Alarm signals from marine zooplankton structure phytoplankton communities.
Abstract
Fear of predation may influence food webs more than actual predation. However, the mechanisms and magnitude of nonconsumptive predator effects are largely unknown in unicellular-dominated food webs such as marine plankton. We report a general mechanism of chemically induced predator effects in marine plankton. Copepods, the most abundant zooplankton in the oceans, imprint seawater with unique polar lipids—copepodamides—which trigger toxin production and bioluminescence in harmful dinoflagellates. We show that copepodamides also elicit defensive traits in other phytoplankton, inducing the harmful algal bloom-forming diatom Pseudo-nitzschia seriata to produce 10 times more toxins, and colony-forming diatoms to decrease colony size by half. A 1-year study in the northeast Atlantic revealed that natural copepodamide concentrations are high enough to induce harmful algal toxins and size reduction in dominant primary producers when copepods are abundant. We conclude that copepodamides will structure marine plankton toward smaller, more defended life forms on basin-wide scales.
INTRODUCTION
In contrast to predation, the effect of fear is not strictly density-dependent; predators can scare many more prey than they consume (1–3). Nonconsumptive effects of predators may consequently amplify in food webs and cause cascading effects that exceed the direct effects of prey consumption (1). Yet, the mechanisms and relative importance of nonconsumptive effects are largely unknown in marine plankton (4). We describe what seems to be a universal chemically-mediated mechanism of nonconsumptive effects of marine copepods on their mainly unicellular prey organisms.
Despite their small size, copepods imbue seawater with a unique chemical signature. Prey organisms sense this molecular fingerprint (5–7) and respond by activating defensive or cryptic traits to evade predation. Some increase toxin production (8, 9) or bioluminescence (10), while others reduce colony size or swimming speed (6, 7). Reducing size and speed reduce encounter rates with predators and thereby protect prey from predation. The effects of algal toxins on herbivory are less predictable. Some grazers avoid toxic algae, whereas others graze on those same algae, seemingly unaffected by the algal toxins (11, 12). In the few cases where algae have been primed with predator cues to produce more toxins, however, a larger proportion of the toxic algae is rejected by the grazers (8, 13). The role of defensive metabolites may consequently have been underestimated because of the use of grazer-free cultures in earlier experiments. The nonconsumptive effects of copepods consequently structure marine food webs beyond effects on actual grazing, but the molecular mechanisms and ecological consequences of these additional effects are still poorly understood (4).
A group of eight closely related polar lipids named copepodamides isolated from copepods were recently shown to induce paralytic shellfish toxin production and bioluminescence in harmful dinoflagellates (10, 14). If copepodamides also elicit defensive traits in other phytoplankton, they have the potential to drive nonconsumptive effects on an unprecedented scale in marine plankton, favoring smaller, more defended phenotypes and harmful algal bloom formation. General elicitors are common in higher plants, where universal alarm signals induce a wide variety of plant defenses (15, 16). Here, we test this hypothesis by exposing centric (Skeletonema marinoi) and pennate (Pseudo-nitzschia seriata) diatoms to copepodamides from the co-occurring copepod Calanus finmarchicus (17, 18). Diatoms are phylogenetically distant from the dinoflagellates known to respond to copepodamides (10). Moreover, along with dinoflagellates, centric and pennate diatoms are the most important constituents of marine microphytoplankton.
Skeletonema and Pseudo-nitzschia have contrasting defense strategies. S. marinoi adjusts its colony size to escape detection (5, 7). Pseudo-nitzschia produces the amnesic shellfish toxin domoic acid, but its defensive effect on copepods is debated (9, 19). Domoic acid accumulates in marine food webs and affects organisms from copepods to seabirds and whales (20). Pseudo-nitzschia increases toxin production in response to grazing copepods (9, 21). The cueing compounds are, however, not known. We exposed Skeletonema and Pseudo-nitzschia to copepodamides isolated from the copepod C. finmarchicus, one of the dominant copepod species on the northern hemisphere (Fig. 1 and fig. S1). By combining dose-response relationships for Skeletonema and Pseudo-nitzschia with measurements of copepodamides in the northeast Atlantic, we predict the level of induction of algal toxins and decreased colony size in nature.
Fig. 1. Illustration of the signaling system.
