type of aortic valve. [B] Age distribution of tricuspid and bicuspid aortic valve patients. [C] Distribution of patients (number, %) with a bicuspid aortic valve according to Sievers’ classification. [D] Distribution of arch replacement during the study period according to valve type. TAV= tricuspid aortic valve; BAV= bicuspid aortic valve.
Operative procedure
Early in the experience the original V-SARR reimplantation technique described by David and Feindel (T. David-I) was employed in 26 patients.3 Thereafter, 19 patients received a T. David-V procedure. The T. David-V-Stanford modification V-SARR technique was used exclusively since December 2002 in 188 patients.13
Total or partial transverse arch replacement was carried out when necessary utilizing the “Peninsula Technique” with a single sigmoid-shaped suture line from the ligamentum to the innominate artery using selective antegrade cerebral perfusion (usually a 6–8 mm arterial perfusion graft sewn to the innominate artery (see TABLE 2 for distribution of cannulation sites) and moderate hypothermic circulatory arrest (HCA) (bladder 25–27 °C).14 Concomitant arch replacement was carried out more frequently in patients with a BAV since their aneurysmal pathology often included the arch15, as illustrated in FIGURE 1D.
Table 2.
Intra-operative data for TAV and BAV patients undergoing Tirone David valve-sparing aortic root replacement.
| TAV (n=170) | BAV (63) | p-Value | |
|---|---|---|---|
| Urgency of surgery | |||
| Elective | 168 | 63 | |
| Urgent | 2 | 0 | |
| Emergent | 0 | 0 | |
| Per-op annular diameter, median (IQR) | 28 (25–30) | 27 (25–30) | p=0.238 |
| CPB Time, min, median (IQR) | 265 (239–301) | 309 (275–330) | p<0.001 |
| Aortic XC time, min, median (IQR) | 211 (184–241) | 242 (212–262) | p<0.001 |
| Arch repair | 17 (10%) | 48 (76%) | p<0.001 |
| SACP time, min, median (IQR) | 25 (22–34) | 27 (23–31) | p=0.758 |
| Axillary/innominate | 5/14 | 1/46 | |
| Concomitant procedures | 39 (23%) | 6 (10%) | p=0.02 |
| Mitral Valve Repair | 13 | 2 | |
| PFO | 29 | 2 | |
| ASD | 6 | 1 | |
| VSD | 1 | 0 | |
| CABG | 4 | 2 | |
| IABP | 4 | 3 | |
| VAD | 3 | 1 | |
| Aortic valve cusp repair | 63 (37%) | 42 (67%) | p<0.001 |
| Cusp FM shortening | 63 | 42 | |
| Gore-Tex neo-chords | 0 | 3 | |
| Triangular resection | 0 | 7 |
CPB= cardiopulmonary bypass; XC= cross-clamp; SACP= selective antegrade cerebral perfusion; PFO= patent foramen ovale; ASD= atrial septal defect; VSD = ventricular septal defect; CABG= coronary artery bypass graft; IABP= intra-aortic balloon pump; VAD= ventricular assist device; FM= free margin; IQR= interquartile range; TAV= tricuspid aortic valve; BAV= bicuspid aortic valve.
Coronary artery reimplantation as full-thickness Carrel “button” anastomoses was done whenever possible. One patient with an anomalous, intramural coronary artery had his left main coronary reconstructed using an arterial (superficial femoral) autograft. Six patients required a Kay-Zubiate right coronary reconstruction (2–3 cm greater saphenous vein interposition graft) due to technical complications. The distribution and type of aortic valve repairs used in the TAV and BAV group are listed in TABLE 2, as well as the concomitant procedures.
Valve repair
Aortic valve cusp repair was performed in 63 of the TAV patients (37%). Cusp repair consisted of shortening the free margin at the nodulus of Arantius in all 63 TAV patients without formal cusp plication sutures. Amongst the BAV patients, 42 patients required cusp free margin shortening (67%) using one or more sutures to correct prolapse and cusp redundancy. A total of 68 sutures were placed, resulting in an average of about 1.4 sutures (range one to four) per patient. Seven of these sutures were centrally placed at the nodulus of Arantius, whereas the remaining 61 sutures were placed further towards the commissures along the cusp free margin. In addition, a small triangular resection of the raphé was performed in 7 BAV patients along with cusp free margin shortening. Creation of commissural neo-suspensory cords using 5–0 Gore-Tex sutures was done in three BAV patients to replace ruptured “truncal” commissural suspensory chords.
End points
Primary end points were: 1.) All cause overall mortality; 2.) Reoperation on the aortic root for any cause; 3.) Structural valve deterioration (SVD); and, 4.) Freedom from > 2+ aortic regurgitation (AR).
Follow-up
Postoperative valve-related adverse events were compiled and analyzed according to the AATS-STS-EACTS Guidelines for Reporting Morbidity and Mortality after Cardiac Valvular Operations.16 Patients were followed clinically on a regular basis; current follow up was done by contacting the patients and their physicians on the telephone. Mean follow-up was 4.7± 3.3 years (± 1 SD); maximum follow-up= 15.1 years, median= 4.2 years, interquartile range [IQR]= 2.3, 6.6 years, cumulative= 1,102 patient-years). At 5 years of follow-up, 88 patients remained at risk, but only 18 at 10 years; for those with MFS or other CTD, these figures were 46 and 7, respectively. Structural valve deterioration (SVD) was categorized according to the valve-reporting guidelines.16
Echocardiography
Transthoracic echocardiograms (TTE) were performed periodically (usually once per year) postoperatively. We obtained late TTE images in 213 of 224 patients (233 minus 4 deaths and 5 who underwent late AVR), equating to 95% late echocardiographic follow-up completeness. Average time of late TTE was 3.9± 3.3 years postoperatively (median= 3.4 years, IQR= 1.24, 5.8 years, maximum= 13.4 years).
