The interaction of the basolateral amygdala and prelimbic cortex plays a crucial role in the reactivation of addiction memory.
Abstract
An important reasons for drug relapse is the retrieval of drug withdrawal memory induced by conditioned context. Previous studies have suggested that the basolateral amygdala (BLA) plays an important role in conditioned context–induced retrieval of morphine withdrawal memory. However, the downstream neuronal circuits of the activation of the BLA in conditioned context–induced retrieval of morphine withdrawal memory remain unknown. Using retrograde labeling, immunohistochemical, and optogenetic approaches, we found that, although BLA neurons projecting to the prelimbic cortex (PrL) played an important role in conditioned context–induced retrieval of morphine withdrawal memory, they do not exhibit increased expression of the neuronal plasticity marker Arc. However, when PrL neurons activated by the BLA send feedback signals to the BLA, a neuronal-related process is induced in other BLA neurons that do not project to the PrL, a finding that is relevant to conditioned context–induced retrieval of morphine withdrawal memory.
INTRODUCTION
Drug addiction is a chronic brain disorder characterized by remissions and relapses (1). One of the most important reasons for the relapses is the retrieval of drug withdrawal memory induced by conditioned context previously associated with withdrawal syndromes (2). Understanding the neuronal circuits underlying conditioned context–induced retrieval of drug withdrawal memory could help in developing new therapeutic approaches to prevent drug relapse.
Previous studies suggested that the basolateral amygdala (BLA) played an important role in conditioned context–induced retrieval of morphine withdrawal memory. Reexposure to conditioned context by morphine withdrawal rats could activate BLA neurons (3, 4). Lesion in the BLA attenuated conditioned context–induced food aversion in morphine withdrawal rats (5). Studies from our laboratory showed that chronic morphine–induced increase in the expression of D1 receptors at presynaptic terminals in the BLA was closely related to conditioned context–induced retrieval of withdrawal memory (6). However, the downstream neuronal circuits of the activation of the BLA in conditioned context–induced retrieval of morphine withdrawal memory remain to be unknown.
It has been known that the BLA sends a dense glutamatergic projection to the brain regions, such as the prelimbic cortex (PrL) (7, 8), the CA1 of the hippocampus (9), and the nucleus accumbens (NAc) (10, 11). Our previous studies showed that chronic morphine sensitized the effect of D1 receptor agonist on presynaptic glutamate release in BLA neurons that projected to the PrL but had no influence on that in BLA neurons that projected to the NAc or the CA1 of the hippocampus (12). Therefore, we proposed that BLA neurons projecting to the PrL might be an important downstream neuronal circuit of the activation of the BLA in conditioned context–induced retrieval of morphine withdrawal memory. To test this hypothesis, we used retrograde labeling method to identify BLA neurons projecting to the PrL and then examined the influence of conditioned context on the expression of c-Fos, a neuronal activity marker (13), in BLA neurons projecting to the PrL using immunohistochemical method and studied the role of BLA neurons projecting to the PrL in conditioned place aversion (CPA) of morphine withdrawal mice by in vivo optogenetic inhibition of axonal terminals or chemical-genetic inhibition of cell bodies of these BLA projecting neurons. CPA was a widely used animal model of conditioned context–induced retrieval of morphine withdrawal memory (3, 4) and was elicited by naloxone-precipitated morphine withdrawal following exposure to chronic morphine as used by other laboratories (14, 15).
There are two possible ways for BLA-PrL projection neurons to participate in conditioned context–induced retrieval of morphine withdrawal memory: one is that they are only a pathway that mediates the passage of signals to induce a retrieval of memory signals at other brain regions, and another is that they exhibit a retrieval of memory signals by themselves. To address whether there was a retrieval of memory signals in BLA-PrL projection neurons after reexposure to conditioned context in morphine withdrawal mice, we examined the influence of conditioned context on the expression of Arc, a neuronal plasticity marker (16), which was closely related to memory retrieval (17), in BLA-PrL projection neurons in morphine withdrawal mice. In addition, it is known that PrL neurons project to a number of brain regions, such as the BLA, the NAc, and the ventral tegmental area (18). Here, we hypothesized that, after BLA neurons projecting to the PrL activate the PrL, PrL neurons send feedback signals to the BLA to induce an increase in Arc expression in other BLA neurons that do not project to the PrL. Preliminary evidence supporting this hypothesis includes findings that the BLA receives glutamatergic projections from the PrL (19–21) and our own data showing that conditioned context could increase the expression of Arc in other BLA neurons that were not projection neurons to the PrL. To test our hypothesis, we studied the influence of in vivo optogenetic inhibition of glutamatergic terminals from the BLA in the PrL or chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL on conditioned context–induced increase in the expression of Arc in BLA neurons that were not projection neurons to the PrL and examined the influence of in vivo optogenetic inhibition of glutamatergic terminals of PrL projection neurons in the BLA on conditioned context–induced increase in the expression of Arc in BLA neurons that were not projection neurons to the PrL in morphine withdrawal mice. In addition, using a two-step virus injection approach (22), we traced the feedback circuit from the PrL to the BLA.
RESULTS
Conditioned context increases c-Fos expression in BLA projection neurons to the PrL
To study whether conditioned context could activate BLA neurons projecting to the PrL in morphine withdrawal mice, we examined the effect of conditioned context on the expression of c-Fos, a marker of neuronal activity (23), in BLA neurons projecting to the PrL in morphine withdrawal mice. We injected FluoroGold (FG) into the PrL to retrograde label BLA neurons projecting to the PrL (Fig. 1A). After recovery from the surgery of FG injection, mice were subjected to behavioral training, as illustrated in the left panel of Fig. 1B. Results showed that mice in the morphine + naloxone group exhibited a strong aversion to the morphine withdrawal–paired compartment and, hence, spent less time in the morphine withdrawal–paired compartment during the post-test than that during the pre-test. Meanwhile, mice from other groups did not exhibit a significant aversion to either compartment. The average CPA score in the morphine + naloxone group was −256.10 ± 22.75 s, which was statistically different from that in the saline + saline group (80.33 ± 47.54 s), the saline + naloxone group (−47.03 ± 29.72 s), and the morphine + saline group (48.05 ± 37.43 s; Fig. 1B, right). After behavioral assay, animals were sacrificed and the effect of conditioned context on the expression of c-Fos in different groups was examined. We could see that the expression of c-Fos and the coexpression of c-Fos and FG in the BLA were significantly increased in the morphine + naloxone group after the reexposure to conditioned context, but they did not change significantly in saline + naloxone or morphine + saline groups compared to the saline + saline group (Fig. 1C, left). The average c-Fos–positive neurons/mm2 in the BLA were 51.79 ± 6.66/mm2 in the morphine + naloxone group, which were significantly higher than those in the saline + saline group (31.85 ± 5.32/mm2), the saline + naloxone group (33.01 ± 2.96/mm2), and the morphine + saline group (34.35 ± 2.23/mm2; Fig. 1C, top right). The average percentage of the coexpression of c-Fos and FG relative to FG in the BLA in the morphine + naloxone group was 13.74 ± 0.96%, which was significantly higher than that in the saline + saline group (7.94 ± 0.43%), the saline + naloxone group (9.59 ± 0.69%), and the morphine + saline group (10.03 ± 0.50%; Fig. 1C, bottom right). This result suggests that conditioned context can activate BLA neurons projecting to the PrL in morphine withdrawal mice to a greater extent than that in control groups.