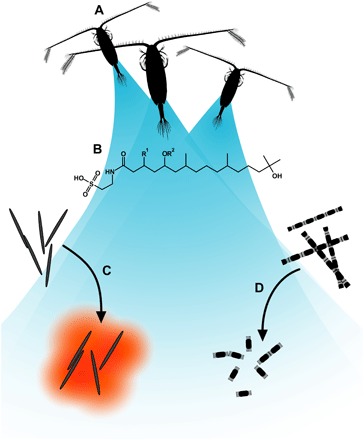
(A) The copepod Calanus sp. emits copepodamides. (B) General structure of copepodamides. Copepodamides are known to induce toxin formation in dinoflagellates. (C) We explore whether copepodamides induce toxin formation in other algae, such as P. seriata, infamous for its production of the neurotoxic alkaloid domoic acid. (D) We also test whether copepodamides are responsible for grazer-induced colony size plasticity seen in other dominating phytoplankton such as S. marinoi.
RESULTS
Both Pseudo-nitzschia and Skeletonema responded to copepodamides in the femto- to picomolar range. Pseudo-nitzschia produced up to 10 times more domoic acid, and Skeletonema reduced its chain length by half (Fig. 2). Half-saturation constants were 0.08 and 0.02 pM for Pseudo-nitzschia and Skeletonema, respectively, which are in the same range as the concentrations observed off the Swedish west coast (40 fM to 2 pM, Fig. 3A). Copepodamide concentrations in the ocean follow the seasonal fluctuation in copepod biomass and peak in the middle of summer (r2 = 0.55; Fig. 3, B and C). The highest concentration recorded, in July, corresponds to levels that induce a ninefold increase in toxicity in Pseudo-nitzschia and a maximum chain length reduction response in Skeletonema in the laboratory. Hydrographic data recorded at the sample site are shown in fig. S2.
Fig. 2. Dose-response relationships for Pseudo-nitzschia and Skeletonema exposed to copepodamides.
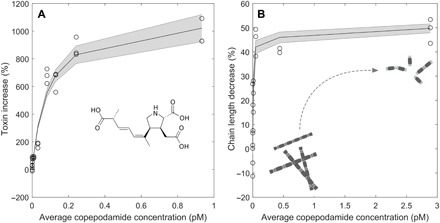
(A) Dose-dependent increase in domoic acid content in P. seriata in response to the composition of copepodamides found in C. finmarchicus, a common copepod in the area. (B) Dose-response relationship showing the percent chain length shortening, compared to controls in S. marinoi, as a function of copepodamide concentration. Note that chain length shortening is expressed relative to untreated control cultures and cannot reach 100%.
Fig. 3. In situ measurements of copepodamides.
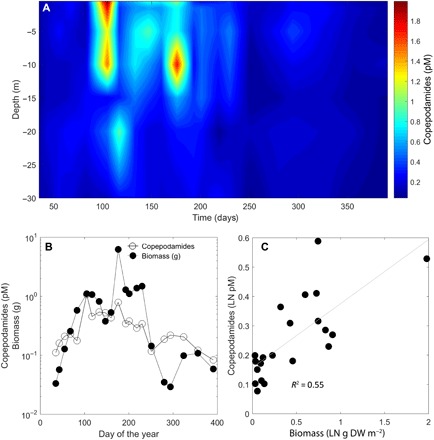
(A) Depth-resolved concentration of copepodamides over a temperate year off the Swedish west coast. Samples were taken biweekly at depths of 0, 1, 5, 10, 15, 20, and 30 m. Color scale shows the concentration (pM) of total copepodamides. (B) Average concentration of copepodamides (pM) in the water column in relation to copepod biomass (g DW m−2) over the year. (C) Copepodamide concentration largely follows the biomass of copepods, which is more evident if the natural logarithm of copepodamide concentration (pM) is plotted against the natural logarithm of the copepod biomass (g DW m−2).