AR was graded as either none or trivial (grade 0), mild (grade 1+), moderate (grade 2+), moderate to severe (grade 3+), severe (grade 4+) based on color flow mapping and continuous-wave and pulsed-wave Doppler by 2 expert echocardiographers (DHL and A-SB) who specifically focused on the vena contracta width, and AR timing and mechanism.
Statistical analysis
Continuous variables are expressed as mean ± 1 standard deviation (SD) or median with interquartile range (IQR). Mann-Whitney Rank Sum Test was used to compare two-group continuous variables. Categorical data were tabulated in 2xn tables and two-group comparisons were made using X2 test or Fisher’s exact probability test. Kaplan-Meier method was used to calculate non-adjusted actuarial survival and freedom from adverse events; statistical comparison of event rates was determined using LogRank calculations. For perspective, age -, gender- and race-matched survival estimates for the U.S. population were calculated. To identify predictors of outcome we used univariable Cox regression proportional hazard models. Commercially available statistics and graphing packages SigmaPlot and SigmaStat 11.0 (Systat Software, San Jose, CA 95110, USA) were used for descriptive and analytical statistical procedures. A probability value of less than 0.05 was considered statistically significant. The Stanford IRB approved this study, and informed consent was obtained from the patients at the time of contact.
RESULTS
Survival
The in-hospital (30 day) mortality rate was 0.9 % (2/233). One death was due to complications arising from reimplantation of a small, non-dominant right coronary artery with subsequent right ventricular failure. The second death was due to cerebral infarction in a patient with a chronic aortic dissection. Early (30 day) morbidity is summarized in TABLE E1, and late complications are listed in TABLE E2.
TABLE E1.
Early postoperative complications among 233 Tirone David valve-sparing aortic root replacement patients.
| Variable | |
|---|---|
| In-hospital mortality (30 days) | 2 (0.9%) |
| Mediastinal re-exploration due to bleeding | 8 (3%) |
| Cardiac complications | |
| Acute myocardial infarction | 1 (0.4%) |
| Atrial fibrillation | 29 (12%) |
| Permanent pacemaker | 8 (3 %) |
| Stroke | 2 (0.9%) |
| Acute renal dysfunction | 0 |
| Length of hospital stay, d, median (IQR) | 6 (5–7) |
IQR= inter-quartile range; d= days.
TABLE E2:
Complications in 231 surviving Tirone David valve-sparing aortic root replacement patients.
| Variable | |
|---|---|
| Superficial wound infection | 2 (0.9%) |
| Deep sternal infection | 1 (0.4%) |
| Late mortality | 2 (0.9%) |
| Thromboembolism | 3 (1.3%) |
| TIA | 2 (0.9%) |
| Stroke | 1 (0.4%) |
| Bleeding events | 0 |
| Endocarditis | 2 (0.9%) |
| Operative management | 2 (0.9%) |
| Acute type B aortic dissection | 6 (2.6%) |
| Conservative therapy | 6 (2.6%) |
| Operative treatment | 0 |
| Reoperation due to structural valve deterioration | 3 (1.3%) |
| AVR | 3 (1.3%) |
| Re-repair | 0 |
| Late Pacemaker | 3 (1.3%) |
| 3rd degree AV block | 3 (1.3%) |
V-SARR= valve-sparing aortic root replacement; TIA= transient ischemic attack; AVR= aortic valve replacement; AV= atrio-ventricular.
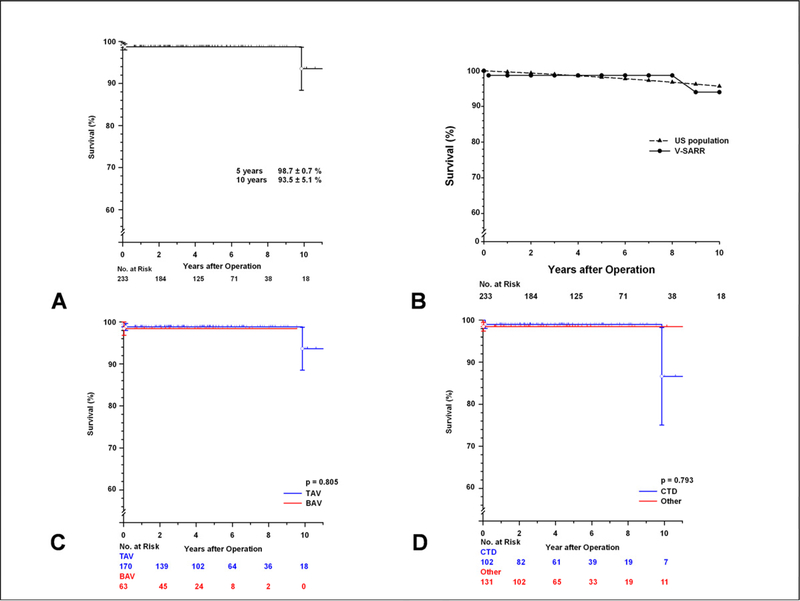
Two late deaths occurred: One patient committed suicide 6 weeks later and one patient with MFS died of cardiomyopathy 10 years postoperatively. Actuarial survival estimates at 5 and 10 years were 98.7± 0.7 % and 93.5± 5.1 %, respectively (Figure 2A).
Figure 2:
[A] Kaplan-Meier survival curve for the 233 patients undergoing valve-sparing aortic root replacement. [B] Kaplan-Meier survival curve of the 233 patients undergoing valve-sparing aortic root replacement compared to the general U.S. population matched for age, gender and race. [C] Kaplan-Meier survival curves comparing the tricuspid and bicuspid aortic valve subsets. [D] Kaplan-Meier survival curves comparing patients with a confirmed diagnosis of MFS or other connective tissue disorders (CTD) versus the remainder of the patients. Vertical bars represent ± 1 SE.