Fig. 1. Effect of conditioned context on the expression of c-Fos in BLA projection neurons to the PrL.
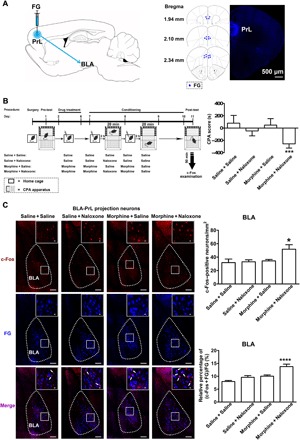
(A) Left: Diagram of FG injection site in the PrL. Right: Anatomical location of the injection site of FG (blue-colored) in the PrL. Numbers indicate coordinates relative to bregma. Scale bar, 500 μm. (B) Left: Experimental timeline for CPA procedure. Right: Average CPA score in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups [n = 7 mice in saline + saline and saline + naloxone groups and n = 8 mice in morphine + saline and morphine + naloxone groups; one-way analysis of variance (ANOVA), ***P < 0.001]. (C) Top line of left panel: c-Fos–positive neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (red-colored). Middle line of left panel: FG-labeling neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (blue-colored). Bottom line of left panel: Colabeling neurons of c-Fos and FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (purple-colored). Scale bars, 100 μm. BLA regions enclosed by white boxes were shown in a higher magnification in upper right square images. Scale bars, 20 μm. Top right: c-Fos–positive neurons/mm2 in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 6 mice in saline + saline, saline + naloxone, and morphine + naloxone groups; n = 7 mice in morphine + saline group; one-way ANOVA, *P < 0.05). Bottom right: Average percentage of the coexpression of c-Fos and FG relative to FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 6 mice in saline + saline, saline + naloxone, and morphine + naloxone groups; n = 7 mice in morphine + saline group; one-way ANOVA, ****P < 0.0001). Data are means ± SEM.
Inhibition of BLA projection neurons to PrL inhibits CPA
To study the role of BLA neurons projecting to the PrL in conditioned context–induced retrieval of morphine withdrawal memory, we examined the influence of in vivo optogenetic inhibition of axonal terminals of these BLA projecting neurons in the PrL on conditioned context–induced place aversion in morphine withdrawal mice. The AAV8-CaMKIIα-eNpHR-eYFP virus or the same viral vectors carrying enhanced yellow fluorescent protein (eYFP) alone were injected into the BLA, and optical fiber was unilaterally implanted over the PrL before CPA (Fig. 2A, left). The expression of eNpHR-eYFP in the BLA after the injection of the virus into the BLA was shown in the middle panel of Fig. 2A. The virus was allowed to express for a minimum of 6 weeks to have sufficient opsin accumulation in axonal terminals in the PrL. To inhibit eNpHR-eYFP–expressed BLA axonal terminals in the PrL, a wireless optical fiber was implanted above the PrL to allow the delivery of 594-nm light (Fig. 2A, right). The mice with the expression of eNpHR-eYFP or eYFP are divided into two groups: one group is the light ON group that will be given a 15-min continuous light, and another group is the light OFF group that will not be given light. Both groups were subjected to behavioral paradigms, as shown in the left panel of Fig. 2B. The result showed that during the light OFF epoch, conditioned context could induce a strong aversion to the morphine withdrawal–paired compartment in eYFP- and eNpHR-eYFP–expressing mice. However, during the light ON epoch, although conditioned context could induce a strong aversion to the morphine withdrawal–paired compartment in eYFP-expressing mice, it could not induce a significant aversion to the morphine withdrawal–paired compartment in eNpHR-eYFP–expressing mice. The average CPA score during the light OFF epoch in eYFP- and eNpHR-eYFP–expressing mice was −148.22 ± 19.04 s and − 177.81 ± 61.27 s, respectively, but during the light ON epoch, the average CPA score in eNpHR-eYFP–expressing mice was 28.09 ± 34.87 s, which was statistically different from that in eYFP-expressing mice (−186.00 ± 57.42 s; Fig. 2B, right). This result suggests that activated BLA neurons projecting to the PrL play an important role in conditioned context–induced retrieval of morphine withdrawal memory in morphine withdrawal mice.
Fig. 2. Influence of the inhibition of BLA projection neurons to the PrL on CPA.
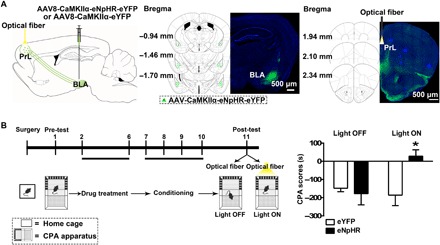
(A) Left: Diagram of virus injection site in the BLA and optical fiber implantation site in the PrL. Middle: Image of coronal brain slice showing the expression of eNpHR-eYFP (green-colored) 6 weeks after virus injection into the BLA. Numbers indicate coordinates relative to bregma. Scale bar, 500 μm. Right: Image of coronal brain slice showing strong eNpHR-eYFP–positive fibers (green-colored) in the PrL and the optical fiber tip (yellow-colored) in the PrL 6 weeks after virus injection into the BLA. Numbers indicate coordinates relative to bregma. Scale bar, 500 μm. (B) Left: Experimental timeline for the CPA procedure. Right: Average CPA score in eNpHR-eYFP– and eYFP-expressing mice in light OFF and light ON groups (n = 8 mice in each group; two-way ANOVA, *P < 0.05). Data are means ± SEM.
To confirm the role of BLA neurons projecting to the PrL in conditioned context–induced retrieval of morphine withdrawal memory, we also examined the influence of chemical-genetic inhibition of cell bodies of these BLA projecting neurons in the BLA on conditioned context–induced place aversion in morphine withdrawal mice. The AAV-hSyn-DIO-hM4Di(Gi)-mCherry virus was injected into the BLA, and the AAV-hSyn-mCherry-IRES-WGA-Cre virus was injected into the PrL before CPA (fig. S1A, left). The expression of mCherry-WGA-Cre in the PrL after the injection of the virus into the PrL was shown in the middle panel of fig. S1A. Four weeks after the injection, the expression of hM4Di(Gi)-mCherry was observed in the BLA due to retrograde transporting mCherry-WGA-Cre from the PrL to the BLA (fig. S1A, right). The mice with the expression of hM4Di(Gi)-mCherry are divided into two groups: one group is the saline group that will be injected with saline 60 min before post-test of CPA, and another group is the clozapine-n-oxide (CNO) group that will be injected with CNO 60 min before post-test of CPA. Both groups were subjected to behavioral paradigms, as shown in the left panel of fig. S1B. The result showed that although conditioned context could induce a strong aversion to the morphine withdrawal–paired compartment in the saline group, conditioned context could not induce a significant aversion to the morphine withdrawal–paired compartment in the CNO group. The average CPA score in the saline group was −178.40 ± 62.66 s, which was statistically different from that in the CNO group (−52.82 ± 72.01 s; fig. S1B, right). This result confirms that activated BLA neurons projecting to the PrL play an important role in conditioned context–induced retrieval of morphine withdrawal memory in morphine withdrawal mice.