DISCUSSION
We show that, in addition to the dinoflagellates previously known to respond to copepodamides (10, 14), other phytoplankton from distant phylogenetic groups also respond to copepodamides. The average copepodamide concentration in the upper 15 m of the Skagerrak sea is sufficient to induce more than a doubling of domoic acid in Pseudo-nitzschia during a substantial part (89%) of the year (Fig. 4A). Similarly, copepodamides will suppress chain formation in Skeletonema during 84% of the year (Fig. 4B). The natural concentrations of copepodamides only permit long-chain formation during short time periods, one of which coincides with the temperate spring bloom. The dominance of large chain-forming diatoms in the spring bloom has traditionally been explained by a combination of the abiotic factors turbulent mixing and nutrient dynamics (22). The subpicomolar sensitivity of Skeletonema to copepodamides, however, suggests that nonconsumptive effects of copepods may be an important factor limiting the occurrence of chain-forming diatoms to short time periods. The sensitivity of Skeletonema to copepodamides is consistent with the observations of Bjærke and colleagues (5), who showed that chain lengths of Skeletonema are negatively correlated with copepod density in the ocean, and that as little as one to two copepods per liter are sufficient to induce chain length shortening (5). The in situ concentrations of copepodamides correspond with the only previous record, 0 to 0.3 pM, obtained during a 2-day sampling effort in the Skagerrak sea (14). Effective concentrations of copepodamides are in the same range as the protein signals that induce defensive morphology in ciliates (23), but higher than the Volvox pheromones (10−16 M) and the rosette-inducing factor that induce colony formation in choanoflagellates (24). Copepods act as moving point sources of copepodamides in nature, creating a heterogeneous concentration field where local concentrations can be substantially higher than the average concentrations reported here.
Fig. 4. Predicted effects of copepodamides in nature.
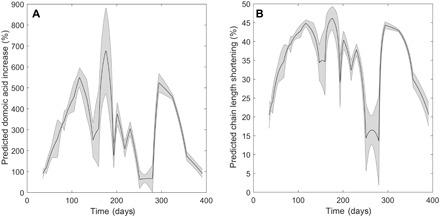
(A) Predicted domoic acid production increase (%) over the year calculated from in situ concentrations of copepodamides in the upper 15 m (Fig. 2) and dose-response relationships (Fig. 3). The shaded error interval represents the SD. (B) Predicted decrease in chain length for S. marinoi calculated the same way. The different shape of the curve in (B) is due to the more sensitive S. marinoi more often approaching the maximum level of induction (here 50%).
Diatoms and dinoflagellates dominate the larger phytoplankton (25). Grazer-induced changes in important traits such as size, toxin content, and swimming speed will consequently have structuring effects on the pelagic food web (26). A decreased colony size redirects carbon from mesozooplankton grazers to microzooplankton such as ciliates and heterotrophic dinoflagellates (5). The formation of large fast sinking aggregates, “marine snow,” increases with chain length (27, 28). Chain length shortening consequently reduces vertical flux of aggregates and constrains the downward flux of organic matter. The decrease in chain length observed here can be predicted to decrease the encounter rate with mesozooplankton by 75% (7, 29) and the critical concentration needed for aggregate formation by 50% (28).
Induced toxin production in Pseudo-nitzschia contributes directly to harmful algal bloom formation. In addition, copepods often selectively reject more toxic cells and target less defended alternative prey (8, 30). Selective grazing is likely a key process driving harmful algal bloom formation. Harmful algal toxins accumulate through food web interactions and poison nontarget organisms such as seabirds, whales, and shellfish-consuming humans (8). Occasionally harmful algal blooms structure entire ecosystems during the Chrysochromulina polylepis bloom in the Skagerrak sea (31), which was accompanied by massive changes in fauna and local extinction of sensitive species. The effect of copepodamides on toxin production is typically larger than that of abiotic factors [Fig. 2; (14)], which suggests that copepodamide-induced toxin production may be an overlooked factor in harmful algal bloom formation.
Copepodamides are the first identified general defense elicitors in marine plankton. General elicitors do, however, exist in higher plants, where methyl jasmonate and volicitin induce a variety of plant defenses (15, 16). Wounded plants are often the source of alarm signals in higher plants [but see (16)]. Unicellular phytoplankton rarely face partial predation the way higher plants do and probably cannot rely on wound-activated signaling pathways. Copepod presence elicits a massive transcriptional response in Skeletonema, with hundreds of genes being up- or down-regulated in response to copepod presence (32). Thus, it is likely that the induction of defensive traits causes allocation costs and trade-offs in induced algae.
We conclude that copepodamides have a unique capacity to broadly induce defensive traits at the base of the marine food web. The chemically mediated nonconsumptive effect of copepods will favor smaller-sized, more defended phenotypes and will specifically trigger production of toxic secondary metabolites leading to harmful algal blooms.