Survival after V-SARR was not significantly different compared to the general U.S. population matched for age, gender and race (FIGURE 2B). There was no significant difference in mid-term survival after V-SARR between the TAV and BAV subsets (p=0.805), but caution in interpreting this finding is necessary because cumulative follow-up was much shorter in the BAV subset (TAV= 886 years, BAV= 216 years, FIGURE 2C). Similarly, there was no statistically significant difference in survival between those patients with a diagnosis of CTD and the rest of the population (p=0.793, FIGURE 2D).
Reoperation
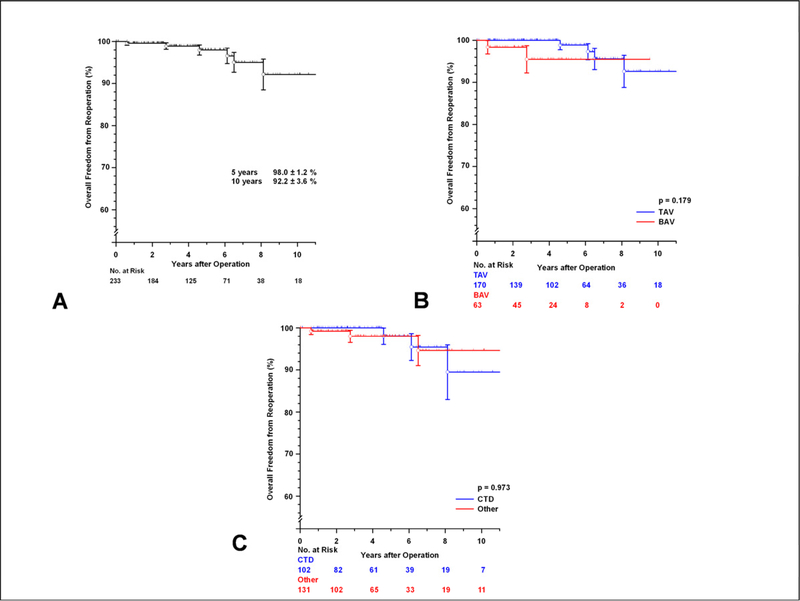
A total of six patients required reoperation on the aortic root due for any cause. Freedom from reoperation at 5 and 10 years was 98.0± 1.2 %, and 92.2± 3.6 %, respectively (FIGURE 3A). There was no demonstrable difference in reoperation between the TAV and BAV subsets (p=0.179, FIGURE 3B) or between those with MFS or other CTD and the rest of the patients (p=0.973, FIGURE 3C). One young patient with MFS required mitral valve replacement due to progressive mitral regurgitation 6 years after V-SARR; he requested replacement of his well functioning native aortic valve at that time to avoid a potential third reoperation since he opted for a mechanical mitral valve.
Figure 3:
[A] Freedom from reoperation on the aortic root after valve-sparing aortic root replacement. [B] Freedom from reoperation on the aortic root comparing the tricuspid and bicuspid aortic valve (TAV and BAV) subsets. [C] Freedom from reoperation on the aortic root after valve-sparing aortic root replacement comparing patients with confirmed MFS or another connective tissue disorder (CTD) and the rest of the patient cohort. Vertical bars represent ± 1 SE.
Endocarditis
Two patients developed endocarditis; both had an uncomplicated early postoperative course. Neither had a positive blood culture at time of reoperation, but both had been treated with antibiotics previously for non-specific symptoms. One infected BAV patient underwent AVR with a homograft one year postoperatively. The second patient (TAV) had a localized infection near the right coronary artery reimplantation site; local resection and debridement was performed successfully.
Late aortic dissection
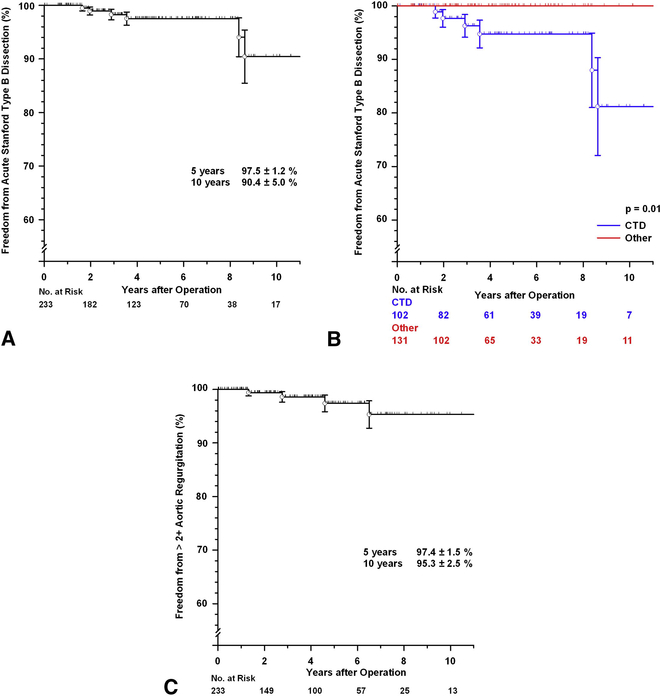
Six patients developed an acute Stanford type B aortic dissection between 19 and 103 months postoperatively, a serious problem. Five were females, and all had an underlying connective tissue disorder (5 MFS and 1 Loeys-Dietz syndrome, TABLE E5). None had had concomitant arch replacement. All patients were managed conservatively and to date are subsequently doing well. Freedom from Stanford type B aortic dissection at 5-years was 97.5± 1.2% and at 10 years 90.4± 5.0% (FIGURE E2A). There was a significant difference in freedom from acute Stanford type B dissection between those with MFS or other CTD and the rest of the patients (p=0.01, FIGURE E2B).