In addition, we noted that when injecting the AAV8-CaMKIIα-eNpHR-eYFP virus into the BLA, there was also expression of eNpHR-eYFP in the infralimbic cortex (IL). This observation is consistent with the report that the BLA has projections to both the PrL and the IL (24). To study the role of BLA neurons projecting to the IL in conditioned context–induced retrieval of morphine withdrawal memory, we examined the influence of in vivo chemical-genetic inhibition of BLA neurons projecting to the IL on conditioned context–induced place aversion in morphine withdrawal mice. The AAV-hSyn-mCherry-IRES-WGA-Cre virus was injected into the IL, and the AAV-hSyn-DIO-hM4Di(Gi)-mCherry virus was injected into the BLA before CPA (fig. S2A, left). The expression of mCherry-WGA-Cre in the IL after the injection of the virus into the IL was shown in the middle panel of fig. S2A. Four weeks after the injection, the expression of hM4D(Gi)-mCherry was observed in the BLA by retrograde tracing mCherry-WGA-Cre from the IL to the BLA (fig. S2A, right). The mice with the expression of hM4D(Gi)-mCherry are divided into two groups: one group is the saline group injected with saline 60 min before post-test of CPA, and another group is the CNO group injected with CNO 60 min before post-test of CPA. Both groups were subjected to behavioral paradigms, and the result showed that conditioned context could induce a strong aversion to the morphine withdrawal–paired compartment in both saline and CNO groups. The average CPA score in the saline group was −114.40 ± 81.20 s, which was not significantly different from that in the CNO group (−160.50 ± 77.35 s; fig. S2B). This result suggests that BLA neurons projecting to the IL do not play an important role in conditioned context–induced retrieval of morphine withdrawal memory in morphine withdrawal mice.
Conditioned context does not increase Arc expression in BLA projection neurons to the PrL
To examine whether conditioned context induced a change in neuronal plasticity of BLA neurons projecting to the PrL in morphine withdrawal mice, we studied the effect of conditioned context on the expression of Arc, a marker of neuronal plasticity (16), in BLA neurons projecting to the PrL in morphine withdrawal mice. We injected FG into the PrL to retrograde label BLA neurons projecting to the PrL, as mentioned above. In behavioral experiments, the average CPA score in the morphine + naloxone group was −186.70 ± 31.67 s, which was statistically different from that in the saline + saline group (92.41 ± 38.67 s), the saline + naloxone group (−33.79 ± 18.31 s), and the morphine + saline group (99.82 ± 52.09 s). Following CPA, the effect of conditioned context on the expression of Arc in different groups was examined. We could see that the coexpression of Arc and FG in the BLA in the morphine + naloxone group had no significant difference compared to that in saline + saline, saline + naloxone, or morphine + saline groups (Fig. 3A). The average percentage of the coexpression of Arc and FG relative to FG in the BLA in the morphine + naloxone group was 5.13 ± 0.56%, which had no significant difference compared to that in the saline + saline group (4.24 ± 0.46%), the saline + naloxone group (3.89 ± 0.53%), or the morphine + saline group (4.56 ± 0.35%; Fig. 3B, left). This result suggests that conditioned context does not increase the expression of neuronal plasticity–related Arc in BLA neurons projecting to the PrL in morphine withdrawal mice.
Fig. 3. Effect of conditioned context on the expression of Arc in BLA projection neurons to the PrL.
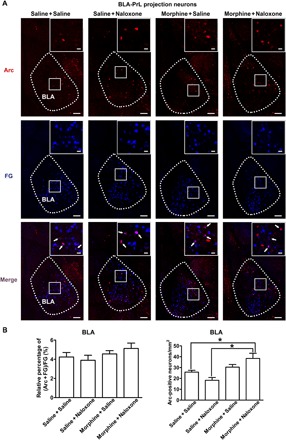
(A) Top: Arc-positive neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (red-colored). Middle: FG-labeling neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (blue-colored). Bottom: Colabeling neurons of Arc and FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (purple-colored). Scale bars, 100 μm. BLA regions enclosed by white boxes were shown in a higher magnification in upper right square images. Scale bars, 20 μm. (B) Left: Average percentage of the coexpression of Arc and FG relative to FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 6 mice in each group; one-way ANOVA, P > 0.05). Right: Arc-positive neurons/mm2 in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 6 mice in each group; one-way ANOVA, *P < 0.05). Data are means ± SEM.
Conditioned context increases Arc expression in other BLA neurons that are not projection neurons to PrL
Although conditioned context did not increase the expression of Arc in BLA neurons projecting to the PrL as described above, it appeared that it could increase the expression of Arc in other BLA neurons because, after the reexposure to conditioned context, there was an increase in the general expression of Arc in the BLA in morphine withdrawal mice. The average Arc-positive neurons/mm2 in the BLA were 38.45 ± 4.79/mm2 in the morphine + naloxone group, which were higher than those in the saline + saline group (25.35 ± 1.37/mm2) and the saline + naloxone group (18.26 ± 2.58/mm2; Fig. 3B, right). This result, combined with that in Fig. 1, suggests that within the BLA, there may be two kinds of neurons that have different responses to conditioned context in morphine withdrawal mice: one is BLA projection neurons to the PrL that exhibit conditioned context–induced increase in the expression of c-Fos, but without an increase in Arc expression, and another is BLA non-PrL projection neurons that exhibit conditioned context–induced increase in the expression of Arc. To confirm this statement, we examined the effect of conditioned context on the coexpression of c-Fos, Arc, and FG in morphine withdrawal mice. We injected FG into the PrL to retrograde label BLA neurons projecting to the PrL as mentioned above. In behavioral experiments, the average CPA score in the morphine + naloxone group was −128.60 ± 29.68 s, which was statistically different from that in the saline + saline group (66.85 ± 50.99 s), the saline + naloxone group (−8.57 ± 51.88 s), and the morphine + saline group (49.79 ± 24.11 s). Following CPA, the effect of conditioned context on the coexpression of c-Fos and Arc in FG labeling neurons in the BLA was examined. We could see that without the expression of Arc, the coexpression of c-Fos and FG in the BLA was increased in the morphine + naloxone group after the reexposure to conditioned context, but it had no significant change in the saline + naloxone or morphine + saline group compared to the saline + saline group (Fig. 4A). The average percentage of the coexpression of c-Fos and FG, but not with Arc, relative to FG in the BLA in the morphine + naloxone group was 7.99 ± 1.05%, which was higher than that in the saline + saline group (4.48 ± 0.63%), the saline + naloxone group (5.22 ± 0.85%), and the morphine + saline group (5.59 ± 0.35%; Fig. 4B, left). Meanwhile, we could also see that the coexpression of c-Fos, Arc, and FG in the BLA had no significant change in the saline + saline, saline + naloxone, morphine + saline, or morphine + naloxone group (Fig. 4A). The average percentage of the coexpression of c-Fos, Arc, and FG relative to FG in the BLA in the morphine + naloxone group was 4.83 ± 0.72%, which had no significant difference with that in the saline + saline group (3.56 ± 0.54%), the saline + naloxone group (3.89 ± 0.57%), and the morphine + saline group (4.52 ± 0.38%; Fig. 4B, middle). However, we could see that the coexpression of c-Fos and Arc without FG labeling in the BLA was increased in the morphine + naloxone group after the reexposure to conditioned context, whereas there was no significant change in the saline + naloxone or morphine + saline group compared to the saline + saline group (Fig. 4A). The average percentage of the coexpression of c-Fos and Arc in the BLA in the morphine + naloxone group was 19.15 ± 2.10%, which was higher than that in the saline + saline group (11.41 ± 1.78%), the saline + naloxone group (12.16 ± 1.26%), and the morphine + saline group (13.49 ± 1.22%; Fig. 4B, right). This result suggests that within the BLA, BLA projection neurons to the PrL exhibit conditioned context–induced increase in the expression of c-Fos but without an increase in the expression of Arc, whereas BLA non-PrL projection neurons exhibit conditioned context–induced increase in the expression of both c-Fos and Arc.