MATERIALS AND METHODS
Experimental design
We exposed Skeletonema and Pseudo-nitzschia to copepodamides purified from C. finmarchicus, as described in (14). At the same time, we measured copepodamides in situ off the Swedish west coast biweekly for 1 year. The dose-response relationships for Skeletonema and Pseudo-nitzschia were combined to predict the level of induction in nature. Sample sizes and duration of experiments were derived from effect size and variance estimates in pilot experiments.
Dose-response experiments
S. marinoi (strain ID: GF 04-7D), isolated from the study area in 2009, was cultivated in silicate-enriched f/2 medium (33) with a salinity of 26 practical salinity units (psu) at 16°C, 12:12 hour light/dark cycles, 100 μmol photons m−2 s−1. P. seriata (strain Disko8), isolated from Disko Bay Greenland in 2013, was grown in L-medium at 22:2 hour light/dark cycles.
Algal cultures were incubated in 8.7-ml scintillation vials in triplicates (Pseudo-nitzschia) or quadruplicates (Skeletonema). Vials were coated with the blend of copepodamides from C. finmarchicus (fig. S1), corresponding to a total concentration of 1, 25, 50, 100, 150, 250, and 500 pM (Pseudo-nitzschia, n = 4 for each concentration) or 0, 1, 5, 10, 50, 100, and 500 pM (Skeletonema, n = 3 for each concentration). We added copepodamides dissolved in methanol or methanol only (negative control) and evaporated the solvent under a stream of nitrogen before adding algal culture. The effective concentrations (copepodamides in solution) were determined in a separate experiment, and dose-response relationships were calculated using the average effective concentration over the incubation time (fig. S3).
We diluted the stock cultures using f/4 medium to a final concentration of ≈5 × 103 cells ml−1 (Skeletonema) or ≈6.5 × 103 cells ml−1 (Pseudo-nitzschia). The vials were incubated on a plankton wheel (0.5 rpm) at 4°C, 22:2 hour light/dark cycles, ~60 μmol photons m−2 s−1 (Pseudo-nitzschia) or at 14.5°C, 12:12 hour light/dark cycles, ~100 μmol photons m−2 s−1 (Skeletonema) for 3 days. Pseudo-nitzschia samples were kept frozen until analysis of total domoic acid content. Skeletonema samples were preserved with acidic Lugol’s solution. Chain length (cells chain−1) of at least 50 chains from each replicate was counted under an inverted microscope.
Domoic acid analysis
Samples were defrosted, acidified with 0.2% formic acid, and loaded onto reversed-phase solid phase extraction (SPE) columns (Bond Elute C18 LRC 10 ml, 200 mg, 40 μm; Agilent) at ~1 ml min−1. SPE columns were preactivated with one column volume of methanol, followed by one column volume of water with 0.2% formic acid. After desalting columns with 5 ml Milli-Q water with 0.2% formic acid, columns were eluted with 750 μl of 50% methanol (aq). The eluent was left in the reservoir for 1 hour to lyse and extract cells. An additional 750 μl of 50% methanol (aq) was added after a 5-min soak step to maximize yield. Samples were analyzed by isocratic elution with 25% acetonitrile (aq) supplemented with 0.1% formic acid at 0.2 ml min−1 on an Agilent 1200 LC system fitted with RP-MS column, 150 mm × 2.1 mm, 2.6 μm (Thermo Scientific) coupled to an Agilent 6410 triple quadrupole detector. The ion source was operated at 300°C and 4500 V in negative mode with a nitrogen flow of 5.8 liters min−1 at 20 psi.
Field sample site
Samples were collected from the mouth of the Gullmar Fjord on the Swedish west coast (58°15′N 11°27′E). Hydrographic data during the sampling period are shown in fig. S2. We aimed to sample every second week but did not sample at wind speeds above 10 m/s. In total, 21 sample occasions were distributed over a 1-year period. Samples were taken between 10 a.m. and 1 p.m.