TABLE E5.
Clinical characteristics of six patients who sustained a Stanford type B dissection.
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| Age at operation, years | 48 | 32 | 32 | 36 | 19 | 26 |
| Gender | F | M | F | F | F | F |
| Connective tissue disorder | MFS | MFS | MFS | MFS | MFS | LDS |
| Operation | TD-I | TD-V | TD-VS-Mod | TD-VS-Mod | TD-VS-Mod | TD-VS-Mod |
| GD | 26 | 32 | 34 | 30 | 30 | 30 |
| Prox GD | 26 | 27 | 25 | 25 | 27 | 25 |
| Dist GD | 26 | 32 | 20 | 22 | 20 | 20 |
| Aortic valve type | TAV | TAV | TAV | BAV | TAV | TAV |
| Pre-op AR | +3 | 0 | +3 | 0 | +3 | 0 |
| Post-op AR | +1 | +1 | +1 | +1 | +1 | 0 |
| Time to dissection, months | 103 | 100 | 34 | 42 | 19 | 23 |
F= female; M= male; MFS= the Marfan syndrome; LDS= Loeys-Dietz syndrome; TAV= tricuspid aortic valve; BAV= bicuspid aortic valve; GD= graft diameter; Prox= proximal or annular end; Dist= distal; AR= aortic regurgitation; TD-I = T. David-I V-SARR; TD-VS-Mod = T. David-V-Stanford modification V-SARR.
Figure E2:
[A] Freedom from Stanford type B aortic dissection for all patients. [B] Freedom from Stanford type B aortic dissection comparing patients with a confirmed diagnosis of MFS or other connective tissue disorder (CTD) with the remainder of the patients. [C] Freedom from more than 2+ aortic regurgitation (AR). Vertical bars represent ± 1 SE
Aortic valve function
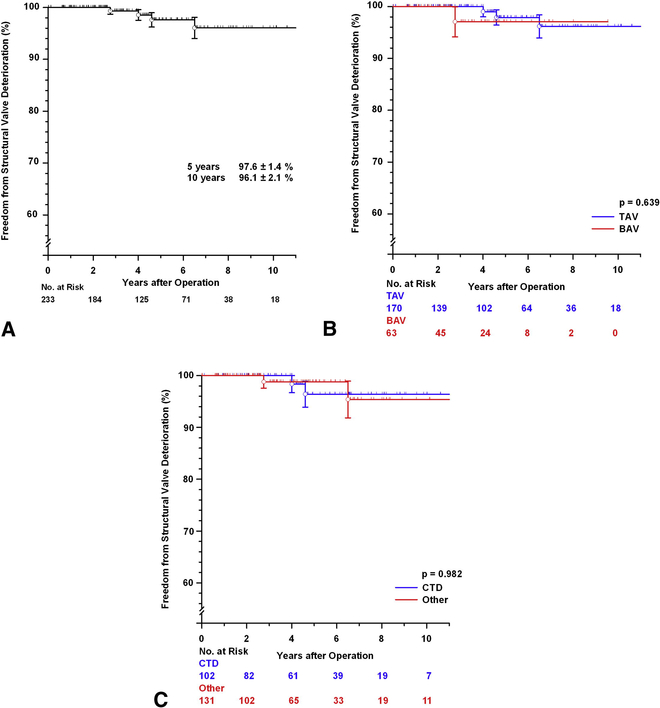
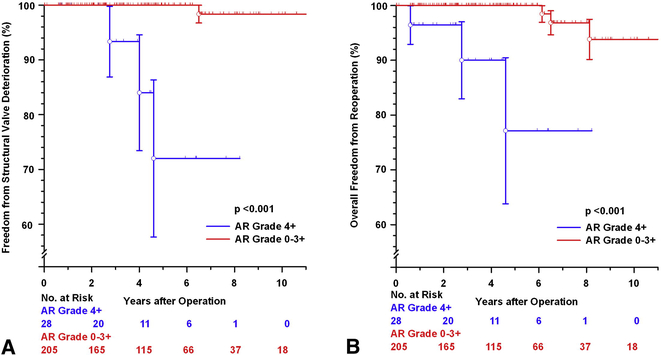
Freedom from SVD was at 5 and 10 years was 97.6± 1.4% and 96.1± 2.1%, respectively (FIGURE E1A). There was no significant difference in SVD between the TAV and BAV subsets (p=0.639 FIGURE E1B) nor between the patients with or without MFS or other CTD (p=0.982, FIGURE E1C). Characteristics of the 3 patients who needed late AVR due to SVD are summarized in TABLE E4 (note one patient with severe AR at the time this study was closed [listed as SVD according to the guidelines] subsequently underwent AVR). The 4 patients who developed SVD during follow-up had either no or mild AR on the first postoperative echocardiogram. There was a significant difference in freedom from SVD and reoperation, however, between those patients with 4+ AR preoperatively compared to the rest of the patients (p<0.001, see FIGURE E3A and FIGURE E3B, respectively).
Figure E1:
[A] Freedom from structural valve deterioration (SVD) after valve-sparing aortic root replacement (V-SARR). [B] Freedom from SVD comparing the tricuspid and bicuspid aortic valve (TAV and BAV) subsets. [C] Freedom from SVD comparing patients with a confirmed diagnosis of MFS or other connective tissue disorder (CTD) and the remainder of the patients. Vertical bars represent ± 1 SE.
TABLE E4.
Clinical characteristics of 4 patients with structural valve deterioration after Tirone David valve-sparing aortic root replacement.