Fig. 4. Effect of conditioned context on the coexpression of c-Fos, Arc, and FG in BLA projection neurons to the PrL.
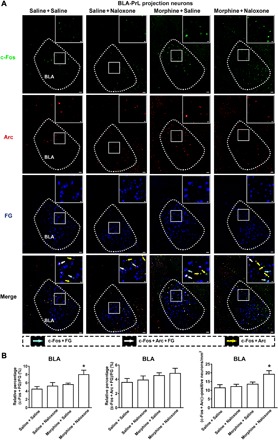
(A) First line: c-Fos–positive neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (green-colored). Second line: Arc-positive neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (red-colored). Third line: FG-labeling neurons in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (blue-colored). Fourth line: Colabeling neurons of c-Fos and FG (cyan-colored); colabeling neurons of c-Fos, Arc, and FG (white-colored); and colabeling neurons of c-Fos and Arc (yellow-colored) in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups. Scale bars, 100 μm. BLA regions enclosed by white boxes were shown in a higher magnification in upper right square images. Scale bars, 20 μm. (B) Left: Average percentage of the expression of c-Fos colabeled with FG, but not with Arc, relative to FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 5 mice in saline + saline, saline + naloxone, and morphine + saline groups; n = 7 mice in morphine + naloxone group; one-way ANOVA, *P < 0.05). Middle: Average percentage of the expression of c-Fos, Arc, and FG relative to FG in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 5 mice in saline + saline, saline + naloxone, and morphine + saline groups; n = 7 mice in morphine + naloxone group; one-way ANOVA, P > 0.05). Right: c-Fos– and Arc-positive neurons/mm2 in the BLA in saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone groups (n = 5 mice in saline + saline, saline + naloxone, and morphine + saline groups; n = 7 mice in morphine + naloxone group; one-way ANOVA, *P < 0.05). Data are means ± SEM.
Inhibition of BLA projection neurons to PrL inhibits conditioned context–induced Arc expression in BLA non-PrL projection neurons
On the basis of the above results, we hypothesized that after BLA neurons projecting to the PrL activated the PrL, PrL neurons sent feedback signals to the BLA again to induce an increase in the expression of Arc in other BLA neurons that were not projection neurons to the PrL. To test this hypothesis, we examined the influence of in vivo optogenetic inhibition of glutamatergic inputs from the BLA to the PrL on conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice. The virus injection, optical fiber implantation, and CPA result were shown in Fig. 2A, and the result of Arc expression was shown in Fig. 5. We could see that conditioned context–induced activation of Arc in the BLA was decreased after in vivo optogenetic inhibition of glutamatergic terminals from the BLA in the PrL in the light ON group (Fig. 5B). The average Arc-positive neurons/mm2 were 24.32 ± 1.28/mm2 in the light ON group, which were lower than those in the light OFF group (38.49 ± 3.46/mm2; Fig. 5C). This result suggests that BLA neurons projecting to the PrL play an important role in conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice. To confirm this statement, we examined the influence of in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL on conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice. The virus injection, CPA result, and the result of Arc expression were shown in fig. S1 (A to C). We could see that conditioned context–induced increase in the expression of Arc in the BLA was decreased after in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL (fig. S1C, left). The average Arc-positive neurons/mm2 were 18.35 ± 0.88/mm2 in the CNO group, which were lower than those in the saline group (34.54 ± 1.64/mm2; fig. S1C, right). This result confirms that BLA neurons projecting to the PrL play an important role in conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice.
Fig. 5. Influence of in vivo optogenetic inhibition of glutamatergic inputs from the BLA to the PrL on conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice.
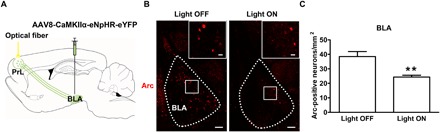
(A) Diagram of virus injection site in the BLA and optical fiber implantation site in the PrL. (B) Arc-positive neurons in the BLA in light OFF and light ON groups (red-colored). Scale bars, 100 μm. BLA regions enclosed by white boxes were shown in a higher magnification in upper right square images. Scale bars, 20 μm. (C) Average Arc-positive neurons/mm2 in the BLA in light OFF and light ON groups (n = 6 mice in each group; unpaired t test, **P < 0.01). Data are means ± SEM.
Inhibition of PrL projection neurons to the BLA inhibits conditioned context–induced Arc expression in these BLA non-PrL projection neurons
We also examined the influence of in vivo optogenetic inhibition of glutamatergic inputs onto the BLA from the PrL on conditioned context–induced activation of Arc in the BLA in morphine withdrawal mice. The AAV8-CaMKIIα-eNpHR-eYFP virus or the same viral vectors carrying eYFP alone were injected into the PrL, and optical fiber was unilaterally implanted over the BLA before CPA (Fig. 6A, left). The expression of eNpHR-eYFP in the PrL after the injection of the virus into the PrL was shown in the middle panel of Fig. 6A. The virus was allowed to express for a minimum of 6 weeks to have sufficient opsin accumulation in axonal terminals in the BLA. To inhibit eNpHR-eYFP–expressed PrL axonal terminals in the BLA, a wireless optical fiber was implanted above the BLA to allow the delivery of 594-nm light (Fig. 6A, right). The mice with expression of eNpHR-eYFP or eYFP are divided into two groups: one group is the light ON group that will be given 15-min continuous light, and another group is the light OFF group that will not be given light. Both groups were subjected to behavioral experiments. Our results showed that during the light OFF periods, conditioned context could induce a strong aversion to the morphine withdrawal–paired compartment in eNpHR-eYFP–expressing mice. However, when the eNpHR-expressed PrL axonal terminals in the BLA were inhibited by 594-nm light in the light ON group, the aversive response of mice to the morphine withdrawal–paired compartment disappeared. The average CPA score in eNpHR-eYFP–expressing mice during the light OFF epoch was −223.40 ± 70.05 s, which was significantly different from that during the light ON epoch (63.84 ± 35.84 s; Fig. 6B, left). On this basis, the effect of conditioned context on the expression of Arc in the BLA in morphine withdrawal mice was examined. The result showed that, after the optogenetic inhibition of glutamatergic inputs onto the BLA from the PrL, conditioned context–induced increase in the expression of Arc in the BLA was decreased (Fig. 6B, middle). The average Arc-positive neurons/mm2 were 25.72 ± 8.51/mm2 in the light ON group, which were lower than those in the light OFF group (48.54 ± 9.30/mm2; Fig. 6B, right). This result suggests that the inhibition of glutamatergic inputs onto the BLA from the PrL inhibits conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice.
Fig. 6. Influence of in vivo optogenetic inhibition of glutamatergic inputs from the PrL to the BLA on conditioned context–induced increase in the expression of Arc in the BLA in morphine withdrawal mice.