Copepodamide sampling in situ
To minimize loss of copepodamides through degradation and handling, the water samples were pulled onto SPE columns in situ, as described in (14). Syringes (100 ml) were interspersed on a submerged line and connected by silicone tubing to the Luer end of the SPE columns (Evolute ABN, 100 mg of sorbent). Pull speed, sorbent, and sample volume were optimized in pilot experiments with natural seawater spiked with known amounts of copepodamides. Copepodamides were sampled at depths of 0, 1, 5, 10, 15, 20, and 30 m. SPE columns were conditioned with 1.5 ml of methanol before deployment. The SPE openings were covered with 65-μm nylon plankton mesh to prevent copepods and copepod eggs from entering. Once deployed, the syringe plunger pulled water through the SPE by gravity from a weight connected to the syringe through a line. The weight was adjusted to achieve a flow rate of ~5 ml min−1. The rig was retrieved after 20 to 30 min, and the volume pulled was noted for each syringe. The procedure was repeated once to sample ~200 ml from each depth. Columns were transported to the laboratory (within 1 hour), desalted with 4 ml of Milli-Q water, and eluted in 3 ml of liquid chromatography–mass spectrometry (LC-MS)–grade methanol. One or two replicate profiles were obtained on each sampling occasion. On one occasion, we failed to get a sample from one of the depths; this value was interpolated linearly from the above and below sample at the same occasion. A single outlier sample, 40 times higher than the replicate from the same depth, was considered an artifact and removed. Sample head space was flushed with N2 gas before storage in glass vials at −80°C until analysis.
Field sampling of copepods
Copepods were collected with two replicate vertical hauls. The 90-μm mesh size WP-2 plankton net was pulled from a depth of 30 m to the surface at approximately 0.5 m s−1 and rinsed from the outside to concentrate the catch in the cod end. The concentrated samples were transferred to plastic jars and preserved in 4% formaldehyde buffered with sodium borate (borax). Analysis was carried out by the National Marine Fisheries Research Institute, Gdynia, Poland. Subsamples were counted until at least 100 individuals of each of the two to three most abundant taxa had been counted. Each copepod was staged according to nauplii; C1, C2, C3, C4, and C5 (copepodite stages 1 to 5); adult male; or adult female. The prosome length was obtained for 10 individuals of each stage unless there were less than 10 individuals in the subsample, in which case all individuals were measured. Biomass was calculated using length weight regressions compiled by Bjærke and colleagues (5). Raw data from zooplankton analysis are provided in data file S1.
Hydrographic metadata
A conductivity, temperature, and pressure profiler (CTD, SeaBird/General Oceanic) cast from the surface to 30 m provided standard data for salinity, temperature, and depth on each sampling occasion. The CTD was also equipped with a fluorescence sensor to estimate chlorophyll a. The data are shown in fig. S2.
Copepodamide extraction and analysis
We analyzed the known copepodamides (A to H) and two additional copepodamides sharing the same molecular scaffold with m/z (mass/charge ratio) of 658 and 686 in negative mode. All chemicals were purchased from Thermo Fisher and used as received. Copepodamides were separated by gradient elution on a high-performance liquid chromatography (Agilent 1200 series with a C18 column, 150 mm × 2.1 mm thermostated to 50°C) coupled to a triple quadrupole MS (Agilent 6410). The gradient went from 5 to 83% B during 18 min. Eluent A consisted of 35:35:30 methanol/acetonitrile/water, and eluent B consisted of 2-propanol. Both eluents were supplemented with 0.2% formic acid and 0.1% ammonia. We designed a multiple reaction monitoring method in negative mode with diagnostic fragments: m/z 432.2 for copepodamides A to C; m/z 430.2 for copepodamides D to F and the two new copepodamides with m/z 659 and 686 (34); m/z 124.0 for copepodamides G and H; fragmentor voltage 250 V; collision energy 44 for A to F, 659 and 686 and 40 for G and H. The ion source was operated at 300°C and 4500 V in negative mode with a nitrogen flow of 6 liters min−1 at 25 psi. Concentrations were determined by comparing with authentic standard available from previous work (14), assuming equal ionization efficiency for the individual copepodamides. Copepodamides for dose-response experiments were isolated from frozen C. finmarchicus, as described in (14). C. finmarchicus is a dominating copepod with geographical distribution overlapping with both Skeletonema and Pseudo-nitzschia.
Statistical analysis and calculations
All depth-resolved data were interpolated (interp2, MATLAB R2016a) for visualization. The average amount of copepodamides in the water column over time was calculated by averaging columns of the interpolated copepodamide concentration matrix. The predicted level of induction was calculated from dose-response relationships and the average copepodamide concentration in the upper 15 m at the study site. Only intact copepodamides were used, as deacylated copepodamides G and H are inactive at ecologically relevant concentrations [fig. S4; (14)]. To be conservative, we doubled the average concentration used to calculate dose-response relationships for this purpose. The dose-response curve fits were performed in MATLAB R2016a based on the Michaelis-Menten equation.