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | |
|---|---|---|---|---|
| Age at operation, yr | 44 | 18 | 27 | 11 |
| Gender | Female | Male | Male | Male |
| Connective tissue disorder | Other | MFS | None | MFS |
| Preoperative AR | None | Severe | Severe | Severe |
| V-SARR procedure type | TD-I | TD-VS-Mod | TD-VS-Mod | TD-VS-Mod |
| Aortic valve type | TAV | TAV | BAV, 1/L-R/r | TAV |
| Valve-repair | No | No | Yes | Yes |
| Procedure | Gore-Tex Neo chord | Cusp FM shortening | ||
| Post-operative AR | None | Mild | Mild | None |
| AR before reop | Severe | Severe | Severe | Severe |
| Time to reop, months | 77 | 55 | 33 | N/A* |
Pt= patient; MFS= the Marfan syndrome; TAV= tricuspid aortic valve; BAV= bicuspid aortic valve; FM= cusp free margin; AR= aortic regurgitation; reop= reoperation; TD-I = T. David-I V-SARR; TD-VS-Mod = T.David-V-Stanford modification V-SARR.
This patient underwent aortic valve replacement after the end of the study closing interval 53 months after valve-sparing aortic root replacement and mitral repair.
Figure E3:
[A] Freedom from structural valve deterioration comparing patients with 4+ aortic regurgitation preoperatively versus the remainder of the patients. [B] Freedom from reoperation on the aortic root comparing patients with 4+ aortic regurgitation preoperatively versus the remainder of the patients.
Based on the latest TTE, 202 (94.8%) patients had either no or trace AR, 10 (4.7%) had mild AR, 0 had moderate to severe AR, and 1 (0.5%) had severe AR (who subsequently underwent AVR). Freedom from more than 2+ AR at 5-years was 97.4± 1.5% and at 10 years 95.3± 2.5 (FIGURE E2B). Only 4 patients developed more than 2+ AR during follow-up.
Predictors of adverse events
For the Cox regression analysis we constructed a composite endpoint, but only 14 adverse events occurred (4 deaths [2 in-hospital and 2 late], 4 SVD, 3 reoperations [not due to SVD, 2 caused by endocarditis], 1 stroke, and 2 TIA). This small number of events made the Cox analysis model unstable, which resulted in unreliable results without clinical relevance, as summarized in TABLE E3. The extremely wide 95% confidence intervals for the variables with a significant hazard ratio (age, arch replacement, AR > 2+ preoperatively) are noteworthy.
TABLE E3. Overview of the variables tested in the Cox proportional hazard regression analysis.
For this analysis we constructed a composite endpoint but only 14 total adverse events occurred (4 deaths [2 in-hospital and 2 late], 4 SVD, 3 reoperations [not due to SVD, 2 caused by endocarditis], 1 stroke and 2 TIA). The small number of events made the Cox model unstable, which resulted in unreliable results without clinical relevance, as evidenced by the extremely wide 95% confidence levels.
| Tirone David valve-sparing aortic root replacement patient variables (n=233) | ||||
|---|---|---|---|---|
| HR | 95 % Conf-L | 95 % Conf-U | P-value | |
| Age (y) | 0.902 | 0.845 | 0.962 | 0.002 |
| Gender | 1.963 | 0.393 | 9.794 | 0.411 |
| Weight (kg) | 1.015 | 0.967 | 1.066 | 0.550 |
| Height (m) | 0.944 | 0.887 | 1.005 | 0.073 |
| STBSA (m2) | 0.818 | 0.0216 | 30.903 | 0.915 |
| LVEF (%) | 0.953 | 0.871 | 1.042 | 0.289 |
| MFS or other CTD | 3.506 | 0.567 | 21.675 | 0.177 |
| BAV | 6.426 | 0.747 | 55.318 | 0.090 |
| Aortic cusp repair | 0.686 | 0.166 | 2.832 | 0.603 |
| Arch replacement | 12.377 | 1.289 | 118.879 | 0.029 |
| CPB (min) | 1.000 | 0.984 | 1.016 | 0.994 |
| XC (min) | 1.002 | 0.978 | 1.027 | 0.879 |
| AR > 2+ preoperative | 6.038 | 1.361 | 26.795 | 0.018 |
HR= hazard ratio; 95% Conf-L and 95 Conf-U= lower and upper 95% confidence levels; LVEF= left ventricular ejection fraction; MFS= the Marfan Syndrome; CTD= connective tissue disorder; BAV= bicuspid aortic valve; CPB= cardio-pulmonary bypass; XC= aortic crossclamp; AR= aortic regurgitation.
DISCUSSION
In this retrospective analysis we observed that survival after elective T. David V-SARR in carefully selected, relatively young patients is excellent out to 10 years. So far it is a safe and – most importantly - durable procedure irrespective of valve type (TAV or BAV) or the presence of MFS or another CTD. The one technique employed for root replacement is a strength compared to other reports.17, 18
Different V-SARR techniques and modifications of the original T. David procedure
Amongst 450 patients undergoing T. David-I V-SARR, the long-term results in 126 patients (20.6% MFS, only 4.0% BAV, no information provided regarding aortic cusp repair) operated upon between 1993 and 2000 was recently reported by Haverich’s group from Hannover.19 Mean follow-up was 10 ±2 years; 44 patients remained at risk at 10 years. Survival estimates were 93%, 85% and 70% at 1, 5 and 10 years, respectively. While lower than survival in our patients, any difference was probably due to differences in patient substrate, for example inclusion of patients with acute type A aortic dissection in the Hannover series. It is theoretically plausible, however, that this difference was due in part to more physiological postoperative aortic root physiology because we used the T. David-V-Stanford modification procedure. Freedom from AVR was 96%, 91% and 87% at 1, 5 and 10 years, respectively 19. MFS was a possible predictor of reoperation and overall mortality.