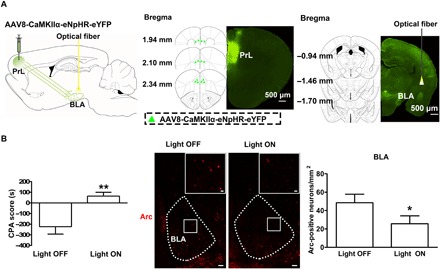
(A) Left: Diagram of virus injection site in the PrL and optical fiber implantation site in the BLA. Middle: Image of coronal brain slice showing the expression of eNpHR-eYFP (green-colored) 6 weeks after virus injection into the PrL. Numbers indicate coordinates relative to bregma. Scale bar, 500 μm. Right: Image of coronal brain slice showing strong eNpHR-eYFP–positive fibers (green-colored) in the BLA and the optical fiber tip (yellow-colored) in the BLA 6 weeks after virus injection into the PrL. Numbers indicate coordinates relative to bregma. Scale bar, 500 μm. (B) Left: Average CPA score in eNpHR-eYFP mice in light OFF and light ON groups (n = 6 mice in each group; unpaired t test, **P < 0.01). Middle: Arc-positive neurons in the BLA in light OFF and light ON groups (red-colored). Scale bars, 100 μm. BLA regions enclosed by white boxes were shown in a higher magnification in upper right square images. Scale bars, 20 μm. Right: Average Arc-positive neurons/mm2 in the BLA in light OFF and light ON groups (n = 6 mice in each group; unpaired t test, *P < 0.05). Data are means ± SEM.
There is a feedback circuit from the PrL to the BLA
We examined whether there was a feedback circuit from the PrL to the BLA using a two-step virus injection approach. We first injected the pAAV-hSyn-Cre-EGFP virus into the BLA to trans-synaptic label PrL neurons that received input from the BLA. If PrL neurons received the input from the BLA, they would be labeled with Cre protein. One week later, the pAAV-EF1α-DIO-hChR2-mCherry virus was injected into the PrL to detect the projecting regions of those Cre-positive PrL neurons. In the PrL, only those neurons receiving input from the BLA could express the hChR2 protein in a Cre-dependent manner. Then, the hChR2 protein anterograde transported to the BLA. Four weeks later, the expression of hChR2-mCherry–expressed PrL axonal terminals was examined in the BLA. The result showed that (i) after the injection of the pAAV-hSyn-Cre-EGFP virus into the BLA, the BLA was labeled with EGFP protein (green-colored, fig. S3B); (ii) after the injection of the pAAV-EF1α-DIO-hChR2-mCherry virus into the PrL, the PrL was labeled with mCherry protein (red-colored, fig. S3C); and (iii) after the two-step viral injection, axonal terminals of PrL neurons in the BLA were labeled with mCherry protein (red-colored, fig. S3D). This result suggests that there is a feedback circuit from the PrL to the BLA.
DISCUSSION
The main findings of the present study are that (i) conditioned context increases the expression of c-Fos in BLA neurons projecting to the PrL in morphine withdrawal mice and that inhibition of BLA neurons projecting to the PrL inhibits the conditioned context–induced place aversion of morphine withdrawal mice; (ii) conditioned context does not increase the expression of Arc in BLA neurons projecting to the PrL, but it increases the expression of Arc in other BLA neurons that are not projection neurons to the PrL in morphine withdrawal mice; (iii) in vivo optogenetic inhibition of glutamatergic terminals from the BLA in the PrL or in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL or in vivo optogenetic inhibition of glutamatergic terminals from the PrL in the BLA inhibits conditioned context–induced increase in the expression of Arc in these BLA neurons in morphine withdrawal mice; and (iv) there is a feedback circuit from the PrL to the BLA.
Previous studies by Stefanik and Kalivas (25) showed that in vivo optogenetic inhibition of BLA neurons projecting to the PrL inhibited the cue-induced reinstatement of cocaine seeking. This result is similar in many ways to our own data indicating that in vivo optogenetic inhibition of BLA neurons projecting to the PrL inhibits conditioned context–induced place aversion of morphine withdrawal mice. However, the memory types retrieved in these two studies are different. In Stefanik and Kalivas’s experiment, during the training stage, cue is paired with cocaine-induced reward memory such that the memory retrieved by cue in their study is reward memory (25); in our study, in contrast, context is paired with morphine withdrawal memory such that the memory retrieved by context in our study is withdrawal memory. This phenomenon suggests that BLA neurons projecting to the PrL may be a common neural substrate that underlies the retrieval of both reward and withdrawal memory. This role of BLA neurons projecting to the PrL in cue- or context-induced retrieval of reward and withdrawal memory may be related to its function of conveying information from the BLA to the PrL about cue or context that has been previously paired (26, 27). However, after this information is conveyed, the downstream circuits may be different for reward and withdrawal memory.
There are two possible roles of the activated BLA neurons projecting to the PrL in the retrieval of morphine withdrawal memory. One possibility is that after activation, these neurons serve only as a pathway that mediates the passage of signals to induce a retrieval of memory signals at other brain regions; another is that these neurons exhibit a retrieval of memory signals themselves. c-Fos is an immediate early gene (IEG), which is responsive to a number of extrinsic cellular stimuli and couples action potential firing to gene expression (13, 28). The signal transduction cascade by which membrane depolarization activates c-Fos gene transcription has been characterized in great detail (28). In a quiescent neuron, c-Fos expression is extremely low, but when excitatory synaptic inputs increase, depolarization causes an influx of extracellular calcium. In response to elevated intracellular calcium, CREB (adenosine 3′,5′-monophosphate calcium response element–binding protein) is rapidly phosphorylated so that CREB and its DNA binding site, Ca/CRE, can function as a regulatory element that activates the c-Fos gene (29). Therefore, the result that conditioned context increases the expression of c-Fos in BLA neurons projecting to the PrL in morphine withdrawal mice suggests that conditioned context can activate BLA neurons projecting to the PrL. However, whether there is a retrieval of memory signals in these projecting neurons after activation has yet to be determined. Current views on the dynamic nature of the memory trace suggest that memory retrieval is a neural process of memory expression (30). Accompanying the retrieval of memory, there should be an increase in expression of a number of molecules that are related to memory expression. Among such events, the increase in Arc expression is known to play an important role in the retrieval of memory. Arc is an IEG that not only participates in memory formation (16) but also is involved in the retrieval of memory (17). Arc knockout mice failed to form long-lasting memories (31), and the inhibition of Arc expression disrupted the retrieval of memory (32). Therefore, our finding that conditioned context does not increase the expression of Arc in BLA neurons projecting to the PrL in morphine withdrawal mice suggests that, after the activation of BLA neurons projecting to the PrL by conditioned context, there may not be a retrieval of memory signals in these projecting neurons. In addition, the lack of Arc expression in the BLA neurons projecting to PrL may be due to potential effects of FG on the physiology of BLA neurons, because FG has been shown to produce some extent of degeneration of neuronal neurons at the injection site. However, the concentration we used here was referred to the one used by Petrovich et al. (33), who showed that this concentration of FG was able to check the increase in the expression of Arc. In addition, in our c-Fos experiments, we also could check the conditioned context–induced increase in the expression of c-Fos in FG labeling neurons. Therefore, we propose that the activated BLA neurons projecting to the PrL by conditioned context in morphine withdrawal mice may only be a pathway that mediates the passage of signals to induce a retrieval of morphine withdrawal memory at other brain regions, rather than showing a retrieval of memory signals in these neurons.