Supplementary Material
Acknowledgments
We thank K. Rigby and L. Gamfeldt for commenting on an earlier draft of the manuscript. Funding: This work was supported by Swedish Research Council VR 2015-05491—Signals in the Sea (to E.S.), Olle Engkvist Byggmästare Foundation (to E.S.), Danish Research Council (DFF) 1323-00258 (to N.L.), and German Research Foundation (DFG) GR 4716/1-1 (to W.G.). Author contributions: All authors designed and performed the study, and E.S. wrote the manuscript with input from all authors. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat5096/DC1
Fig. S1. Composition of copepodamides from C. finmarchicus used in experiment.
Fig. S2. Hydrographic data.
Fig. S3. Effective concentrations of copepodamides.
Fig. S4. Copepodamides G and H do not induce chain length shortening in Skeletonema.
Data file S1. Zooplankton raw data.
Data file S2. MS raw data.
REFERENCES AND NOTES
- 1.Preisser E. L., Bolnick D. I., Benard M. F., Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology 86, 501–509 (2005). [Google Scholar]
- 2.Schmitz O. J., Effects of predator hunting mode on grassland ecosystem function. Science 319, 952–954 (2008). [DOI] [PubMed] [Google Scholar]
- 3.Suraci J. P., Clinchy M., Dill L. M., Roberts D., Zanette L. Y., Fear of large carnivores causes a trophic cascade. Nat. Commun. 7, 10698 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stibor H., Vadstein O., Diehl S., Gelzleichter A., Hansen T., Hantzsche F., Katechakis A., Lippert B., Løseth K., Peters C., Roederer W., Sandow M., Sundt-Hansen L., Olsen Y., Copepods act as a switch between alternative trophic cascades in marine pelagic food webs. Ecol. Lett. 7, 321–328 (2004). [Google Scholar]
- 5.Bjærke O., Jonsson P. R., Alam A., Selander E., Is chain length in phytoplankton regulated to evade predation? J. Plankton Res. 37, 1110–1119 (2015). [Google Scholar]
- 6.Long J. D., Smalley G. W., Barsby T., Anderson J. T., Hay M. E., Chemical cues induce consumer-specific defenses in a bloom-forming marine phytoplankton. Proc. Natl. Acad. Sci. U.S.A. 104, 10512–10517 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selander E., Jakobsen H. H., Lombard F., Kiørboe T., Grazer cues induce stealth behavior in marine dinoflagellates. Proc. Natl. Acad. Sci. U.S.A. 108, 4030–4034 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selander E., Thor P., Toth G., Pavia H., Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc. Biol. Sci. 273, 1673–1680 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tammilehto A., Nielsen T. G., Krock B., Møller E. F., Lundholm N., Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods. Aquat. Toxicol. 159, 52–61 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Lindström J., Grebner W., Rigby K., Selander E., Effects of predator lipids on dinoflagellate defence mechanisms—Increased bioluminescence capacity. Sci. Rep. 7, 13104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tammilehto A., Nielsen T. G., Krock B., Møller E. F., Lundholm N., Calanus spp.—Vectors for the biotoxin, domoic acid, in the Arctic marine ecosystem? Harmful Algae 20, 165–174 (2012). [Google Scholar]
- 12.Turner J. T., Planktonic marine copepods and harmful algae. Harmful Algae 32, 81–93 (2014). [Google Scholar]
- 13.Xu J., Nielsen L. T., Kiørboe T., Foraging response and acclimation of ambush feeding and feeding-current feeding copepods to toxic dinoflagellates. Limnol. Oceanogr. 63, 1449–1461 (2018). [Google Scholar]
- 14.Selander E., Kubanek J., Hamberg M., Andersson M. X., Cervin G., Pavia H., Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc. Natl. Acad. Sci. U.S.A. 112, 6395–6400 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farmer E. E., Ryan C. A., Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. U.S.A. 87, 7713–7716 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber H., Fatty acid-derived signals in plants. Trends Plant Sci. 7, 217–224 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Båmstedt U., Håkanson J. L., Brenner-Larsen J., Björnsen P. K., Geertz-Hansen O., Tiselius P., Copepod nutritional condition and pelagic production during autumn in Kosterfjorden, western Sweden. Mar. Biol. 104, 197–208 (1990). [Google Scholar]