David et al. from Toronto reported results in 167 patients (38% MFS, 7% BAV) undergoing V-SARR. Ten-year survival and freedom from moderate to severe AR estimates were 92±3% and 94±4%, respectively.20 These survival and durability observations parallel our findings.
The high rate of recurrent AR due to annular dilatation eventually mandating reoperation after only 5 years using the Yacoub remodeling V-SARR procedure21 has convinced most to abandon its use, especially in patients with MFS. The Yacoub remodeling procedure (or T. David-II or -III) was used in only 3 other adult patients at Stanford since 1993.
In 2010 De Paulis et al. reported results in 278 patients (15% MFS, 11% BAV, aortic cusp repair in 9%) from four Italian centers using a prefabricated Valsalva graft for David reimplantation V-SARR. Survival at 10-years and freedom from AR requiring reintervention were 91% and 88%, respectively22, inferior to the outcomes reported in this analysis. This commercial Valsalva graft is popular; our T. David-V Stanford modification technique also creates large billowing neo-sinuses, but is customized for each individual patient’s pathological anatomy.
The Hospital Universitaria 12 Octubre group in Madrid reported on 120 patients (43% MFS, 12% BAV, 76% receiving a T. David-V-Stanford modification V-SARR) and showed 5-year survival and freedom from more than 2+ AR estimates of 97% and 96 %, respectively23. This corroborates our findings and demonstrates reproducibility of the T. David-V-Stanford modification procedure at other experienced institutions.
V-SARR for patients with BAV
Even though patients with a BAV had significantly longer cardiopulmonary bypass and aortic cross-clamp times and more commonly required concomitant aortic arch replacement or cusp free margin repair (see TABLE 2) compared to the TAV cohort, the clinical outcome in this subset was not different than the TAV subcohort to date. This underlines that the T. David procedure, with proper patient selection, is an excellent option for patients with regurgitant BAVs, but longer follow-up is necessary. These results are consistent with other investigators24; however, the largest experience with V-SARR for BAV employed aortic root remodeling (Yacoub), isolated BAV repair without root-replacement, or ascending aortic replacement alone, but not V-SARR utilizing the reimplantation technique.25 Since substantial annular dilatation is a hallmark of a BAV and causes AR due to inadequate cusp coaptation height, use of a V-SARR procedure that does not reduce aortic annular size is not prudent. Despite equal durability so far in our experience, we believe V-SARR long-term durability in patients with a BAV will eventually prove to be inferior compared to that for patients with a TAV since the bicuspid valves inexorably and progressively become more fibrotic and eventually stenotic.8 This makes careful patient selection crucial: We avoid V-SARR in older patients with a BAV and those with moderately severe fibrosis or calcification of the valve.
Fazel and we showed that a large number of BAV patients (> 70%) have aortic arch dilatation (i.e., Stanford-Fazel clusters III and IV BAV-associated aortopathy).15 This is reflected in our V-SARR experience where arch replacement was performed in 76% of the BAV patients compared to only 10% of the TAV patients. BAV aortopathy and arch dilatation is addressed more aggressively at Stanford in these young patients (average age= 43±12 years) undergoing elective procedures than recommended by others.26 While the natural history of BAV indicates a relative low risk for reintervention on the arch in patients after isolated aortic valve replacement27, most of these Ontario patients were older. The Mayo Clinic group recommended not replacing the transverse aortic arch in patients with bicuspid aortic valve disease26, but average age was 55.8± 14.9 years, the arch diameter was only 3.4± .6 cm, 50% had aortic stenosis, 7% had mixed AS/AR, and 89% underwent AVR; in such a patient population, we also would not replace the arch. These are different phenotypes of BAV disease. While we do not proclaim “nearly routine” replacement of the aortic arch in patients with BAV, being aware that in most institutions arch procedures under HCA carries substantial additional morbidity and mortality, it is performed often at Stanford without any added risk of death or stroke.
This series included 63 BAV patients (or 27%) whereas only 4% of the Hannover T. David-I series had a BAV19; comparing outcomes with respect to valve morphology is difficult. Our cohort contains a fairly large fraction of patients with El Khoury type II AR (i.e., cusp prolapse). Most BAV patients with AR due to cusp prolapse had an eccentric AR jet visualized on the preoperative echocardiogram. Sievers’ group has advocated avoiding V-SARR in such patients due to a higher likelihood of AR recurrence.6 Our BAV patients with type II AR had excellent clinical and echocardiographic results so far, but those with 4+ AR preoperatively did not do as well (FIGURE E3AB). The fairly high number of cusp repair procedures (TAV 37%, BAV 67%) had no demonstrable adverse effect on outcome in this analysis, contrary to what Sievers observed6, but we simply shortened the cusp free margin without resorting to complete cusp plication, which reduces available cusp area.
Regarding the distribution of BAV types encountered compared to Sievers’ original seminal publication12, the 304 patients reported by Sievers had undergone either valve replacement or preservation and were subdivided as type 0 (“naturally perfect BAV”) in 7%, type 1 (one raphé) in 88%, and type 2 (2 raphés) in 5%. In our V-SARR series the distribution was: Type 0 in 30%, type 1 in 70%, and type 2 in none. This dramatic difference is explained by far fewer V-SARR procedures in Sievers’ experience, where reimplantation or remodeling V-SARR was used in only 1.6% and 1% of patients, respectively.12
V-SARR in the setting of CTD
The Johns Hopkins’ group reported good early results after V-SARR in 31 patients with Loeys-Dietz syndrome.28 We only had 8 patients with Loeys-Dietz syndrome, and all did well except one patient who later sustained an acute type B dissection. Since many patients with CTD are actively lobbying for a V-SARR procedure, a randomization trial between V-SARR and CVG is unrealistic. The safety and long-term durability of the CVG procedure in patients with MFS has been well documented. In the world-wide series of 675 patients with MFS compiled by Gott et al. in 1999, survival was 94%, 91% and 59% at 5, 10, and 20 years respectively (159 and 4 patients at risk at 10 and 20 years, respectively).9 Most MFS patients are young and therefore usually receive a mechanical valve as part of the CVG (89 % in Gott’s report) due to the limited durability of bioprosthetic and homograft valves. Amongst the patients who received a CVG and were discharged alive from hospital, 90% were free of a thromboembolic event at 20 years.9 Three patients in our T. David V-SARR series sustained a thromboembolic event (2 transient ischemic attacks and one stroke), and none had a hemorrhagic complication.