It is known that BLA projections to the PrL consist primarily of excitatory fibers (29). Most of these excitatory synapses are on pyramidal neurons of the PrL, whereas inhibitory interneurons of the PrL receive a relatively light excitatory innervation (34, 35). Therefore, BLA activation might be expected to cause direct excitation of PrL pyramidal neurons or indirect inhibition of PrL pyramidal neurons through the activation of inhibitory interneurons, which had a local inhibitory input onto PrL pyramidal neurons (29). However, based on our finding that in vivo optogenetic inhibition of glutamatergic terminals from the BLA in the PrL or in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL could inhibit conditioned context–induced place aversion in morphine withdrawal mice, it was more possible that BLA activation might cause a direct excitation of PrL pyramidal neurons. However, the role of the feed-forward inhibition of BLA excitatory projection–PrL local interneurons–PrL pyramidal neurons in the retrieval of morphine withdrawal memory remains to be studied.
The downstream neuronal circuit, where there is conditioned context–induced increase in the expression of Arc, of activation of BLA neurons projecting to the PrL is an interesting question to be investigated. On the basis of evidence that the BLA received glutamatergic projections from the PrL (19–21) and our results showing that conditioned context could increase the expression of Arc in other BLA neurons that were not projection neurons to the PrL, we proposed a hypothesis that, after BLA neurons projecting to the PrL activated the PrL, PrL neurons sent feedback signals to the BLA again to induce an increase in the expression of Arc in other BLA neurons that were not projection neurons to the PrL. To confirm this hypothesis, it requires two kinds of evidence: one is that, when inhibiting the Arc-negative BLA output neurons from the BLA to the PrL, it should cancel conditioned context–induced increase in the expression of Arc in Arc-positive BLA neurons and the inhibition of PrL projection neurons to the BLA should also cancel conditioned context–induced increase in the expression of Arc in Arc-positive BLA neurons, and another is that using retrograde or trans-synaptic approaches to trace the feedback circuit from the PrL to the BLA. Now, we already have these evidences.
In conclusion, although BLA neurons projecting to the PrL play an important role in conditioned context–induced retrieval of morphine withdrawal memory, they do not exhibit an increase in the expression of the neuronal plasticity marker Arc. However, when activated PrL neurons by the BLA send feedback signals to the BLA, it induces a neuronal plasticity–related process in BLA neurons, which is relevant to conditioned context–induced retrieval of morphine withdrawal memory (fig. S4). However, we still do not know whether these BLA neurons with different Arc responses to conditioned context in morphine withdrawal mice are of the same or different neuron type.
MATERIALS AND METHODS
Experimental animals
Male adult (8 to 12 weeks) C57BL/6J mice were housed singly in a 12-hour light/12-hour dark cycle in a temperature- and humidity-controlled environment with food and water freely available. All experimental procedures conformed to Fudan University and international guidelines on the ethical use of animals. All efforts were made to minimize animal suffering and reduce the number of animals used.
Animal surgery
Mice were anesthetized with ketamine and xylazine (160 mg/kg and 12 mg/kg body weight, respectively) before the stereotaxic surgery was performed. For retrograde labeling experiments, mice received bilateral injections (0.5 μl in each side, infused over 10 min) of FG (Fluorochrome, USA) into the PrL [anteroposterior (AP), +1.98 mm; mediolateral (ML), ±0.30 mm; dorsoventral (DV), −2.20 mm]. For in vivo optogenetic inhibition in CPA experiments, mice were injected with the AAV8-CaMKIIα-eNpHR-eYFP virus or the same viral vectors carrying eYFP alone (2.45 × 1012 vector genomes/ml; Neuron Biotech Company, China) bilaterally into the BLA (AP, −1.60 mm; ML, ±3.35 mm; DV, −4.80 mm) or the PrL at a volume of 0.5 μl for 10 min. To allow projection-specific targeting, the optical fiber was held at least 500 μm above eYFP-expressing axonal terminals. For all the above stereotaxic injections, the needle was retained in place for an additional 10 min to allow diffusion of the injected solutions.
Chronic morphine treatment
Mice were treated with morphine (Shenyang No.1 Pharmaceutical Factory, China), as described before (36). Briefly, morphine dependence was induced in mice by repeated intraperitoneal injections of morphine twice daily. Morphine doses were progressively increased over 5 days from 10 mg/kg to 40 mg/kg: day 1, 2 × 10 mg/kg; day 2, 2 × 20 mg/kg; day 3, 2 × 30 mg/kg; days 4 and 5, 2 × 40 mg/kg. Control mice were treated with saline.
Conditioned place aversion
CPA was conducted using a three-compartment place conditioning apparatus (Med Associates, USA) with distinct visual and tactile context, and the procedure was similar to that described previously (14, 37, 38). The chamber and drop pan were thoroughly cleaned with 75% ethanol before each behavioral session. The CPA procedure entailed naloxone-precipitated morphine withdrawal aversion following chronic morphine treatment. As shown in the left panel of Fig. 1B, the CPA procedure included four phases: pre-test (day 1), drug treatment (days 2 to 6), conditioning (days 7 to 10), and post-test (day 11).
On pre-test day (day 1), mice were given an initial preference testing to assess their baseline place preference. Mice showing strong unconditioned preference (>75% of the session time) or aversion (<25% of the session time) for any compartment were eliminated from the study. All mice with unbiased preferences were randomly divided into four groups: saline + saline, saline + naloxone, morphine + saline, and morphine + naloxone.
On drug treatment days (days 2 to 6), morphine treatment in morphine + saline and morphine + naloxone groups was commenced. Mice in saline + saline and saline + naloxone groups were treated with saline.
On conditioning days (days 7 to 10), for each mouse, morphine withdrawal was paired with the compartment that it spent more time in (baseline place preference side) during the pre-test period. On days 7 and 9, each mouse in the morphine + naloxone group was injected with naloxone (0.3 mg/kg, intraperitoneally) 2 hours after receiving morphine injection (40 mg/kg, intraperitoneally) to induce enhanced withdrawal and confined in its morphine withdrawal–paired compartment for 20 min. On alternating days (8 and 10), the mouse in the morphine + naloxone group was injected with saline injection (0.1 ml, intraperitoneally) 2 hours after receiving morphine injection (40 mg/kg, intraperitoneally) and confined in its saline-paired compartment for 20 min. To confirm that the CPA seen in the current study was induced by naloxone-precipitated morphine withdrawal aversion but not by spontaneous withdrawal after morphine administration or naloxone alone, we compared the effects of morphine-naloxone treatment on the acquisition of CPA with three control groups (saline + saline, saline + naloxone, and morphine + saline). All CPA procedures with three control groups were the same as described above, except drug treatment with saline + saline, saline + naloxone, or morphine + saline instead of morphine + naloxone.
On post-test day (day 11), each mouse was placed in the same apparatus for 15 min to assess place aversion response. CPA score was defined as the time in the naloxone-paired compartment in the post-test minus that in the pre-test.
In vivo optogenetic approach for CPA
After injection with the AAV8-CaMKIIα-eNpHR-eYFP virus in the BLA or the PrL for a minimum of 6 weeks, mice were habituated to connect the mimical optical receivers (Teleopt, Japan) in their home cages (3 days, 5 min/day). As shown in the left panel of Fig. 2B, the pre-test (day 1), drug treatment (days 2 to 6), and conditioning (days 7 to 10) phases were similar as described above. On post-test day (day 11), the wireless optical receivers were connected to chronically implanted optical fiber and suspended above the behavioral testing arena to allow mice to move freely while receiving laser stimulation. The wireless optical receivers were controlled by a remote controller. All laser output was manipulated with a stimulator (Teleopt, Japan) to output TTL Hi signal. The mice with the expression of eNpHR-eYFP or eYFP were divided into two groups: light ON and light OFF. For mice in the light ON group, optogenetic inhibition was achieved through the optical fiber connected to the wireless optical receivers by using 10 mW (~35.35 mW/mm2) of 15 min of constant 594-nm light. For mice in the light OFF group, no light was given.