- 18.Niehoff B., Madsen S., Hansen B., Nielsen T., Reproductive cycles of three dominant Calanus species in Disko Bay, West Greenland. Mar. Biol. 140, 567–576 (2002). [Google Scholar]
- 19.Lundholm N., Skov J., Pocklington R., Moestrup Ø., Domoic acid, the toxic amino acid responsible for amnesic shellfish poisoning, now in Pseudonitzschia seriata (Bacillariophyceae) in Europe. Phycologia 33, 475–478 (1994). [Google Scholar]
- 20.Trainer V. L., Bates S. S., Lundholm N., Thessen A. E., Cochlan W. P., Adams N. G., Trick C. G., Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae 14, 271–300 (2012). [Google Scholar]
- 21.Harðardóttir S., Pančić M., Tammilehto A., Krock B., Møller E., Nielsen T. G., Lundholm N., Dangerous relations in the Arctic marine food web: Interactions between toxin producing Pseudo-nitzschia diatoms and Calanus copepodites. Mar. Drugs 13, 3809–3835 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margalef R., Life-forms of phytoplankton as survival alternatives in an unstable environment. Oceanol. Acta 1, 493–509 (1978). [Google Scholar]
- 23.Kusch J., Heckmann K., Isolation of the Lembadion-Factor, a morphogenetically active signal, that induces Euplotes cells to change from their ovoid form into a larger lateral winged morph. Dev. Genet. 13, 241–246 (1992). [Google Scholar]
- 24.Alegado R. A., Brown L. W., Cao S., Dermenjian R. K., Zuzow R., Fairclough S. R., Clardy J., King N., A bacterial sulfonolipid triggers multicellular development in the closest living relatives of animals. eLife 1, e00013 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Falkowski P. G., Katz M. E., Knoll A. H., Quigg A., Raven J. A., Schofield O., Taylor F. J. R., The evolution of modern eukaryotic phytoplankton. Science 305, 354–360 (2004). [DOI] [PubMed] [Google Scholar]
- 26.Hansen B., Bjornsen P. K., Hansen P. J., The size ratio between planktonic predators and their prey. Limnol. Oceanogr. 39, 395–403 (1994). [Google Scholar]
- 27.Alldredge A. L., Gotschalk C., In situ settling behavior of marine snow. Limnol. Oceanogr. 33, 339–351 (1988). [Google Scholar]
- 28.Jackson G. A., A model of the formation of marine algal flocs by physical coagulation processes. Deep Sea Res. Part A Oceanogr. Res. Pap. 37, 1197–1211 (1990). [Google Scholar]
- 29.Bergkvist J., Thor P., Jakobsen H. H., Wängberg S.-Å., Selander E., Grazer-induced chain length plasticity reduces grazing risk in a marine diatom. Limnol. Oceanogr. 57, 318–324 (2012). [Google Scholar]
- 30.Irigoien X., Flynn K. J., Harris R. P., Phytoplankton blooms: A ‘loophole’ in microzooplankton grazing impact? J. Plankton Res. 27, 313–321 (2005). [Google Scholar]
- 31.Underdal B., Skulberg O. M., Dahl E., Aune T., Disastrous bloom of Chrysochromulina polylepis (Prymnesiophyceae) in Norwegian coastal waters 1988: Mortality in marine biota. Ambio 18, 265–270 (1989). [Google Scholar]
- 32.Amato A., Sabatino V., Nylund G. M., Bergkvist J., Basu S., Andersson M. X., Sanges R., Godhe A., Kiørboe T., Selander E., Ferrante M. I., Grazer-induced transcriptomic and metabolomic response of the chain-forming diatom Skeletonema marinoi. ISME J. 12, 1594–1604 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R. R. L. Guillard, in Culture of Marine Invertebrate Animals (Springer, 1975), pp. 29–60. [Google Scholar]
- 34.Grebner W., Berglund E. C., Berggren F., Eklund J., Harðadóttir S., Andersson M. X., Selander E., Induction of defensive traits in marine plankton—New copepodamide structures. Limnol. Oceanogr. (2018). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/2/eaat5096/DC1
Fig. S1. Composition of copepodamides from C. finmarchicus used in experiment.
Fig. S2. Hydrographic data.
Fig. S3. Effective concentrations of copepodamides.
Fig. S4. Copepodamides G and H do not induce chain length shortening in Skeletonema.
Data file S1. Zooplankton raw data.
Data file S2. MS raw data.