In 2009 David reported on 103 patients with MFS who underwent either a Yacoub procedure or T. David reimplantation V-SARR over 18 years.7 Overall results were satisfactory, but inferior for those who had a Yacoub remodeling procedure. It is import to emphasize that only 29 V-SARR patients remained at risk at 10 years; therefore, we still do not know with certainty how durable V-SARR for patients with MFS will be 10–20 years later.
All 6 patients in our series who sustained a new type B aortic dissection had a CTD (5 MFS, TABLE E5, FIGURE E2A and E2B). Most had been or were currently on losartan, another angiotensin II receptor blocker (ARB) or an ACE inhibitor (ACEi); while anecdotal, this has prompted us to avoid ARB or ACEi therapy postoperatively in patients with a CTD. David et al. also noted that the majority of late deaths was related to downstream aortic dissection-related complications.7 Furthermore, computational fluid dynamic studies have suggested that an ascending aortic graft increases distal pulse pressure and might exacerbate systolic hypertension. 29
A David V-SARR procedure is an excellent option for most patients with aortic root aneurysm if the aortic valve cusps are structurally normal, even if AR is present due to sinotubular junction or annular dilatation or cusp prolapse since the aortic regurgitation can be corrected by restoring normal annular and sinotubular junction geometry (with or without cusp reconstruction). Yacoub remodeling should be avoided if there is annular dilatation. Structurally abnormal valve cusps, including severe fibrosis, calcification, or multiple perforations, are contraindication to V-SARR. The 20-year prognosis of patients undergoing V-SARR compared to CVG remains unknown, especially in terms of all valve-related morbidity and mortality. Therefore, patients who desire V-SARR to avoid anticoagulation must accept an unknown - but finite -- risk of reoperation in their lifetimes as a trade-off.
Limitations
This investigation was a retrospective analysis, albeit data were collected prospectively. Only 18 patients remained at risk beyond 10 years (7 with MFS); thus, only cautious statements about late results can be supported. Only 14 adverse events occurred, which precluded use of a hazard model to identify patient- or disease-related variables or technical factors associated with a higher likelihood of complications; this highlights the need for rigorous inspection of larger numbers of patients over longer follow-up before we learn conclusively what the long-term durability of T. David V-SARR really is. Clinically important adverse events (such as stroke, hemorrhagic events, infection, SVD, reoperation) occurred rarely, which would make randomized, prospective studies prohibitively expensive and unrealistic. This report included only selected patients undergoing elective, first-time V-SARR; more studies similar to that by Chen’s group from Emory30 are needed to assess comprehensively the results of V-SARR in acute settings, especially for patients with acute type A aortic dissection, but the Hannover group is urging caution about performing V-SARR in patients with acute dissections due to the higher mortality.19
Aortic regurgitation generally is well tolerated; therefore it is essential to report severity of echocardiographic residual/recurrent AR over time in all surviving patients and not just rely on the need for reoperation. Since different groups use different AR grading systems and some report only severe AR postoperatively, meaningful comparison between reports is difficult. One surgeon (DCM) operated on nearly all these Stanford patients, making generalization of the results to other surgeons or institutions speculative. Finally, patient-referral and patient-selection biases are likely present.
In conclusion, Tirone David reimplantation V-SARR was associated with excellent clinical and functional outcome out to 5–10 years in patients with or without associated CTD (primarily MFS) or with a BAV. The incidence of more than 2+ AR or reoperation at 10 years was very low. Further follow-up is required, however, to characterize the risks, hazards and outcomes of V-SARR beyond 10 years.