Immunohistochemistry and imaging
After the end of CPA testing, all mice were perfused with 0.9% saline, followed by ice-cold solution of 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS) (pH 7.4). The brains were removed and fixed in 4% PFA overnight. The brains were cut in 50-μm coronal sections using a vibratome (Leica, USA) and collected in PBS. To examine the injection site and the expression of each tracer or virus, brain slices were rinsed in PBS for three times (5 min for each wash) and incubated with 4′,6-diamidino-2-phenylindole (C1002, Beyotime Biotechnology, USA) diluted into 0.5 μg/ml for 10 min at room temperature. To do immunohistochemistry experiments, brain slices were rinsed in PBS for three times (5 min for each wash) and incubated with blocking solution containing 10% normal goat serum and 0.3% Triton X-100 in PBS for 2 hours at 4°C. For the analysis of c-Fos expression or Arc expression in FG-labeling BLA neurons, brain slices were incubated with rabbit anti–c-Fos antibody (#5348, Cell Signaling Technology, USA) diluted 1:500 or rabbit anti-Arc/Arg 3.1 antibody (#I56003, Synaptic Systems, Germany) diluted 1:1000 overnight at 4°C. Subsequently, they were rinsed in PBS for three times (5 min for each wash), followed by application of Alexa Fluor 594–conjugated goat anti-rabbit antibody (Jackson ImmunoResearch Laboratory, USA) diluted 1:200 for 2 hours at 37°C. For the analysis of c-Fos and Arc expression in FG-labeling BLA neurons, brain slices were incubated with guinea pig anti–c-Fos antibody (#226004, Synaptic Systems, Germany) diluted 1:500 overnight at 4°C. Then, they were rinsed in PBS for three times (5 min for each wash) and incubated with goat anti-guinea pig immunoglobulin G (IgG) antibody (Vector, US) diluted 1:200 for 1 hour at 37°C, followed by Alexa Fluor 488–conjugated streptavidin (Jackson ImmunoResearch Laboratory, USA) diluted 1:1000 for 1 hour at 37°C. Subsequently, they were rinsed in PBS for three times (5 min for each wash) and incubated with anti-Arc/Arg 3.1 antibody (#I56003, Synaptic Systems, Germany) diluted 1:1000 overnight at 4°C. Then, they were rinsed in PBS for three times (5 min for each wash) and incubated with goat anti-rabbit IgG antibody (Vector, USA) diluted 1:200 for 1 hour at 37°C, followed by cyanine 3–conjugated streptavidin (Jackson ImmunoResearch Laboratory, USA) diluted 1:1000 for 1 hour at 37°C. Last, immunolabeled sections were rinsed in PBS for three times (5 min for each wash), mounted on glass slides, and imaged by confocal microscopy (Nikon AIR MP, Japan). All c-Fos and Arc antibodies were dissolved into 10% normal goat serum and 0.3% Triton X-100 in PBS, and other antibodies were dissolved into 10% normal goat serum in PBS.
A series of slices containing the BLA were imaged by confocal microscopy with a 20× immersion lens and collected at a resolution of 1024 × 1024 pixels. The same laser and scanning settings were used for all confocal images within an experiment to allow comparison across groups. In general, coronal sections from five to eight mice were used for quantitative analysis. Five to eight images of each mouse and one image of each slice were averaged to determine a value for the slice. Series of images were captured from the confocal microscope and converted to 8-bit gray scale images, and then the area and mean gray values of white color clusters were measured using the Image-Pro Plus 6.0 software. Quantification of c-Fos, FG, and c-Fos + FG labeling neurons was estimated in the form of optical density with the same threshold. The positive neurons were defined with large nuclei stained diffusely and staining above basal background. Quantitative analysis of Arc labeling neurons and quantitative analysis of c-Fos + Arc + FG labeling neurons were performed as described above.
Statistical analysis
All data were analyzed using GraphPad Prism 5. Numerical data were expressed as the mean ± SEM. Statistical significance was determined using Student’s t test for comparisons between two groups or analyses of variance (ANOVAs) for comparisons among three or more groups. One-way ANOVA and two-way ANOVA were followed by Bonferroni post hoc test. In all cases, n refers to the number of animals. For all results, P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Program of Basic Research sponsored by the Ministry of Science and Technology of China (2015CB553500, 2009CB52201, and 2013CB835100), Science and Technology Program of Yunnan Province (2013GA003), and Project of Foundation of National Natural Science of China (31121061, 91332204, 31421091, 81371466, and 31070932). Author contributions: J.S.: conception and design, acquisition of data, analysis and interpretation of data, and drafting or revising the article; D.S., X.G., Y.Z., D.C., Q.M., and H.S.: acquisition of data as well as analysis and interpretation of data; L.M.: conception and design; B.L. and M.C.: conception and design as well as analysis and interpretation of data; P.Z.: conception and design, analysis and interpretation of data, and drafting or revising the article. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat3210/DC1
Supplementary Materials and Methods
Fig. S1. Influence of in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL on conditioned context–induced place aversion in morphine withdrawal mice.
Fig. S2. Influence of in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the IL on conditioned context–induced place aversion in morphine withdrawal mice.
Fig. S3. Tracing the feedback circuit from the PrL to the BLA.
Fig. S4. Diagram of the roles of BLA-PrL-BLA neuronal circuit in conditioned context–induced Arc activation in the BLA and the retrieval of morphine withdrawal memory.