Acknowledgments
The authors thank Stanford Professor Mark Hlatky, M.D. for providing comparative U.S. survival data. We also appreciate and acknowledge the efforts of Michael Sheehan, MSN, RNFA, NPc in following up patients and capturing late echocardiograms. This work was supported by the U.S.-Norway Fulbright Foundation, the Swedish Heart-Lung Foundation and the Swedish Society for Medical Research (Dr. Kvitting).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Miller DC. Valve-sparing aortic root replacement: current state of the art and where are we headed? Ann Thorac Surg 2007;83:S736–9; [DOI] [PubMed] [Google Scholar]
- 2.Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435–8. [PubMed] [Google Scholar]
- 3.David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617–21; [PubMed] [Google Scholar]
- 4.Kallenbach K, Karck M, Pak D, Salcher R, Khaladj N, Leyh R, et al. Decade of aortic valve sparing reimplantation: are we pushing the limits too far? Circulation 2005;112:I253–9. [DOI] [PubMed] [Google Scholar]
- 5.Zehr KJ, Thubrikar MJ, Gong GG, Headrick JR, Robicsek F. Clinical introduction of a novel prosthesis for valve-preserving aortic root reconstruction for annuloaortic ectasia. J Thorac Cardiovasc Surg 2000;120:692–8. [DOI] [PubMed] [Google Scholar]
- 6.Hanke T, Charitos EI, Stierle U, Robinson D, Gorski A, Sievers HH, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg 2009;137:314–9. [DOI] [PubMed] [Google Scholar]
- 7.David TE, Armstrong S, Maganti M, Colman J, Bradley TJ. Long-term results of aortic valve-sparing operations in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2009;138:859–64; [DOI] [PubMed] [Google Scholar]
- 8.Svensson LG, Batizy LH, Blackstone EH, Gillinov AM, Moon MC, D’Agostino RS, et al. Results of matching valve and root repair to aortic valve and root pathology. J Thorac Cardiovasc Surg 2011;142:1491–8 e7. [DOI] [PubMed] [Google Scholar]
- 9.Gott VL, Greene PS, Alejo DE, Cameron DE, Naftel DC, Miller DC, et al. Replacement of the aortic root in patients with Marfan’s syndrome. N Engl J Med 1999;340:1307–13. [DOI] [PubMed] [Google Scholar]
- 10.Gott VL, Cameron DE, Alejo DE, Greene PS, Shake JG, Caparrelli DJ, et al. Aortic root replacement in 271 Marfan patients: a 24-year experience. Ann Thorac Surg 2002;73:438–43. [DOI] [PubMed] [Google Scholar]
- 11.Tang GH, Maganti M, David TE, Feindel CM, Scully HE, Borger MA. Effect of prior valve type on mortality in reoperative valve surgery. Ann Thorac Surg 2007;83:938–45. [DOI] [PubMed] [Google Scholar]
- 12.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007;133:1226–33. [DOI] [PubMed] [Google Scholar]
- 13.Demers P, Miller DC. Simple modification of “T. David-V” valve-sparing aortic root replacement to create graft pseudosinuses. Ann Thorac Surg 2004;78:1479–81. [DOI] [PubMed] [Google Scholar]
- 14.Itoh A, Fischbein M, Arata K, Miller DC. “Peninsula-style” transverse aortic arch replacement in patients with bicuspid aortic valve. Ann Thorac Surg 2010;90:1369–71. [DOI] [PubMed] [Google Scholar]
- 15.Fazel SS, Mallidi HR, Lee RS, Sheehan MP, Liang D, Fleischman D, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg 2008;135:901–7, 7 e1–2. [DOI] [PubMed] [Google Scholar]
- 16.Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. J Thorac Cardiovasc Surg 2008;135:732–8. [DOI] [PubMed] [Google Scholar]
- 17.de Kerchove L, Boodhwani M, Glineur D, Poncelet A, Verhelst R, Astarci P, et al. Effects of preoperative aortic insufficiency on outcome after aortic valve-sparing surgery. Circulation 2009;120:S120–6. [DOI] [PubMed] [Google Scholar]
- 18.Aicher D, Fries R, Rodionycheva S, Schmidt K, Langer F, Schafers HJ. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2009;37:127–32. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha M, Baraki H, Maeding I, Fitzner S, Sarikouch S, Khaladj N, et al. Long-term results after aortic valve-sparing operation (David I). Eur J Cardiothorac Surg 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Aortic valve preservation in patients with aortic root aneurysm: results of the reimplantation technique. Ann Thorac Surg 2007;83:S732–5; [DOI] [PubMed] [Google Scholar]
- 21.Birks EJ, Webb C, Child A, Radley-Smith R, Yacoub MH. Early and long-term results of a valve-sparing operation for Marfan syndrome. Circulation 1999;100:II29–35. [DOI] [PubMed] [Google Scholar]
- 22.De Paulis R, Scaffa R, Nardella S, Maselli D, Weltert L, Bertoldo F, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S23–7; discussion S45–51. [DOI] [PubMed] [Google Scholar]
- 23.Forteza A, Centeno J, Bellot R, Lopez Gude MJ, Perez de la Sota E, Sanchez V, et al. [Aortic valve sparing in 120 patients with aortic root aneurysms]. Rev Esp Cardiol 2011;64:470–5. [DOI] [PubMed] [Google Scholar]
- 24.Boodhwani M, de Kerchove L, Watremez C, Glineur D, Vanoverschelde JL, Noirhomme P, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg 2011;141:917–25. [DOI] [PubMed] [Google Scholar]
- 25.Schafers HJ, Kunihara T, Fries P, Brittner B, Aicher D. Valve-preserving root replacement in bicuspid aortic valves. J Thorac Cardiovasc Surg 2010;140:S36–40; discussion S5–51. [DOI] [PubMed] [Google Scholar]
- 26.Park CB, Greason KL, Suri RM, Michelena HI, Schaff HV, Sundt TM 3rd. Should the proximal arch be routinely replaced in patients with bicuspid aortic valve disease and ascending aortic aneurysm? J Thorac Cardiovasc Surg 2011;142:602–7. [DOI] [PubMed] [Google Scholar]
- 27.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, et al. Outcomes in adults with bicuspid aortic valves. JAMA 2008;300:1317–25. [DOI] [PubMed] [Google Scholar]
- 28.Patel ND, Arnaoutakis GJ, George TJ, Allen JG, Alejo DE, Dietz HC, et al. Valve-sparing aortic root replacement in Loeys-Dietz syndrome. Ann Thorac Surg 2011;92:556–60; discussion 60–1. [DOI] [PubMed] [Google Scholar]
- 29.Vardoulis O, Coppens E, Martin B, Reymond P, Tozzi P, Stergiopulos N. Impact of aortic grafts on arterial pressure: a computational fluid dynamics study. Eur J Vasc Endovasc Surg 2011;42:704–10. [DOI] [PubMed] [Google Scholar]
- 30.Leshnower BG, Guyton RA, Myung RJ, Puskas JD, Kilgo PD, McPherson L, et al. Expanding the indications for the David V aortic root replacement: Early results. J Thorac Cardiovasc Surg 2012;143:879–84. [DOI] [PubMed] [Google Scholar]