REFERENCES AND NOTES
- 1.Daglish M. R. C., Weinstein A., Malizia A. L., Wilson S., Melichar J. K., Britten S., Brewer C., Lingford-Hughes A., Myles J. S., Grasby P., Nutt D. J., Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am. J. Psychiatry 158, 1680–1686 (2001). [DOI] [PubMed] [Google Scholar]
- 2.Hellemans K. G. C., Everitt B. J., Lee J. L. C., Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J. Neurosci. 26, 12694–12699 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas M., Frenois F., Vouillac C., Stinus L., Cador M., Le Moine C., Reactivity and plasticity in the amygdala nuclei during opiate withdrawal conditioning: Differential expression of c-fos and arc immediate early genes. Neuroscience 154, 1021–1033 (2008). [DOI] [PubMed] [Google Scholar]
- 4.Frenois F., Stinus L., Di Blasi F., Cador M., Le Moine C., A specific limbic circuit underlies opiate withdrawal memories. J. Neurosci. 25, 1366–1374 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulteis G., Ahmed S. H., Morse A. C., Koob G. F., Everitt B. J., Conditioning and opiate withdrawal. Nature 405, 1013–1014 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Li Z., Luan W., Chen Y., Chen M., Dong Y., Lai B., Ma L., Zheng P., Chronic morphine treatment switches the effect of dopamine on excitatory synaptic transmission from inhibition to excitation in pyramidal cells of the basolateral amygdala. J. Neurosci. 31, 17527–17536 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bishop S. F., Lauzon N. M., Bechard M., Gholizadeh S., Laviolette S. R., NMDA receptor hypofunction in the prelimbic cortex increases sensitivity to the rewarding properties of opiates via dopaminergic and amygdalar substrates. Cereb. Cortex 21, 68–80 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Senn V., Wolff S. B. E., Herry C., Grenier F., Ehrlich I., Gründemann J., Fadok J. P., Müller C., Letzkus J. J., Lüthi A., Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Richter-Levin G., Akirav I., Amygdala-hippocampus dynamic interaction in relation to memory. Mol. Neurobiol. 22, 11–20 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Stamatakis A. M., Sparta D. R., Jennings J. H., McElligott Z. A., Decot H., Stuber G. D., Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology 76 Pt B, 320–328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuber G. D., Sparta D. R., Stamatakis A. M., van Leeuwen W. A., Hardjoprajitno J. E., Cho S., Tye K. M., Kempadoo K. A., Zhang F., Deisseroth K., Bonci A., Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J., Chen M., Dong Y., Lai B., Zheng P., Chronic morphine selectively sensitizes the effect of D1 receptor agonist on presynaptic glutamate release in basolateral amygdala neurons that project to prelimbic cortex. Neuropharmacology 133, 375–384 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Fields R. D., Eshete F., Stevens B., Itoh K., Action potential-dependent regulation of gene expression: Temporal specificity in ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J. Neurosci. 17, 7252–7266 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li M., Hou Y.-y., Lu B., Chen J., Chi Z.-q., Liu J.-g., Expression pattern of neural synaptic plasticity marker-Arc in different brain regions induced by conditioned drug withdrawal from acute morphine-dependent rats. Acta Pharmacol. Sin. 30, 282–290 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blokhina E. A., Sukhotina I. A., Bespalov A. Y., Pretreatment with morphine potentiates naloxone-conditioned place aversion in mice: Effects of NMDA receptor antagonists. Eur. J. Pharmacol. 406, 227–232 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Tzingounis A. V., Nicoll R. A., Arc/Arg3.1: Linking gene expression to synaptic plasticity and memory. Neuron 52, 403–407 (2006). [DOI] [PubMed] [Google Scholar]
- 17.Alaghband Y., O’Dell S. J., Azarnia S., Khalaj A. J., Guzowski J. F., Marshall J. F., Retrieval-induced NMDA receptor-dependent Arc expression in two models of cocaine-cue memory. Neurobiol. Learn. Mem. 116, 79–89 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heidbreder C. A., Groenewegen H. J., The medial prefrontal cortex in the rat: Evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 27, 555–579 (2003). [DOI] [PubMed] [Google Scholar]
- 19.Roozendaal B., McReynolds J. R., Van der Zee E. A., Lee S., McGaugh J. L., McIntyre C. K., Glucocorticoid effects on memory consolidation depend on functional interactions between the medial prefrontal cortex and basolateral amygdala. J. Neurosci. 29, 14299–14308 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenkranz J. A., Grace A. A., Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J. Neurosci. 21, 4090–4103 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao Y., Zhang J., Yang H., Cui D., Song J., Ma Q., Luan W., Lai B., Ma L., Chen M., Zheng P., Memory retrieval in addiction: A role for miR-105-mediated regulation of D1 receptors in mPFC neurons projecting to the basolateral amygdala. BMC Biol. 15, 128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zingg B., Chou X.-l., Zhang Z.-g., Mesik L., Liang F., Tao H. W., Zhang L. I., AAV-mediated anterograde transsynaptic tagging: Mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron 93, 33–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dragunow M., Faull R., The use of c-fos as a metabolic marker in neuronal pathway tracing. J. Neurosci. Methods 29, 261–265 (1989). [DOI] [PubMed] [Google Scholar]
- 24.Cheriyan J., Kaushik M. K., Ferreira A. N., Sheets P. L., Specific targeting of the basolateral amygdala to projectionally defined pyramidal neurons in prelimbic and infralimbic cortex. eNeuro 3, ENEURO.0002-16.2016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stefanik M. T., Kalivas P. W., Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Front. Behav. Neurosci. 7, 213 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fuchs R. A., Eaddy J. L., Su Z.-I., Bell G. H., Interactions of the basolateral amygdala with the dorsal hippocampus and dorsomedial prefrontal cortex regulate drug context-induced reinstatement of cocaine-seeking in rats. Eur. J. Neurosci. 26, 487–498 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Mashhoon Y., Wells A. M., Kantak K. M., Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol. Biochem. Behav. 96, 347–353 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng H. Z., Fields R. D., Nelson P. G., Specific regulation of immediate early genes by patterned neuronal activity. J. Neurosci. Res. 35, 459–467 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Dilgen J., Tejeda H. A., O’Donnell P., Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. J. Neurophysiol. 110, 221–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J., Kwon J.-T., Kim H.-S., Josselyn S. A., Han J.-H., Memory recall and modifications by activating neurons with elevated CREB. Nat. Neurosci. 17, 65–72 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Plath N., Ohana O., Dammermann B., Errington M. L., Schmitz D., Gross C., Mao X., Engelsberg A., Mahlke C., Welzl H., Kobalz U., Stawrakakis A., Fernandez E., Waltereit R., Bick-Sander A., Therstappen E., Cooke S. F., Blanquet V., Wurst W., Salmen B., Bösl M. R., Lipp H.-P., Grant S. G. N., Bliss T. V., Wolfer D. P., Kuhl D., Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron 52, 437–444 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Nakayama D., Iwata H., Teshirogi C., Ikegaya Y., Matsuki N., Nomura H., Long-delayed expression of the immediate early gene Arc/Arg3.1 refines neuronal circuits to perpetuate fear memory. J. Neurosci. 35, 819–830 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrovich G. D., Holland P. C., Gallagher M., Amygdalar and prefrontal pathways to the lateral hypothalamus are activated by a learned cue that stimulates eating. J. Neurosci. 25, 8295–8302 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham M. G., Bhattacharyya S., Benes F. M., Increasing Interaction of amygdalar afferents with GABAergic interneurons between birth and adulthood. Cereb. Cortex 18, 1529–1535 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Gabbott P. L. A., Warner T. A., Busby S. J., Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience 139, 1039–1048 (2006). [DOI] [PubMed] [Google Scholar]
- 36.Desjardins S., Belkai E., Crete D., Cordonnier L., Scherrmann J.-M., Noble F., Marie-Claire C., Effects of chronic morphine and morphine withdrawal on gene expression in rat peripheral blood mononuclear cells. Neuropharmacology 55, 1347–1354 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Jin C., Araki H., Nagata M., Suemaru K., Shibata K., Kawasaki H., Hamamura T., Gomita Y., Withdrawal-induced c-Fos expression in the rat centromedial amygdala 24 h following a single morphine exposure. Psychopharmacology 175, 428–435 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Gracy K. N., Dankiewicz L. A., Koob G. F., Opiate withdrawal-induced fos immunoreactivity in the rat extended amygdala parallels the development of conditioned place aversion. Neuropsychopharmacology 24, 152–160 (2001). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat3210/DC1
Supplementary Materials and Methods
Fig. S1. Influence of in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the PrL on conditioned context–induced place aversion in morphine withdrawal mice.
Fig. S2. Influence of in vivo chemical-genetic inhibition of cell bodies of BLA neurons projecting to the IL on conditioned context–induced place aversion in morphine withdrawal mice.
Fig. S3. Tracing the feedback circuit from the PrL to the BLA.
Fig. S4. Diagram of the roles of BLA-PrL-BLA neuronal circuit in conditioned context–induced Arc activation in the BLA and the retrieval of morphine withdrawal memory.


